Abstract
In autoimmune hemolytic anemia autoantibodies against erythrocytes lead to increased clearance of the erythrocytes, which in turn results in a potentially fatal hemolytic anemia. Depending on whether IgG or IgM antibodies are involved, response to therapy is different. Proper identification of the isotype of the anti-erythrocyte autoantibodies is, therefore, crucial. However, detection of IgM autoantibodies can be challenging. We, therefore, set out to improve the detection of anti-erythrocyte IgM. Direct detection using a flow cytometry-based approach did not yield satisfactory improvements. Next, we analyzed whether the presence of complement C3 on a patient’s erythrocytes could be used for indirect detection of anti-erythrocyte IgM. To this end, we fractionated patients’ sera by size exclusion chromatography and tested which fractions yielded complement deposition on erythrocytes. Strikingly, we found that all patients with C3 on their erythrocytes according to standard diagnostic tests had an IgM anti-erythrocyte component that could activate complement, even if no such autoantibody had been detected with any other test. This also included all tested patients with only IgG and C3 on their erythrocytes, who would previously have been classified as having an IgG-only mediated autoimmune hemolytic anemia. Depleting patients’ sera of either IgG or IgM and testing the remaining complement activation confirmed this result. In conclusion, complement activation in autoimmune hemolytic anemia is mostly IgM-mediated and the presence of covalent C3 on patients’ erythrocytes can be taken as a footprint of the presence of anti-erythrocyte IgM. Based on this finding, we propose a diagnostic workflow that will aid in choosing the optimal treatment strategy.
Introduction
Autoimmune hemolytic anemia (AIHA) is a rare autoimmune disease characterized by the presence of autoantibodies against red blood cells (RBC). The clinical course of AIHA can be variable and life-threatening in certain cases. It is, therefore, important to have an appropriate laboratory work-up to fine-tune the treatment and clinical management of patients with AIHA.
AIHA has traditionally been subdivided into two main types based on the optimal binding temperature of the autoantibodies involved.1 In warm AIHA, mainly polyclonal RBC autoantibodies of IgG class and sometimes of IgA class are involved and react optimally around 37°C.2 Sensitization of RBC with this type of antibodies will lead to destruction via IgG-Fc receptors (FcγR) or IgA-Fc receptors (FcαR), respectively, on phagocytes, mainly in the spleen. Autoantibodies in so-called cold AIHA react optimally at temperatures below 30°C and are mainly of IgM class.3 RBC IgM autoantibodies will activate complement, leading to either complement deposition on the RBC membrane with extravascular destruction of the RBC via complement receptor-mediated phagocytosis or even to intravascular hemolysis if a membrane attack complex is formed. Mixed cold/warm AIHA has also been described, with RBC autoantibodies of IgG class and IgM antibodies with sometimes a high thermal amplitude, in which patients usually present with more severe and chronic hemolysis.3 It is important to realize that RBC IgM autoantibodies may also be involved in a considerable percentage of the warm AIHA,4 which may alter the clinical course and response to therapy. A third, rare, type of AIHA exists (Donath-Landsteiner hemolytic anemia), in which RBC destruction occurs via an IgG that binds at low temperatures and activates complement at higher temperatures.
In current routine diagnostic practice the direct antiglobulin test (DAT) is used to detect bound autoantibodies or the d/g part of complement factor 3 (C3) on patients’ RBC. The indirect antiglobulin test (IAT) is used to detect the autoantibodies in patients’ serum or in eluates from patients’ RBC.5 Both methods are based on RBC agglutination for detection. In addition, some diagnostic laboratories also offer a test to judge the potency of a patient’s serum at inducing complement-mediated hemolysis (the hemolysin test).5
Historically, the therapy of AIHA has been based on the temperature characteristics of the autoantibody rather than of the isotype. In warm (mostly IgG-mediated) AIHA, prednisone is the first-line treatment and is successful in around 70% of the cases with complete remission in 15% of the cases, while the remaining patients require a maintenance dose of steroids.6 Splenectomy is used as second-line therapy, which leads to remission in 50% of patients.7 Rituximab has also been seen to be a successful treatment for IgG-mediated AIHA,8 and – despite its high cost and side effects – is recommended as second-line therapy in steroid-refractory AIHA. Cold (IgM-mediated) AIHA usually does not respond to prednisone. In some cases hemolysis can be prevented by protection from cold, but otherwise the therapeutic anti-CD20 antibody rituximab seems to be a promising strategy for treatment of this group of patients, showing a response rate of around 50%.9,10 In general, patients with mixed AIHA initially respond well to steroids, but usually go on to develop chronic hemolysis.11,12
To determine the optimal therapy, it is crucial to identify the causative RBC autoantibodies correctly, and to evaluate the presence of anti-RBC IgM autoantibodies in AIHA. However, with the current diagnostic techniques, it can be challenging to detect IgM because of its frequently low avidity. Moreover, a distinction is not routinely made between IgG and IgM in the IAT and can be difficult. We, therefore, studied alternative methods to detect autoantibodies in AIHA, focusing on the detection of IgM. First, we adapted the DAT and IAT to a fluorescence-activated cell sorting (FACS)-based assay, since a similar strategy had previously been shown to be successful for the detection of IgG and C3 on RBC.13–16 Secondly, we studied a method for detecting IgM based on the presence of deposited complement factors on patients’ RBC. It is known that IgM activates complement more efficiently than IgG and it has been shown that if a patient’s serum induces hemolysis in vitro, this is often caused by anti-RBC IgM17. Instigated by this, we studied whether the presence or extent of complement C3 deposition, as routinely measured in the DAT, can indicate the presence of anti-RBC IgM by separating IgG and IgM in the sera from AIHA patients and subsequently testing which fractions of the samples activated complement.
