Abstract
Chronic oral treatment of tetrabromobisphenol A (TBBPA) to female Wistar Han rats resulted in increased incidence of cell proliferation at 250 mg/kg and tumor formation in the uterus at higher doses. The present study was designed to test the hypothesis that disruption of estrogen homeostasis was a major mode-of-action for the observed effects. Biological changes were assessed in serum, liver, and the proximal (nearest the cervix) and distal (nearest the ovaries) sections of the uterine horn of Wistar Han rats 24 hours following administration of the last of five daily oral doses of 250 mg/kg. Expression of genes associated with receptors, biosynthesis, and metabolism of estrogen was altered in the liver and uterus. TBBPA treatment also resulted in changes in expression of genes associated with cell division and growth. Changes were also observed in the concentration of thyroxine in serum and in expression of genes in the liver and uterus associated with thyroid hormone receptors. Differential expression of some genes was tissue-dependent or specific to tissue location in the uterus. The biological responses observed in the present study support the hypothesis that perturbation of estrogen homeostasis is a major mode-of-action for TBBPA-mediated cell proliferation and tumorigenesis previously observed in the uterus of TBBPA-treated Wistar Han rats.
Keywords: Tetrabromobisphenol A, TBBPA, uterine toxicity, rats, gene expression, estrogen homeostasis
1. Introduction
Some high production volume brominated flame retardants (BFRs) such as polybrominated diphenyl ether (PBDEs) mixtures have been, or are being phased-out due to their environmental persistence and potential for bioaccumulation and toxicity (USEPA, 2014). In contrast, production of tetrabromobisphenol A (TBBPA), used primarily as a reactive BFR in epoxy resins for printed circuit boards, continues at a high volume (Canada, 2012; de Wit, 2010; ECB, 2006). TBBPA is well absorbed in rats, rapidly conjugated and eliminated from systemic circulation, has little potential to accumulate in tissues, and acute toxicity is in the g/kg range (EU, 2006; Hakk et al., 2000; Knudsen et al., 2014; Kuester et al., 2007; USEPA, 2008). TBBPA may be degraded by photochemical, thermal, and bacterial decomposition in the environment (Li et al., 2014). Even so, TBBPA is categorized as moderately persistent (USEPA, 2008). TBBPA has minimal potential to leach from products when used as a reactive BFR (ECB, 2006; USEPA, 2008); however, the chemical more easily enters the environment when used in an additive fashion similar to that of PBDEs (Canada, 2012; de Wit et al., 2010).
The safety of TBBPA is of concern based on effects on the endocrine system in animals and a recent study reporting carcinogenic activity in TBBPA-treated rats and mice (Dunnick et al., 2015). Studies conducted in vitro indicate that TBBPA competes with thyroxine (T4) for binding to transthyretin (TTR), inhibits binding of triiodothyronine (T3) to thyroid hormone receptors (TR), affects gene or protein expression of TR and peroxisome proliferator-activated receptors (PPARs), and may bind to PPARγ (Hamers et al., 2006; Kitamura et al., 2002; Meerts et al., 2000; Riu et al., 2011). TBBPA was estrogenic in a uterotropic assay in ovariectomized mice and competed with 17β-estradiol (E2) for receptor binding in MtT/E-2 cells (Kitamura et al., 2002, 2005). However, other studies indicate that TBBPA has minimal estrogenic activity. TBBPA had no activity in estrogen receptor (ER)-CALUX® assays and had little affinity for ERα in MCF-7 or HepG2 cells (Dorosh et al., 2010; Hamers et al., 2006; Meerts et al., 2001; Riu et al., 2011). Recently, the National Toxicology Program (NTP) reported a positive 2-year carcinogenicity study for TBBPA in both Wistar Han rats and B6C3F1 mice (NTP, 2014). Results indicated an increased incidence of epithelial atypical hyperplasia in uterine tissue of rats at the lowest dose tested (250 mg/kg) and an increased incidence of uterine tumors (adenomas, adenocarcinomas, and malignant Müllerian cell tumors) at higher doses. This response in rats raises concern for human exposure to TBBPA. TBBPA has been detected in serum, milk, and/or adipose samples from disparate populations (NTP, 2014). Although not correlated with any specific chemical exposure, cancers of the uterine corpus are estimated to be the fourth most common of new cancer cases in U.S. women (Siegel et al. 2013).
The mechanism(s) of the carcinogenic response in the uterus of TBBPA-treated rats may involve tissue insult from accumulation of TBBPA or a reactive metabolite. TBBPA can be metabolized to a 2,6-dibromobenzoquinone radical in male Sprague-Dawley rats (Chignell et al., 2008). However, several studies investigating the fate of TBBPA in rats detected no evidence of oxidative cleavage of the molecule (Hakk et al., 2000; Knudsen et al., 2014; Kuester et al., 2007). Furthermore, neither persistence nor accumulation of TBBPA-derived material was observed in uterine tissue following repeated oral administration of 14C-labeled TBBPA to female Wistar Han rats (Knudsen et al., 2014). TBBPA-mediated disruption of estrogen homeostasis may be the best explanation for observed carcinogenic effects in the uterus (Dunnick et al., 2015). Excessive estrogen exposure has been linked to uterine tumors in humans (Lax, 2004). Inhibition, induction, or saturation of enzymes involved in estrogen synthesis, metabolism, and elimination could result in increased concentrations of the hormone in uterine tissue of TBBPA-treated rats.
Conjugation with glucuronic acid and/or sulfate are the major pathways for TBBPA metabolism in female Wistar Han rats (Knudsen et al., 2014). Estrogen is also metabolized through these conjugation pathways, with sulfation being the major mechanism for regulation of serum concentrations (Raftogianis et al., 2000). TBBPA and E2 have similar binding affinities for human estrogen sulfotransferase (Gosavi et al., 2013) and TBBPA is a potent inhibitor of E2 sulfation in vitro (Hamers et al., 2006). Decreased availability of the enzyme following TBBPA exposure could lead to prolonged or elevated levels of estrogen in target tissues.
Cell proliferation occurred in the uterus after chronic administration of 250 mg/kg to rats (Dunnick et al., 2015). This dose could be considered to be well above a threshold needed to alter kinetics of TBBPA and/or estrogen metabolism following short-term repeated administration. Therefore, it was postulated that effects on estrogen biosynthesis and/or metabolism would be observed after administration of as few as five daily doses of 250 mg/kg. The results of the present study confirmed the study design and supported the proposed hypothesis that disruption of estrogen homeostasis is a major mode-of-action for the histological changes observed in the uterus of Wistar Han rats chronically exposed to TBBPA.
