Abstract
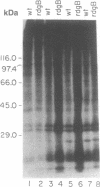
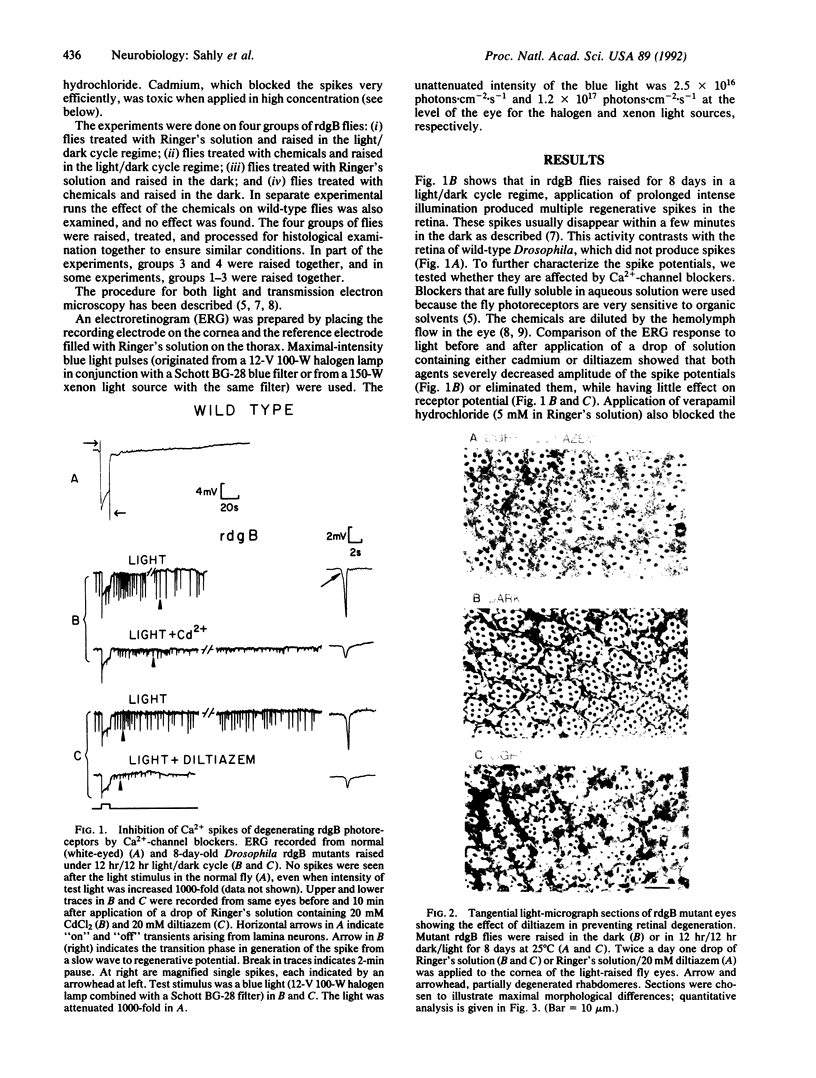
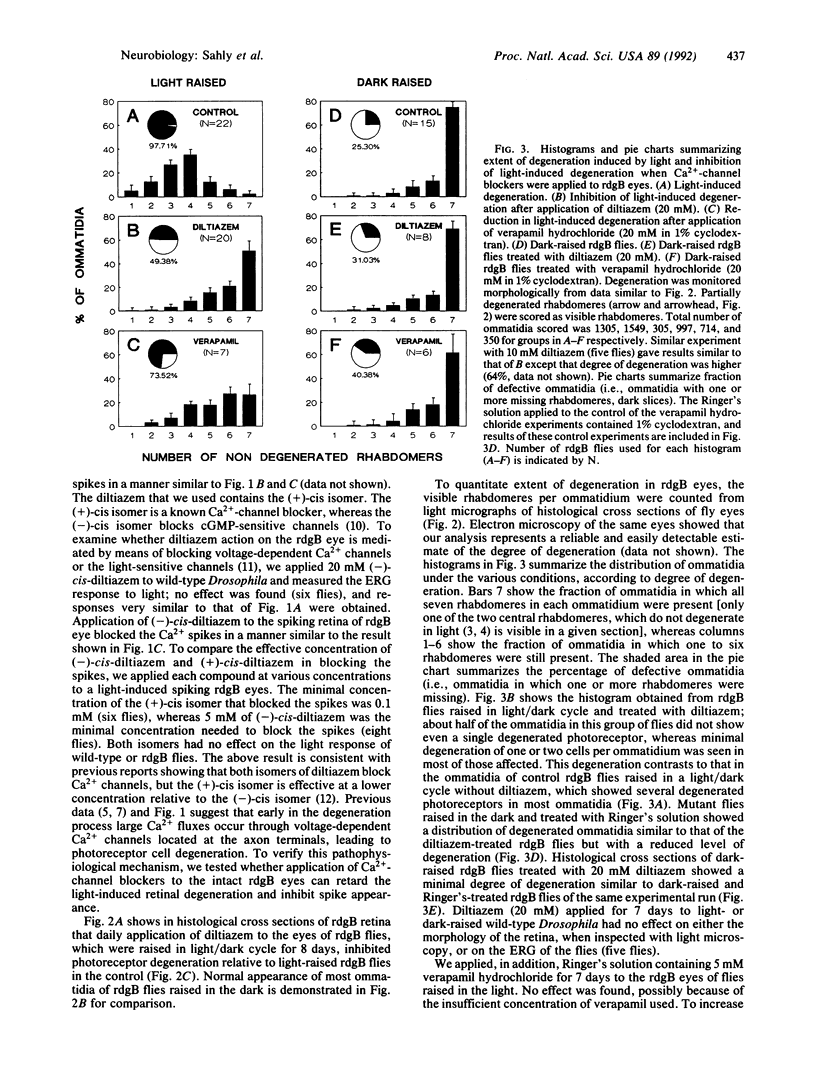
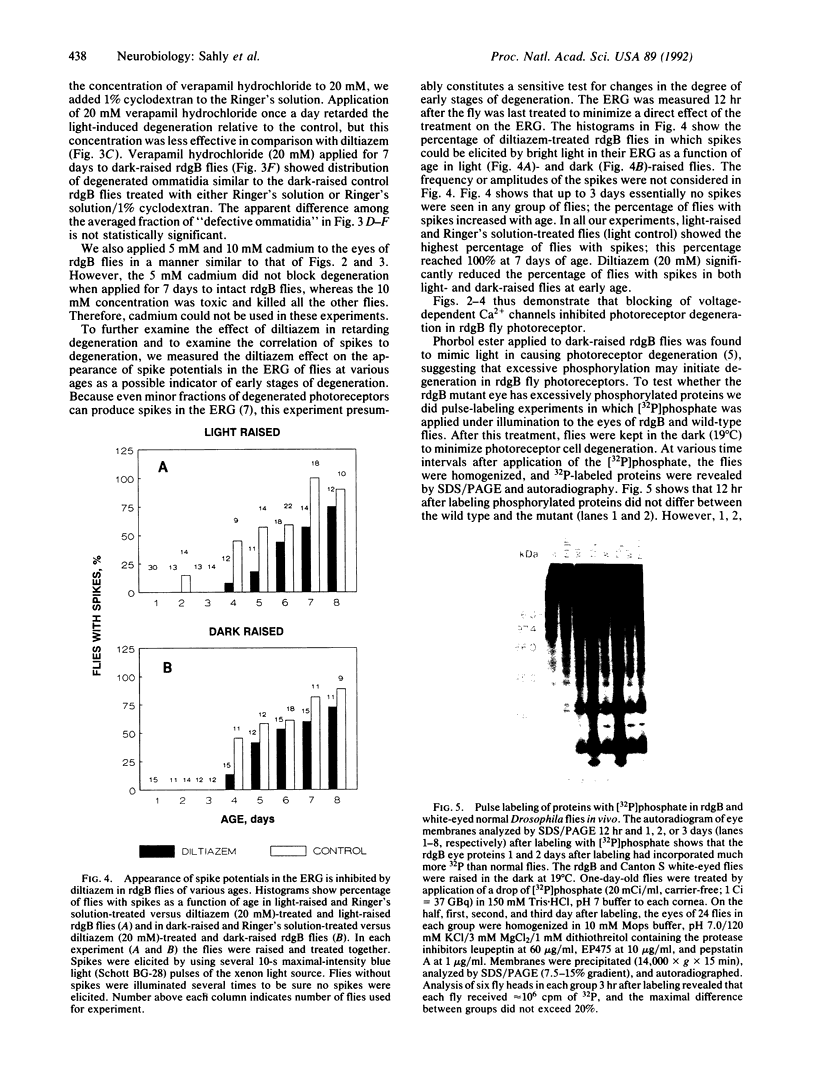
Light accelerates degeneration of photoreceptor cells of the retinal degeneration B (rdgB) mutant of Drosophila. During early stages of degeneration, light stimuli evoke spikes from photoreceptors of the mutant fly; no spikes can be recorded from photoreceptors of the wild-type fly. Production of spike potentials from mutant photoreceptors was blocked by diltiazem, verapamil hydrochloride, and cadmium. Little, if any, effect of the (-)-cis isomer or (+)-cis isomer of diltiazem on the light response was seen. Further, the (+)-cis isomer was approximately 50 times more effective than the (-)-cis isomer in blocking the Ca2+ spikes, indicating that diltiazem action on the rdgB eye is mediated by means of blocking voltage-sensitive Ca2+ channels, rather than by blocking the light-sensitive channels. Application of the Ca(2+)-channel blockers (+)-cis-diltiazem and verapamil hydrochloride to the eyes of rdgB flies over a 7-day period largely inhibited light-dependent degeneration of the photoreceptor cells. Pulse labeling with [32P]phosphate showed much greater incorporation into eye proteins of [32P]phosphate in rdgB flies than in wild-type flies. Retarding the light-induced photoreceptor degeneration in the mutant by Ca(2+)-channel blockers, thus, suggests that toxic increase in intracellular Ca2+ by means of voltage-gated Ca2+ channels, possibly secondary to excessive phosphorylation, leads to photoreceptor degeneration in the rdgB mutant.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquirre G., Farber D., Lolley R., Fletcher R. T., Chader G. J. Rod-cone dysplasia in Irish setters: a defect in cyclic GMP metabolism in visual cells. Science. 