Methods
Patients’ material
For the FACS-based IAT and DAT studies, anonymized residual samples from patients with AIHA (34 and 50 patients, respectively) were used. The samples were collected in 2013 and 2014 and had been sent in for diagnostic tests to the Dutch reference laboratory for immunohematology and had a positive DAT for at least C3d and positivity in the hemolysin test. For the fractionation/depletion experiments, 16 additional samples were used that were positive for at least C3d in the DAT. Details can be found in Online Supplementary Table S1. The presence of clinical hemolysis was confirmed using the following parameters, if available: low hemoglobin, high lactate dehydrogenase, decreased haptoglobin, increased indirect bilirubin or a confirmed diagnosis by the treating physician.
Fractionation of serum or eluate from patients with autoimmune hemolytic anemia
AIHA patients’ serum, recalcified plasma or eluate was filtered through a 0.22 μm filter before loading 0.5 mL on a 10/300 Superdex200 column (GE healthcare) equilibrated with two column volumes of phosphate-buffered saline or veronal buffer (5 mM barbital/sodium barbital + 150 mM NaCl, pH = 7.4) and run in the same buffer at 0.5 mL/min for 1.5 column volumes. Fractions of 0.5 mL were collected.
Depletion of serum of IgM or IgG
AIHA patients’ serum or recalcified plasma was depleted, batchwise, of IgM using CaptureSelect IgM affinity Matrix (BAC; now Life Technologies) or of IgG using protein G sepharose as follows. The resin was washed with water and equilibrated in phosphate-buffered saline. Next, 100 μL patients’ serum or recalcified plasma were added to 200 μL packed resin. After incubation for 15–20 min with gentle shaking, the samples were spun down and the depleted serum or recalcified plasma was collected. The IgM affinity matrix was regenerated by eluting bound antibodies with glycine buffer pH 2.9 and washing the material extensively with phosphate-buffered saline. The protein G sepharose was discarded after use.
IgM and IgG enzyme-linked immunosorbent assays
IgM and IgG concentrations were measured by an IgM or IgG enzyme-linked immunosorbent assay (ELISA), respectively. For this, Nunc Maxisorp plates (Thermo Scientific) were coated with polyclonal anti-human IgM (SH15; Sanquin Reagents) or monoclonal anti-human IgG (MH16-1; Sanquin Reagents). After washing, titrations of samples were applied in High Performance ELISA buffer (HPE; Sanquin Reagents). Horseradish peroxidase (HRP)-labeled monoclonal anti-human IgM (MH15-HRP; Sanquin Reagents) or HRP-labeled monoclonal anti-human IgG (MH16-1-HRP; Sanquin Reagents) was used for the detection. Tetramethylbenzidine was used for staining. For ease of visual presentation, IgG and IgM concentrations are expressed in arbitrary units, which consist of the percentage of the highest measured concentration (per ELISA).
C3 and C4 deposition assay
The C3 and C4 deposition assay was performed as described previously.18 In brief, samples were incubated for 2 h at 37°C with bromelain-treated O-typed RBC, AB serum and inhibitory anti-C5 monoclonal antibody in veronal buffer supplemented with 10 mM CaCl2, 2 mM MgCl2 and 0.05% gelatin (VBG++). After washing the RBC, samples were stained with fluorescently labeled anti-C3-FITC (clone anti-C3-19, Sanquin Research) and anti-C4-APC (clone anti-C4-10, Sanquin Research) and analyzed by flow cytometry. Gating was done on single RBC using a FSC-A versus FSC-W plot.
Additional details of the methods can be found in the Online Supplementary Material.