2. Materials and methods
2.1 Animals and treatments
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the National Institute of Environmental Health Sciences (NIEHS). Female Wistar Han rats (circa 9 weeks old) were obtained from Charles River, Raleigh NC. Upon receipt into the animal facilities, bedding from male rat cages was added to the cages housing the females to stage the estrous cycle prior to dosing with TBBPA (Whitten, 1958). The estrous cycle of each rat was monitored daily using the vaginal cytology assay as described by Hubscher et al. (2005). The randomized rats were initially dosed on a morning when the assay indicated that 18 of 20 rats were in diestrus (one TBBPA-treated rat was in metestrus and one vehicle control rat was in proestrus; see Supplemental Material, Table S1). The rats were circa 12 weeks old and weighed 197 ± 17 g at dosing. Each rat (n = 10/group) received either five consecutive daily doses of 250 mg/kg of TBBPA or vehicle (1:1:3 ratio of ethanol, Cremophore EL®, and water) by gavage. The TBBPA (3,3′,5,5′-tetrabromobisphenol A) used in this study was obtained from Sigma-Aldrich (St. Louis, MO) and had a chemical purity of 97%. The doses were administered in a volume of 4 ml/kg using a #16 ball-tipped feeding needle attached to a syringe. Rats of the same treatment group were housed two per cage and received water and food (NIH #31) for ad libitum consumption. The stage of the estrous cycle was determined and recorded daily. All rats were euthanized by CO2 asphyxiation 24 hours following the final dose. Blood, liver, and uterine tissue were collected rapidly for processing.
2.2. Tissue collection and preparation
Blood was collected by cardiac puncture at time of death, allowed to clot, and centrifuged at 2000 g for 10 minutes to produce serum. The liver and uterus were excised, the central portion of the left lobe of the liver was collected and cubed, and the uterus was collected in three sections by locating the approximate midpoint of the branched uterine horns and cutting one to two mm to either side of the midpoint. The uterine collection technique followed a protocol used by the NTP in the chronic study; however, the histopathologic data were reported for the complete uterus, not by section (NTP, 2014). The proximal section (nearest the cervix) and the distal portion (nearest the ovaries) of the uterus were analyzed separately as described below. The small portion of tissue between the proximal and distal sections was not analyzed. The uterine and liver tissues were flash-frozen in liquid nitrogen upon collection and were stored along with the serum at −80° C. The concentrations of T3, T4, and E2 were determined in serum using RIA kits (Siemans Diagnostics, Los Angeles, CA) and an APEX automatic gamma counter (ICN Micromedic Systems, Inc., Huntsville AL). The concentration of thyroid stimulating hormone (TSH) was determined in serum using an EIA assay kit (ALPCO, Salem, NH) and a SpectraMAX 340PC plate reader with SOFTmax PRO software (Molecular Devices Corp., Sunnyvale, CA). Frozen tissue samples (50–60 mg) were weighed quickly and minced in Qiagen (Germantown, MD) RLT buffer containing β-mercaptoethanol (1:100). Sample volume was brought to 1.4 ml (liver) or 1 ml (uterus) and samples were processed with an Omni H handheld homogenizer (Omni International, Kennesaw, GA) using disposable OmniTip homogenizer probes (soft tissue for liver, hard tissue for uterus) to prevent cross-contamination. Homogenates were centrifuged at maximum speed in an Eppendorf 5430R Microcentrifuge and 3 × 350-μl aliquots of lysate were transferred into 2-ml tubes (additional buffer was added to bring the triplicate uterine samples to 350 μl each). Total RNA was isolated from the lysate in a QIAcube (Qiagen) following the standard QIAcube Protocol for animal tissues and cells using the RNeasy Mini Kit with DNase treatment; elution volume was 50 μl (liver) or 30 μl (uterus). A second 30 μl elution of uterus samples was performed manually to maximize sample recovery. RNA concentrations were determined for individual eluates using a Nanodrop 2000c (Thermo Scientific, Wilmington, DE). Aliquots with similar concentrations from the same sample were pooled prior to further analysis. RNA integrity was measured on a QIAxcel instrument (Qiagen) using a QIAxcel RNA QC Kit V2.0, with analysis performed with QIAxcel ScreenGel version 1.2.0. All samples were stored at −80° C.
cDNA was prepared using 2.5 U/μl Moloney Murine Leukemia Virus (MuLV) Reverse Transcriptase (Applied Biosystems, Life Technologies, Grand Island, NY), 5 mM MgCl2, PCR Buffer II (ABI), 1 mM dNTP mix, 2.5 μM Random Hexamers, 0.25 U/μl RNase Inhibitor, and either 100 ng/μl or 20 ng/μl RNA. Reactions were incubated in a Bio-Rad T100 thermal cycler (Bio-Rad Laboratories, Hercules, CA) for one cycle of 10 min at 25° C, 60 min at 42° C, 5 min at 95° C, then held at 4° C until removal to storage at −20° C.
TaqMan® Gene Expression Assays (20x) and TaqMan Universal PCR Master (2x) were purchased from Applied Biosystems. Quantitative PCR was performed in 10 μl assays containing master mix, primer/probe (0.9 μM/0.25 μM), RNase-free water, and the indicated amount of cDNA in a Bio-Rad CFX384 Real-Time PCR System. Cycling parameters included an initial activation of 10 min at 95° C, followed by 40 cycles of 15 seconds at 95° C and 1 min at 60° C. Assays for Cyp19a1 and Hsd3b5 were purchased from Bio-Rad. Quantitative PCR was performed for these assays using Bio-Rad SsoAdvanced™ Universal Probe Supermix with methods modifications as per manufacturer’s instructions.
All assays were assessed for efficiency and dynamic range by running a quantitative PCR standard curve using pooled rat liver and uterus cDNA diluted in 4-fold increments from 100 ng to 0.00152 ng. Efficiency, defined as Efficiency = (10−1/slope−1) × 100, was calculated by plotting the resulting Cq values versus log input and determining the resulting slope (Applied Biosystems, 2008). Dynamic range was estimated by calculating the difference between adjacent points of the curve (a 4-fold dilution in input amount should produce a 2-fold difference in Cq value within the linear range of the assay). Assays with efficiencies below 90% were disqualified.
2.3 Reference genes
Ten candidate reference genes were chosen for testing (see Supplemental Material, Table S2). Five were frequently-used reference genes from the literature. The other five were the most stable genes in similar samples as determined using the RefGenes function of Genevestigator (http://www.refgenes.org/rg/). Quantitative PCR was performed for each gene using 20 ng cDNA from each of 12 liver samples and 18 uterus samples. Resulting Cq values were analyzed using the geNormPLUS function of qbasePLUS (Biogazelle, Zwijnaarde, Belgium) to determine the optimum number (see Vandesompele et al., 2002) and best reference genes for use with the individual sample tissues, with the best choice for liver being Elk4 and Sdha, and those for uterus being Hprt1 and Kdm2a.
2.4. Target genes
The representative genes of interest were chosen to investigate hypothesized biological pathways affected by TBBPA treatment. The genes were grouped by type into three sets as shown in Table 1. The sets consisted of: 1) genes associated with transport and receptors; 2) genes associated with cell division and growth; and 3) genes associated with metabolism of TBBPA and/or estrogen. The major type and function of protein encoded by each assayed gene are listed in Table 1. Quantitative PCR was performed as above using 20 ng cDNA for each assay, except Cyp19a1 (100 ng used).
Table 1.