1978 Sep 22;201(4361):1133–1134. doi: 10.1126/science.210508. [DOI] [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blest A. D., Carter M., Clausen J. A., Stowe S., Trowell S. C., Tsukitani Y. Induction of retinal degeneration in a crab by light and okadaic acid in vitro: comparison with the Drosophila light-dependent retinal degeneration mutant w rdgBKS222. Vis Neurosci. 1991 Jul-Aug;7(1-2):35–48. doi: 10.1017/s0952523800010920. [DOI] [PubMed] [Google Scholar]
- Bowes C., Li T., Danciger M., Baxter L. C., Applebury M. L., Farber D. B. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990 Oct 18;347(6294):677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988 Oct;11(10):465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Devary O., Heichal O., Blumenfeld A., Cassel D., Suss E., Barash S., Rubinstein C. T., Minke B., Selinger Z. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6939–6943. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Farber D. B., Flannery J. G., Bird A. C., Shuster T., Bok D. Histopathological and biochemical studies on donor eyes affected with retinitis pigmentosa. Prog Clin Biol Res. 1987;247:53–67. [PubMed] [Google Scholar]
- Farber D. B., Lolley R. N. Cyclic guanosine monophosphate: elevation in degenerating photoreceptor cells of the C3H mouse retina. Science. 1974 Nov 1;186(4162):449–451. doi: 10.1126/science.186.4162.449. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R. Assay for calcium channels. Methods Enzymol. 1985;109:513–550. doi: 10.1016/0076-6879(85)09112-1. [DOI] [PubMed] [Google Scholar]
- Harris W. A., Stark W. S. Hereditary retinal degeneration in Drosophila melanogaster. A mutant defect associated with the phototransduction process. J Gen Physiol. 1977 Mar;69(3):261–291. doi: 10.1085/jgp.69.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Benzer S. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1156–1163. doi: 10.1073/pnas.67.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. C., Robinson P. R., Lisman J. E. Cyclic GMP is involved in the excitation of invertebrate photoreceptors. Nature. 1986 Dec 4;324(6096):468–470. doi: 10.1038/324468a0. [DOI] [PubMed] [Google Scholar]
- Minke B., Rubinstein C. T., Sahly I., Bar-Nachum S., Timberg R., Selinger Z. Phorbol ester induces photoreceptor-specific degeneration in a Drosophila mutant. Proc Natl Acad Sci U S A. 1990 Jan;87(1):113–117. doi: 10.1073/pnas.87.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein C. T., Bar-Nachum S., Selinger Z., Minke B. Chemically induced retinal degeneration in the rdgB (retinal degeneration B) mutant of Drosophila. Vis Neurosci. 1989;2(6):541–551. doi: 10.1017/s0952523800003485. [DOI] [PubMed] [Google Scholar]
- Rubinstein C. T., Bar-Nachum S., Selinger Z., Minke B. Light-induced retinal degeneration in rdgB (retinal degeneration B) mutant of Drosophila: electrophysiological and morphological manifestations of degeneration. Vis Neurosci. 1989;2(6):529–539. doi: 10.1017/s0952523800003473. [DOI] [PubMed] [Google Scholar]
- Stark W. S., Sapp R. Retinal degeneration and photoreceptor maintenance in Drosophila: rdgB and its interaction with other mutants. Prog Clin Biol Res. 1989;314:467–489. [PubMed] [Google Scholar]
- Stern J. H., Kaupp U. B., MacLeish P. R. Control of the light-regulated current in rod photoreceptors by cyclic GMP, calcium, and l-cis-diltiazem. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1163–1167. doi: 10.1073/pnas.83.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic T. S., Hyde D. R., O'Tousa J. E. Isolation and characterization of the Drosophila retinal degeneration B (rdgB) gene. Genetics. 1991 Apr;127(4):761–768. doi: 10.1093/genetics/127.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]