Target genes
| Genes associated with transport and receptors | Assay Identification | Associated protein(s)/function |
|---|---|---|
| Esr1, Esr2 | Rn01640372_m1, Rn00562610_m1 | Estrogen receptors α and β/mediate biological activity of estrogen |
| Nr1i2 | Rn00583887_m1 | Pregnane X receptor (PXR)/regulates xenobiotic metabolism |
| Nr1i3 | Rn00576085_m1 | Constitutive androstane receptor (CAR)/regulates xenobiotic metabolism |
| Nr3c1 | Rn00561369_m1 | Glucocorticoid receptor/regulates carbohydrate, fat, and protein metabolism |
| Ppara, Pparg | Rn00566193_m1, Rn00440945_m1 | Peroxisome proliferator-activated receptors α and γ/Regulate genes involved in cell proliferation and differentiation and immune and inflammatory responses |
| Slc16a2 | Rn00596041_m1 | Solute carrier/transports thyroid hormone |
| Thra, Thrb | Rn01464140_m1, Rn00562044_m1 | Thyroid receptor α and β/mediate biological activity of thyroid hormone |
| Ttr | Rn00562124_m1 | Transthyretin/transports thyroid hormone |
| Genes associated with Cell division and growth | ||
| Birc5 | Rn00574012_m1 | BIRC protein/inhibitor of apoptosis |
| Ccnb1, Ccnb2 | Rn01494177_m1, Rn02346769_m1 | Cyclin B/regulates mitosis |
| Ccnd1, Ccnd2, Ccnd3 | Rn00432360_m1, Rn03020897_m1, Rn00562751_m1 | Cyclin D/regulates cell cycle from G1 to S |
| Cdk1 | Rn00570728_m1 | Kinase/forms complex with Cyclin B for cell cycle progression |
| Cdk4 | Rn99999090_m1 | Kinase/forms complex with Cyclin D for cell cycle progression |
| Igf1 | Rn00710603_m1 | Insulin-like growth factor 1/mediates cell growth |
| Genes associated with Metabolism of TBBPA and/or estrogen | ||
| Cyp1b1, Cyp2b1, Cyp2b2 | Rn00564055_m1, Rn01457875_m1, Rn02786833_m1 | Cytochrome P450/oxidative metabolism |
| Cyp11a1, Cyp17a1, Cyp19a1 | Rn00568733_m1, Rn00562601_m1, qRnoCIP0029624 | Cytochrome P450/steroidogenesis |
| Comt | Rn01404927_g1 | Catechol-O-methyl transferase/conjugative metabolism |
| Hsd3b5 | qRnoCEP0041702 | 3β Hydroxysteroid dehydrogenase/steroidogenesis |
| Hsd17b2, Hsd17b4 | Rn00577779_m1, Rn00577789_m1 | 17β Hydroxysteroid dehydrogenase/E2 metabolism |
| Sult1a1, Sult1e1, Sult2a1, Sult2a2 | Rn01510633_m1, Rn00820646_g1, Rn04223057_mH, Rn02586796_g1 | Sulfotransferases/conjugative metabolism |
| Ugt1a1, Ugt2b, Ugt2b17 | Rn00754947_m1, Rn00756519_m1, Rn01790037_g1 | Glucuronosyltransferases/conjugative metabolism |
2.5 Data analysis
The data presented in Figure 1 are from n = 10 rats/treatment group, whereas, the data presented in Figures 2, 3, and 4 are from 6–10 rats/group. The number of rats per group for the gene expression assays was variable due to quality control rejection of some total RNA. The expression of each target gene was determined 1 to 3 separate times in triplicate, depending on availability of the sample. Fold expression was calculated as 2−ΔΔCq. The average fold expression for each gene expression assay was presented as the mean of all biological replicates +/− standard error. The data were subjected to statistical analysis using Sigma Plot version 12.5 (Systat Software, Inc., San Jose, CA). The serum hormone and some gene expression data followed normal distributions, therefore, significance of differences between treatment groups was determined using a two-tailed t-test. However, much of the gene expression data was not normally distributed; therefore, significance of those data was established using the Mann-Whitney Rank Sum test. Data were considered to be significantly different at p < 0.05.
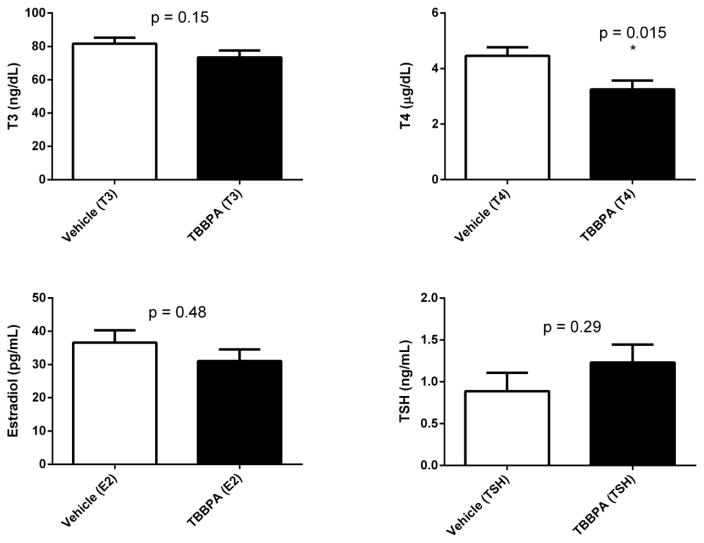
Fig 1.
Serum hormone concentrations in rats 24 hours following 5 days oral administration of TBBPA or vehicle. Each value is the mean ± SE of 10 rats/group. *The value is significantly different from vehicle control (p<0.05).
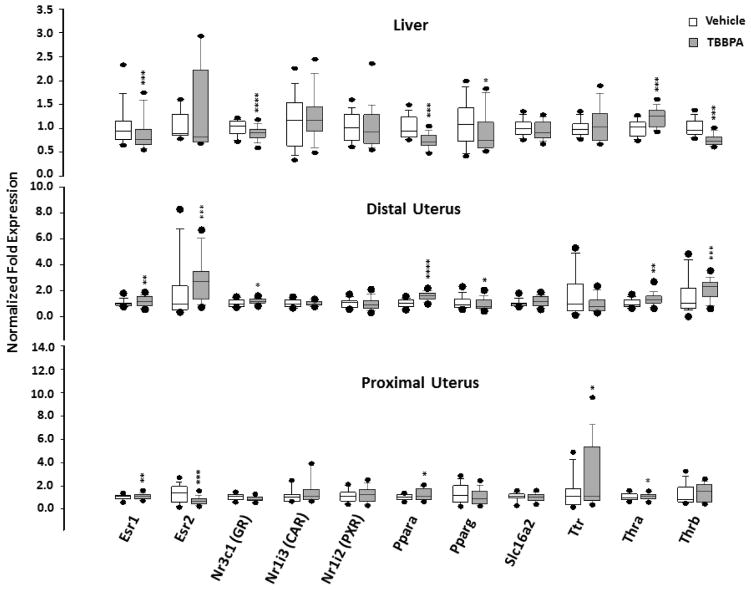
Fig. 2.
Box plots of the expression of genes associated with transport or receptors in tissues of rats 24 hours following 5 days oral administration of TBBPA or vehicle. Each value is the mean ± SE of 6–10 rats/group with 3–9 replicates/rat. The value is significantly different from vehicle control at *p<0.05, ** p<0.01, ***p<0.001, ****p<0.0001.
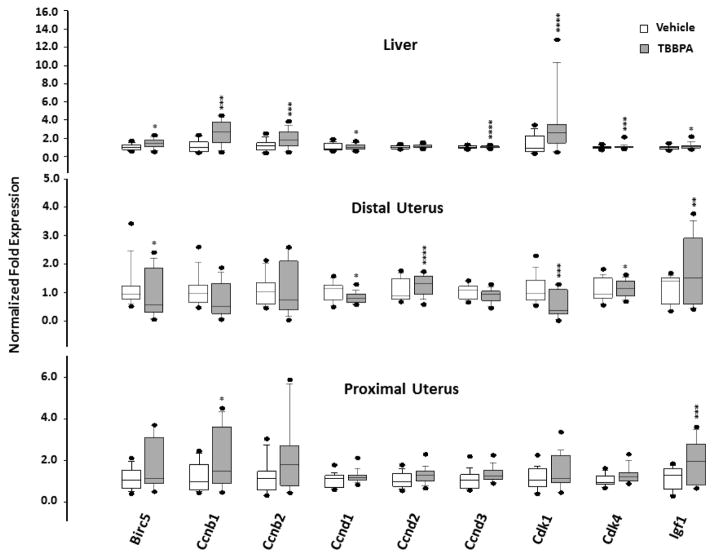
Fig. 3.
Box plots of the expression of genes associated with cell growth in tissues of rats 24 hours following 5 days oral administration of TBBPA or vehicle. Each value is the mean ± SE of 6–10 rats/group and 3–9 replicates/rat. The value is significantly different from vehicle control at *p<0.05, ** p<0.01, ***p<0.001, and ****p<0.0001.
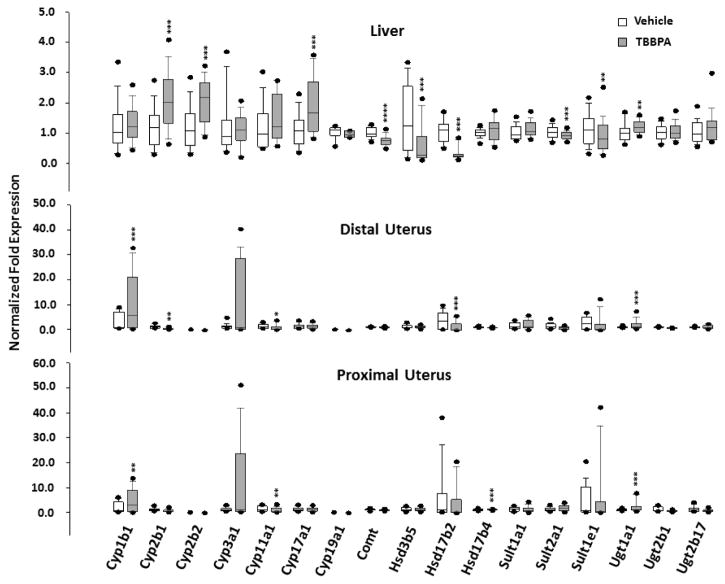
Fig. 4.
Box plots of the expression of genes associated with metabolism of TBBPA and/or estrogen in tissues of rats 24 hours following 5 days oral administration of TBBPA or vehicle. Each value is the mean ± SE of 6–10 rats/group and 3–9 replicates/rat. * The value is significantly different from vehicle control at *p<0.05, ** p<0.01, ***p<0.001, and ****p<0.0001.
3. Results
Body weight increased over the course of the study for 18 of 20 rats (data not shown). The increases were in the same range across both treatment groups (0.5 to 8%) and no clinical signs of toxicity were observed in these rats. The terminal weights of two of the TBBPA-treated rats were lower than those observed prior to the first dose. Neither rat exhibited overt signs of toxicity and the nature of the weight loss indicated transitory gavage effects rather than TBBPA-mediated toxicity (data not shown). TBBPA did not have an obvious effect on the estrous cycle during five days of administration of 250 mg/kg (see Supplemental Material, Table S1). The final vaginal cytology assay indicated that 17 of 20 rats were in diestrus. Two vehicle-treated rats and one TBBPA-treated rat were in metestrus. TBBPA-treatment resulted in a statistically significant decline in T4 compared to vehicle treated rats (Figure 1). No significant differences were observed between vehicle- and TBBPA-treated rats for E2, T3, or TSH concentrations in serum (Figure 1).
The effect on gene expression in the liver and uterus of rats treated with TBBPA was investigated. The first set of target genes was associated with transport and receptors, including markers for endocrine disruption (Table 1). Expression of three genes in TBBPA-treated rats was significantly different from vehicle controls in both tissues (liver and uterus) and in the proximal and distal portions of the uterus (Figure 2; Tables 2 and 3). The gene for TRα (Thra) was up-regulated at all three sites. In contrast, the genes for ERα (Esr1) and PPARα (Ppara) were down-regulated in liver and up-regulated in both sections of the uterus. Effects on other genes varied by tissue type and location. Change in expression of the gene for ERβ (Esr2) was observed in the proximal and distal portions of the uterus, expression of the gene for TTR (Ttr) changed only in the proximal uterus, expression of the gene for TRβ (Thrb) changed in the liver and distal uterus, and expression of Nr3c1, the gene for the glucocorticoid receptor (GR), changed in the liver and proximal uterus. The directional response in expression of these genes varied with tissue location. Expression of the genes for CAR (Nr1i3) and PXR (Nr1i2) was unaffected in TBBPA-treated rats.
Table 2.
Expression of target genes in liver with significant differences between vehicle and TBBPA-treated rats
| Tissue/Gene | Vehicle Mean | Vehicle SE | Vehicle Median | TBBPA Mean | TBBPA SE | TBBPA Median | Increase/Decrease | P-value (Mann-Whitney Rank Sum test) | P-value (2-tailed t-test) |
|---|---|---|---|---|---|---|---|---|---|
| Birc5 | 1.06 | 0.12 | 1.03 | 1.26 | 0.16 | 1.24 | ↑ | 0.012 | - |
| Ccnb1 | 1.16 | 0.19 | 1.04 | 2.45 | 0.41 | 2.51 | ↑ | <0.001 | - |
| Ccnb2 | 1.16 | 0.20 | 1.15 | 1.76 | 0.30 | 1.62 | ↑ | <0.001 | - |
| Ccnd1 | 1.08 | 0.13 | 0.85 | 0.88 | 0.10 | 0.86 | ↓ | 0.034 | - |
| Ccnd3 | 1.01 | 0.05 | na | 0.90 | 0.04 | na | ↓ | - | <0.0001 |
| Cdk1 | 1.34 | 0.32 | na | 3.16 | 1.02 | na | ↑ | - | <0.0001 |
| Cdk4 | 1.01 | 0.05 | 0.98 | 0.98 | 0.17 | 0.88 | ↓ | <0.001 | - |
| Comt | 1.02 | 0.06 | na | 0.71 | 0.06 | na | ↓ | - | <0.0001 |
| Cyp2b1 | 1.21 | 0.23 | 1.19 | 2.05 | 0.30 | 1.97 | ↑ | <0.001 | - |
| Cyp2b2 | 1.01 | 0.23 | 1.09 | 2.00 | 0.23 | 2.12 | ↑ | <0.001 | - |
| Cyp17a1 | 1.14 | 0.17 | 1.08 | 1.86 | 0.29 | 1.61 | ↑ | <0.001 | - |
| Esr1 | 1.07 | 0.14 | 0.99 | 0.87 | 0.11 | 0.76 | ↓ | <0.001 | - |
| Igf1 | 1.03 | 0.07 | 1.02 | 0.97 | 0.11 | 0.88 | ↓ | 0.012 | - |
| Hsd3b5 | 1.50 | 0.36 | 1.26 | 0.56 | 0.20 | 0.23 | ↓ | <0.001 | - |
| Hsd17b2 | 1.07 | 0.12 | 1.11 | 0.26 | 0.06 | 0.20 | ↓ | <0.001 | - |
| Nr3c1 | 1.01 | 0.05 | na | 0.89 | 0.05 | na | ↓ | - | <0.0001 |
| Ppara | 1.03 | 0.08 | 0.95 | 0.73 | 0.05 | 0.71 | ↓ | <0.001 | - |
| Pparg | 1.11 | 0.16 | 1.09 | 0.92 | 0.13 | 0.74 | ↓ | 0.022 | - |
| Sult1e1 | 1.15 | 0.18 | na | 0.88 | 0.19 | na | ↓ | - | 0.005 |
| Sult2a1 | 1.03 | 0.07 | 1.02 | 0.88 | 0.04 | 0.86 | ↓ | <0.001 | - |
| Thra | 1.02 | 0.06 | 1.02 | 1.23 | 0.06 | 1.24 | ↑ | <0.001 | - |
| Thrb | 1.01 | 0.05 | 0.96 | 0.76 | 0.04 | 0.73 | ↓ | <0.001 | - |
| Ugt1a1 | 1.05 | 0.10 | 1.00 | 1.17 | 0.07 | 1.14 | ↑ | 0.003 | - |
na = not applicable for determining significance using the 2-tailed t-test
Table 3.
Expression of target genes in uterus with significant differences between vehicle and TBBPA-treated rats
| Tissue/Gene | Vehicle Mean | Vehicle SE | Vehicle Median | TBBPA Mean | TBBPA SE | TBBPA Median | Increase/Decrease | P-value (Mann-Whitney Rank Sum test) | P-value (2-tailed t-test) |
|---|---|---|---|---|---|---|---|---|---|
| Proximal Uterus | |||||||||
| Ccnb1 | 1.18 | 0.24 | 0.98 | 1.92 | 0.46 | 1.29 | ↑ | 0.042 | - |
| Cyp1b1 | 1.94 | 0.76 | 0.56 | 4.81 | 1.46 | 3.08 | ↑ | 0.006 | - |
| Cyp11a1 | 1.37 | 0.35 | 1.08 | 0.98 | 0.31 | 0.43 | ↓ | 0.004 | - |
| Esr1 | 1.03 | 0.08 | na | 1.16 | 0.08 | na | ↑ | - | 0.008 |
| Esr2 | 1.33 | 0.28 | 1.41 | 0.79 | 0.11 | 0.74 | ↓ | <0.001 | - |
| Hsd17b4 | 1.04 | 0.11 | 1.08 | 1.23 | 0.06 | 1.26 | ↑ | <0.001 | - |
| Igf1 | 1.16 | 0.18 | 1.30 | 1.80 | 0.31 | 1.78 | ↑ | <0.001 | - |
| Ppara | 1.03 | 0.08 | 1.03 | 1.30 | 0.15 | 1.19 | ↑ | 0.016 | - |
| Thra | 1.04 | 0.10 | na | na | 1.15 | 0.09 | ↑ | - | 0.049 |
| Ttr | 1.64 | 0.55 | 1.00 | 2.91 | 0.96 | 1.19 | ↑ | 0.029 | - |
| Ugt1a1 | 1.06 | 0.12 | 1.08 | 2.59 | 0.76 | 1.28 | ↑ | <0.001 | - |
|
| |||||||||
| Distal Uterus | |||||||||
| Birc5 | 1.14 | 0.30 | 0.93 | 1.04 | 0.25 | 0.68 | ↓ | 0.036 | - |
| Ccnd1 | 1.06 | 0.13 | 1.14 | 0.93 | 0.07 | 0.90 | ↓ | 0.026 | - |
| Ccnd2 | 1.06 | 0.15 | na | 1.37 | 0.11 | na | ↑ | - | <0.0001 |
| Cdk1 | 1.09 | 0.20 | 0.98 | 0.70 | 0.14 | 0.48 | ↓ | <0.001 | - |
| Cdk4 | 1.07 | 0.16 | 0.93 | 1.26 | 0.10 | 1.26 | ↑ | 0.011 | - |
| Cyp1b1 | 2.64 | 1.36 | 0.69 | 11.21 | 3.66 | 5.88 | ↑ | <0.001 | - |
| Cyp2b1 | 1.13 | 0.34 | 1.03 | 0.57 | 0.11 | 0.44 | ↓ | 0.002 | - |
| Cyp11a1 | 1.27 | 0.35 | 1.02 | 1.11 | 0.38 | 0.49 | ↓ | 0.03 | - |
| Esr1 | 1.03 | 0.09 | na | 1.22 | 0.10 | na | ↑ | - | 0.002 |
| Esr2 | 1.95 | 0.94 | 0.97 | 2.96 | 0.61 | 2.76 | ↑ | <0.001 | - |
| Hsd17b2 | 3.54 | 1.36 | 3.33 | 1.35 | 0.61 | 0.06 | ↓ | <0.001 | - |
| Igf1 | 1.15 | 0.20 | 1.40 | 1.87 | 0.37 | 1.62 | ↑ | 0.008 | - |
| Nr3c1 | 1.03 | 0.11 | na | 1.17 | 0.07 | na | ↑ | - | 0.013 |
| Ppara | 1.05 | 0.13 | na | 1.57 | 0.12 | na | ↑ | - | <0.0001 |
| Pparg | 1.08 | 0.19 | 0.94 | 0.92 | 0.15 | 0.76 | ↓ | 0.032 | - |
| Thra | 1.05 | 0.14 | na | 1.34 | 0.17 | na | ↑ | - | 0.004 |
| Thrb | 1.63 | 0.54 | 1.06 | 2.13 | 0.27 | 2.37 | ↑ | 0.001 | - |
| Ugt1a1 | 1.04 | 0.13 | 0.98 | 2.28 | 0.65 | 1.45 | ↑ | 0.001 | - |
na = not applicable for determining significance using the 2-tailed t-test
The second set of target genes was associated with cell division and growth (Table 1). The expression of most of these genes was affected in the liver, with fewer significant responses observed in the uterus, especially the proximal section. (Figure 3; Tables 2 and 3). Overall, the greatest fold-increase in expression of this set of genes was observed in the liver for genes (Ccnb1, Ccnb2, and Cdk1) encoding the Cyclin B complex of the cell cycle. The greatest change in genes encoding the Cyclin D complex of the cell cycle consisted of up-regulation of Ccnd2 in the distal uterus. A significant increase in the expression of Igf1, the gene encoding insulin-like growth factor (IGF-1), was observed in both sections of the uterus.
Some of the greatest fold-changes in expression of target genes occurred in the set associated with metabolism of TBBPA and/or estrogen (Table 1). The fold change in Cyp1b1 was relatively high in the distal and proximal uterus of TBBPA-treated rats, but not affected in the liver (Figure 4, Tables 2 and 3). Cyp2b1 and Cyp2b2 were up-regulated in the liver, whereas, expression of these genes was low to non-detectable in either section of the uterus. Cyp17a1 was up-regulated in liver, but unchanged in the uterus. Cyp11a1 was down-regulated in the uterus, but unchanged in the liver. The expression of Cyp19a1 was unaffected in the liver of TBBPA-treated rats and was below the limit of quantitation in the uterus in both treatment groups. The greatest fold down-regulation in this gene set was observed for Hsd17b2 in the distal uterus. Genes encoding other hydroxysteroid dehydrogenases were affected variably in tissues of TBBPA-treated rats, as were genes associated with conjugating enzymes. In liver, Comt, Sult2a1, and Sult1e1 were down-regulated. Ugt1a1 was up-regulated in the liver and in both sections of the uterus. There was no effect on Comt, Sult2a1, or Sult1e1 in the uterus of TBBPA-treated rats. Message for Sult2a2 was detected in proximal and distal uterus of vehicle-treated rats, but not in either uterine section of TBBPA-treated rats (data not shown).
4. Discussion
The goal of the present project was to characterize the mechanism(s) responsible for the increased incidence of uterine lesions in TBBPA-treated rats observed in a chronic toxicity study conducted by the National Toxicology Program (NTP). A previous study conducted in this laboratory found no evidence for accumulation of [14C]-labeled TBBPA or metabolites that could account for uterine tissue insult (Knudsen et al., 2014). The present work tested a hypothesis that uterine lesions correlate with TBBPA-mediated disruption of estrogen homeostasis at the site-of-action (Dunnick et al., 2015). Atypical hyperplasia, adenomas, adenocarcinomas, and malignant mixed Müllerian tumors were observed in the uterus of TBBPA-treated Wistar Han rats in the NTP study (NTP, 2014). Atypical hyperplasia and carcinomas in the endometrium may be associated with elevated estrogen concentrations in humans (Lax, 2004). Further, malignant mixed Mullerian tumors in the myometrium may also arise from estrogen exposure and correlate with dysregulation of the cell cycle and apoptosis (Kanthan et al., 2010; Wang et al., 2005). A measurement of changes in estrogen concentrations in rat uterus was not technologically feasible for this short-term TBBPA exposure. Instead, it was determined that the most sensitive and efficient method to test the hypothesis was to search for TBBPA-mediated effects on genes associated with specific pathways of estrogen biosynthesis and metabolism. This approach resulted in observed changes in expression of some of these target genes after only five days of TBBPA treatment to rats. These changes may correlate with increased E2 or estrogen-derived reactive metabolites in the tissue. In contrast, no change in E2 serum concentration was observed. This result is not without precedent. In a reported human study, the concentration of E2 in serum did not predict the much higher concentration of E2 detected in healthy or ectopic endometrium (Huhtinen et al., 2012).
Exposure to TBBPA resulted in changes in expression of genes for some nuclear receptors. Genes encoding both ERα and ERβ were up-regulated in the distal uterus, indicating potential for greatest receptor binding with E2 nearest the ovaries. Expression of genes for TR, PPARs and GR were also affected by TBBPA exposure. These receptors may modulate estrogen homeostasis and/or undergo estrogen-dependent regulation (Foryst-Ludwig et al., 2008; Gong et al., 2008; Santin and Furlanetto, 2011). It is uncertain if these genes were directly affected by TBBPA or if the responses are indicative of TBBPA-perturbed estrogen homeostasis.
TBBPA is rapidly excreted in the female Wistar Han rat primarily as the result of conjugation with sulfate or glucuronic acid (Knudsen et al., 2014). This metabolic pathway is shared by endogenous estrogen, with sulfation viewed as more important than glucuronidation for regulation of estrogen homeostasis (Raftogianis et al., 2000). Other work has shown a similar binding affinity of TBBPA and E2 for human estrogen sulfotransferase (Gosavi et al., 2013). Consequently, competition between TBBPA and estrogen for enzymes involved in conjugation may serve to increase the concentration of the hormone in tissues. UDP-glucuronosyl transferase (UGT) activity was decreased in female rats at doses of 50 mg/kg or greater of TBBPA in a subchronic study conducted by the NTP (2014). Sulfotransferase activity was not measured. In rodents, saturation of sulfotransferases (SULTs) generally precedes that of UGTs (Burka et al., 1996; Hjelle and Klaassen, 1984); therefore, it can be assumed that the conjugation capacity for endogenous estrogen was compromised in the NTP study. In the present study, the gene responses for the assayed UGT and SULT isoforms were variable in tissues; however, the data indicated potential for change in the ratio of sulfation to glucuronidation after five days of treatment with 250 mg TBBPA/kg. Glucuronidation of TBBPA may have increased based on up-regulation of Ugt1a1 in both liver and uterus, whereas, sulfation of TPPBA may have decreased relative to glucuronidation, based on down-regulation of Sult2a1 and Sult1e1 in liver and no change in expression of these genes in the uterus. The presence of Sult2a2 message in the uterus of vehicle-treated, but not TBBPA-treated rats, may also indicate decreased capacity for sulfation in the tissue.
Effects on other genes involved in the biosynthesis and metabolism of E2 included down-regulation of Hsd17b2 in the distal uterus of TBBPA-treated rats. This gene encodes 17β-HSD type 2, an enzyme that converts E2 to less active estrone (E1) (Ito et al., 2006). This enzyme has been shown to have an inverse correlation with E2 levels in human endometrial disorders, including hyperplasia and carcinoma. Unconjugated estrogen could be oxidized to catechols and quinones, postulated to be involved in mammary carcinogenesis (Cavalieri et al., 1997; Dawling et al., 2001; Liehr, 2000). CYP1B metabolizes E1 and E2 to catechols (hydroxyestrogens), and redox cycling of these metabolites can lead to formation of DNA-reactive semiquinones. Conversely, hydroxyestrogens are metabolized by catechol-O-methyltransferase (COMT). In the present study, Cyp1b1 was significantly up-regulated in both sections of the uterus following TBBPA exposure. The expression of Comt was decreased in liver, but unchanged in the uterus. Together, the observed significant responses of Cyp1b1 and Comt support the potential for increased formation of mutagenic estrogen-derived metabolites in tissues of TBBPA-treated rats. Increased expression of Cyp2b1 and Cyp2b2 was observed in the liver of TBBPA-treated rats. CYP2B1 and CYP2B2 have been shown to increase in the liver of tamoxifen (TAM)-treated Sprague Dawley rats (Nuwaysir et al., 1995). TAM exerts an estrogenic effect on the uterus in humans resulting in an increased risk of cancer in that tissue (Nasu et al., 2008). In a recent study, catechol-estrogen metabolite (4-hydroxyestrone) increased, COMT expression decreased, and expression of CYP1B1 increased in a human endometrial cell line exposed to TAM (Williams-Brown et al., 2011). The similarities in these TAM-mediated protein responses to gene responses observed in the present study support the postulated effect of TBBPA on estrogen metabolism in the uterus.
TBBPA treatment resulted in differential expression of some genes involved in cell division and growth. Genes (Ccnb1, Ccnb2, and Cdk1) associated with the Cyclin B complex were up-regulated in the liver and/or in the proximal uterus and some genes (Ccnd2, Cdk4) involved with the Cyclin D complex were up-regulated in the distal uterus. The active Cyclin B complex drives the cell into mitosis and the active Cyclin D complex is essential for activation of DNA synthesis (Ford and Pardee, 1999). Disruption of cell cycle regulation is implicated in tumor formation in target tissues. Zhao et al. (2006) demonstrated increased gene expression for Cyclin B in cancerous cervical tissue from human subjects. Gene expression for Cyclin D and Cdk-4 has been shown to be up-regulated in BG-1 ovarian cancer cells following exposure to di-n-butyl phthalate and hexabromocyclododecane (another BFR) through putative estrogenic activity (Park et al. 2012). In the present study, expression of Igf1, the gene encoding growth factor IGF-1, increased in both sections of the uterus. Klotz et al. (2000) has shown that activation of IGF-1 may mediate mitogenic effects of E2 in the rat uterus through activation of ERα. Consequently, the observed changes in expression of genes associated with both cell cycle regulation and growth may indicate increased estrogenic activity and potential to influence tumorigenesis in the uterus of rats chronically-exposed to TBBPA.
The Effect of TBBPA on thyroid homeostasis was also investigated in this work. The expression of the gene (Thra) encoding TRα increased in the liver and in both sections of the uterus. TBBPA has been shown to disrupt TRα function in vitro and alter expression of Thra in Pacific tree frogs, Xenopus laevis tadpoles, and zebrafish embryo-larvae (Chan and Chan, 2012; Fini et al., 2012; Lévy-Bimbot et al., 2012; Veldhoen et al., 2006). Further, serum T4 decreased in TBBPA-treated rats, whereas serum T3 was not significantly affected. These results are in agreement with those reported in rats receiving 500 or 1000 mg TBBPA/kg by gavage for five days/week for 14 weeks (NTP, 2014). The mode-of-action is uncertain.
In conclusion, the present work provides evidence to support the hypothesis that disruption of estrogen homeostasis is a major mechanism leading to histological changes in the tissue following chronic administration of 250 mg/kg and higher doses of TBBPA. Additional studies are currently underway in rats to investigate toxicological responses at lower doses and to search for other biological pathways that may be affected by TBBPA exposure. Finally, the approach used here to sample uterine tissue may have maximized the ability to detect baseline changes in some genes and suggests that concentrations of estrogen and estrogenic metabolites may vary in the uterus of TBBPA-treated rats relative to distance from the ovaries. Differential responses observed in the two assayed sections of the uterus may be of importance to future study design involving the tissue.
Supplementary Material
Highlights.
Perturbation of estrogen homeostasis in TBBPA-treated female rats was investigated.
Gene expression changes were observed in the liver and uterus of these rats.
Genes associated with estrogen biosynthesis and metabolism were affected.
Genes associated with thyroid homeostasis and cell division/growth were affected.
A mechanism of uterine toxicity via endocrine disruption was indicated.
Acknowledgments
The authors thank Mr. Ralph Wilson (NIEHS) for serum analysis. This work was supported by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health [Project: ZIA BC 011476].
Footnotes
Conflicts of interest
The authors declare that they have no actual or potential competing financial interests associated with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Applied Biosystems. [accessed 6 Jan 2014];Guide to Performing Relative Quantitation of Gene Expression using Real-Time Quantitative PCR. 2008 Available: http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/generaldocuments/cms_042380.pdf.
- Burka LT, Sanders JM, Matthews HB. Comparative metabolism and disposition of ethoxyquin in rat and mouse. II Metabolism Xenobiotica. 1996;26:597–611. doi: 10.3109/00498259609046736. [DOI] [PubMed] [Google Scholar]
- Canada (Government of Canada) Risk management scope for phenol, 4,4′-(1-methylethylidene) bis[2,6-dibromo-(tetrabromobisphenol A) [accessed 13 Nov 2014];Environment Canada Health Canada. 2012 Nov; Available: http://www.ec.gc.ca/ese-ees/3BC8852B-124A-4D0D-8376-3A62D41530F8/TBBPA_RM%20Scope_EN.pdf.
- Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG. Molecular origin of cancer: Catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Chan KM. Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA, and BPA. Aquat Toxicol. 2012;108:106–111. doi: 10.1016/j.aquatox.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Chignell CF, Han SK, Mouithys-Mickalad A, Sik RH, Stadler K, Kadiiska MB. EPR studies of in vivo radical production by 3,3′,5,5′-tetrabromobisphenol A (TBBPA) in the Sprague-Dawley rat. Toxicol Appl Pharmacol. 2008;230:17–22. doi: 10.1016/j.taap.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Dawling S, Roodi N, Mernaugh R, Wang X, Pari FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: Comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–6722. [PubMed] [Google Scholar]
- de Wit C, Herzke D, Vorkamp K. Brominated flame retardants in the Arctic environment-trends and new candidates. Sci Total Environ. 2010;408:2885–2918. doi: 10.1016/j.scitotenv.2009.08.037. [DOI] [PubMed] [Google Scholar]
- Dorosh A, Děd L, Elzeinová F, Pěknicová J. Assessing oestrogenic effects of brominated flame retardants hexabromocyclododecane and tetrabromobisphenol A on MCF-7 cells. Folia Biologica (Praha) 2010;56:35–39. [PubMed] [Google Scholar]
- Dunnick JK, Sanders JM, Kissling GE, Johnson C, Boyle MH, Elmore SA. Environmental chemical exposure may contribute to uterine cancer development: studies with tetrabromobisphenol A. Toxicol Pathol. 2015;43:464–473. doi: 10.1177/0192623314557335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECB (European Chemicals Bureau) European Union Risk Assessment Report. 2,2′,6,6′-Tetrabromo-4,4′-isopropylidenediphenol (tetrabromobisphenol-A or TBBP-A). Part II-human health. 4th Priority List. 2006;63 [Google Scholar]
- Fini JB, Riu A, Debrauwer L, Hillenweck A, Le Mével S, Chevolleau S, Boulahtouf A, Palmier K, Balaguer P, Cravedi JP, Dermeneix BA, Zalko D. Parallel biotransformation of tetrabromobisphenol A in Xenopus laevis and mammals: Xenopus as a model for endocrine perturbation studies. Toxicol Sci. 2012;125:359–367. doi: 10.1093/toxsci/kfr312. [DOI] [PubMed] [Google Scholar]
- Ford HL, Pardee AB. Cancer and the cell cycle. J Cell Biochem Suppl. 1999;32/33:166–172. doi: 10.1002/(sici)1097-4644(1999)75:32+<166::aid-jcb20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson J-Å, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERβ) are mediated by a negative crosstalk with PPARγ. PloS Genet. 2008;4:1–16. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Jarzynka MJ, Cole TJ, Lee JH, Wada T, Zhang B, Gao J, Song WC, DeFranco DB, Cheng SY, Xie W. Glucocorticoids antagonize estrogens by glucocorticoid receptor-mediated activation of estrogen sulfotransferase. Cancer Res. 2008;68:7386–7393. doi: 10.1158/0008-5472.CAN-08-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosavi RA, Knudsen GA, Birnbaum LS, Pedersen LC. Mimicking of estradiol binding by flame retardants and their metabolites: A crystallographic analysis. Environ Health Perspect. 2013;121:1194–1199. doi: 10.1289/ehp.1306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakk H, Larsen G, Bergman Å, Örn U. Metabolism, excretion, and distribution of the flame retardant tetrabromobisphenol-A in conventional and bile-duct cannulated rats. Xenobiotica. 2000;30:881–890. doi: 10.1080/004982500433309. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Hjelle JJ, Klaassen CD. Glucuronidation and biliary excretion of acetaminophen in rats. J Pharmacol Exp Ther. 1984;228:407–413. [PubMed] [Google Scholar]
- Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem. 2005;80:79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- Huhtinen K, Desai R, Ståhle M, Salminen A, Handelsman DJ, Perheentupa A, Poutanen M. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J Clin Endocrinol Metab. 2012;97:4228–4235. doi: 10.1210/jc.2012-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Utsunomiya H, Suzuki T, Saitou S, Akahira JI, Okamura K, Yaegashi N, Sasano H. 17β-Hydroxysteroid dehydrogenases in human endometrium and its disorders. Mol Cell Endocrinol. 2006;248:136–140. doi: 10.1016/j.mce.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Kanthan R, Senger JLB, Diudea D. Malignant mixed Mullerian tumors of the uterus: histopathological evaluation of cell cycle and apoptotic regulatory proteins. World J Surg Oncol. 2010;8:1–9. doi: 10.1186/1477-7819-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Jinno N, Ohta S, Kuroki H, Fujimoto N. Thyroid hormonal activity of the flame retardant tetrabromobisphenol A and tetrachlorobisphenol A. Biochem Biophys Res Commun. 2002;293:554–559. doi: 10.1016/S0006-291X(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci. 2005;84:249–259. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- Klotz DM, Hewitt SC, Korach KS, Diaugustine RP. Activation of a uterine insulin-like growth factor I signaling pathway by clinical and environmental estrogens: Requirement of estrogen receptor-α. Endocrinol. 2000;141:3430–3439. doi: 10.1210/endo.141.9.7649. [DOI] [PubMed] [Google Scholar]
- Knudsen GA, Sanders JM, Sadik AM, Birnbaum LS. Disposition and kinetics of tetrabromobisphenol A in female Wistar Han rats. Toxicol Rep. 2014;1:214–223. doi: 10.1016/j.toxrep.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuester RK, Sólyom AM, Rodriguez VP, Sipes IG. The effects of dose, route, and repeated dosing on the disposition and kinetics of tetrabromobisphenol A in male F-344 rats. Toxicol Sci. 2007;96:237–245. doi: 10.1093/toxsci/kfm006. [DOI] [PubMed] [Google Scholar]
- Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004;444:213–223. doi: 10.1007/s00428-003-0947-3. [DOI] [PubMed] [Google Scholar]
- Lévy-Bimbot M, Major G, Courilleau D, Blondeau JP, Lévi Y. Tetrabromobisphenol-A disrupts thyroid hormone receptor alpha function in vitro: use of fluorescence polarization to assay corepressor and coactivator peptide binding. Chemosphere. 2012;87:782–788. doi: 10.1016/j.chemosphere.2011.12.080. [DOI] [PubMed] [Google Scholar]
- Li F, Wang J, Nastold P, Jiang B, Sun F, Zenker A, Kolvenbach BA, Ji R, Corvini PFX. Fate and metabolism of tetrabromobisphenol A in soil surries without and with the amendment with the alkylphenol degrading bacterium Sphingomonas sp Strain TTNP3. Environ Pollut. 2014;193:181–188. doi: 10.1016/j.envpol.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- Meerts IATM, van Zanden JJ, Luijks EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman Å, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Meerts IATM, Letcher RJ, Hoving S, Marsh G, Bergman Å, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasu K, Takai N, Nishida M, Narahara H. Tumorigenic effects of tamoxifen on the female genital tract. Clin Med Pathol. 2008;1:17–34. doi: 10.4137/cpath.s487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP (National Toxicology Program) NTP technical report on the toxicology studies of tetrabromobisphenol A (CAS NO. 79-94-7) in F344/NTac rats and B6C3F1 mice and toxicology and carcingogenesis studies of tetrabromobisphenol A in Wistar Han [Crl:WI9Han)] rats and B6C3F1 mice (gavage studies) National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services; 2014. NTP TR 587. NTP publication No. 14-5929. [Google Scholar]
- Nuwaysir EF, Dragan YP, Jefcoate CR, Jordan VC, Pitot HC. Effects of tamoxifen administration on the expression of xenobiotic metabolizing enzymes in rat liver. Cancer Res. 1995;55:1780–1786. [PubMed] [Google Scholar]
- Park MA, Hwang KA, Lee HR, Yi BR, Jeung EB, Choi KC. Cell growth of BG-1 ovarian cancer cells is promoted by di-n-butyl phthalate and hexabromocyclododecane via upregulation of the cyclin D and cyclin-dependent kinase-4 genes. Mol Med Rep. 2012;5:761–766. doi: 10.3892/mmr.2011.712. [DOI] [PubMed] [Google Scholar]
- Raftogianis R, Creveling C, Weinshilboum R, Weisz J. Chapter 6: Estrogen metabolism by conjugation. J Natl Cancer Inst Monogr. 2000;27:113–124. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- Riu A, le Maire A, Grimaldi M, Audebert M, Hillenweck A, Bourguet W, Balaguer P, Zalko D. Characterization of novel ligands of ERα, ERβ, and PPARγ: The case of halogenated bisphenol A and their conjugated metabolites. Toxicol Sci. 2011;122:372–382. doi: 10.1093/toxsci/kfr132. [DOI] [PubMed] [Google Scholar]
- Santin AP, Furlanetto TW. Role of estrogen in thyroid function and growth regulation. J Thyroid Res. 2011;2011:1–7. doi: 10.4061/2011/875125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- USEPA (United States Environmental Protection Agency) [Accessed 13 Nov 2014];Partnership to evaluate flame retardants in printed circuit boards. 2008 Available: http://www.epa.gov/oppt/dfe/pubs/projects/pcb/full_report_pcb_flame_retardants_report_draft_11_10_08_to_e.pdf.
- USEPA (United States Environmental Protection Agency) [Accessed 14 Oct 2015];Technical fact sheet-Polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyls (PBBs) 2014 Available: http://www2.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_perchlorate_january2014_final_0.pdf.
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen N, Boggs A, Walzak K, Helbing CC. Exposure to tetrabromobisphenol-A alters TH-associated gene expression and tadpole metamorphosis in the Pacific tree frog Pseudacris regilla. Aquat Toxicol. 2006;78:292–302. doi: 10.1016/j.aquatox.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Tangjitgamol S, Liu J, Kavanagh JJ. Response of recurrent uterine high-grade malignant mixed Müllerian tumor to letrozole. Int J Gynecol Cancer. 2005;15:1243–1248. doi: 10.1111/j.1525-1438.2005.00193.x. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Modification of the oestrous cycle of the mouse by external stimuli associated with the male. Changes in the oestrous cycle determined by vagina smears. J Endocrin. 1958;17:307–313. doi: 10.1677/joe.0.0170307. [DOI] [PubMed] [Google Scholar]
- Williams-Brown MY, Salih SM, Xu X, Veenstra TD, Saeed M, Theiler SK, Diaz-Arrastia CR, Salama SA. The effect of tamoxifen and roloxifene on estrogen metabolism and endometrial cancer risk. J Steroid Biochem Mol Biol. 2011;126:78–86. doi: 10.1016/j.jsbmb.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Kim YT, Yoon BS, Kim SW, Kang MH, Kim SH, Kim JH, Kim JW, Park YW. Expression profiling of Cyclin B1 and D1 in cervical carcinoma, Exp. Oncol. 2006;28:44–48. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.