Results
Flow cytometry-based indirect and direct antiglobulin testing
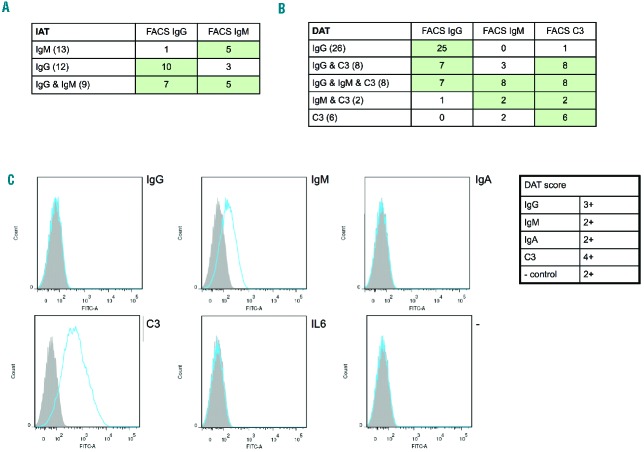
To obtain a sensitive method for detecting autoantibodies, especially RBC autoantibodies of the IgM class in AIHA, we developed a variant of the IAT and the DAT with detection by FACS instead of by agglutination. After optimization of the assays (Online Supplementary Figure S1), we measured anti-RBC IgM and IgG in serum or plasma of 34 AIHA patients, and IgM, IgG and C3 bound to the RBC of 50 patients. As can be seen in Figure 1A, IgG detection in serum by FACS in both IgG-positive and IgG & IgM-positive patients is slightly less sensitive than the conventional method by IAT, while in a large proportion of IgM or IgG & IgM positive samples, the IgM was missed by FACS (Online Supplementary Figure S2A), probably because it was washed away in the washing steps necessary before the FACS staining. Detection of (small amounts of) IgM in three samples that were determined as having only IgG in serum was probably due to the IAT by FACS being better able to distinguish IgG and IgM and thus being capable of discerning small amounts of IgM in the presence of IgG if the IgM bound strongly enough to remain bound during the washing steps.
Figure 1.
IAT and DAT by FACS on AIHA samples characterized by the conventional (agglutination-based) IAT and DAT. (A) Results of IAT by FACS on samples in which IgM, IgG or IgM & IgG were detected by routine diagnostics, showing that the FACS-based method is less sensitive than the agglutination-based method especially for IgM. The number of patients tested per group is indicated in brackets. (B) Results of the FACS-based DAT on samples that were characterized as having IgG, IgG & C3, IgG & IgM & C3, IgM & C3 or C3 by routine diagnostic DAT (column method), showing strong similarity between the two techniques. The number of patients tested per group is indicated in brackets. (C) The FACS (figure) and diagnostic DAT (table) results are shown for a patient for whom the diagnostic DAT was problematic because of a positive phosphate-buffered saline control, but for whom the FACS result was very clearly only positive for IgM and C3 with nice negative controls.
The results from the FACS-based DAT were very similar to those from conventional DAT by the column method (Figure 1B; Online Supplementary Figure S2B). In the IgG & C3 and C3 samples in which IgM was detected only by FACS-based DAT, cold agglutinins (weak or strong) were detected in the serum by conventional methods, so the difference might be due to a difference in temperature during the experiment, leading to better detection of IgM by FACS. The DAT by FACS has one advantage over the conventional DAT, exemplified by a case that was problematic in routine diagnostics because of direct agglutination of the cells, which gave a clear result in the FACS-based assay (Figure 1C). Routine diagnostics of this sample may have been complicated by the IgM still binding and causing agglutination, even after washing of the cells.
Development of a complement-based method
Because the FACS-based assays did not improve the detection of anti-RBC IgM substantially, we attempted a different strategy. It is often presumed that when C3d is detected in the DAT and there is no autoantibody, complement deposition has been caused by a weakly binding IgM. By investigating the initiator of complement activation in AIHA, we studied whether this assumption is true and whether perhaps a large amount of C3 in the DAT is in general indicative of a (hidden) anti-RBC IgM. This would then yield an indirect method for IgM detection.
To this end, we took two approaches to separate IgG and IgM in patients’ serum and subsequently to evaluate complement activation on RBC. We first validated these techniques using ABO mismatching as a model system, since naturally occurring anti-A and anti-B IgM in serum from donors with group O are known to be the cause of complement activation on AB-typed RBC. For the first method, group O-typed serum was fractionated by size exclusion and the fractions’ capacity to activate complement was determined. Total IgM and IgG concentrations in the fractions were determined by ELISA in order to know in which fractions IgM and IgG elute from the column. As can be seen in Figure 2A, complement activation on group AB-typed RBC occured only in the IgM-containing fractions, showing that this method works. No complement activation was seen in any fraction nor by the serum itself on group O-typed RBC, as expected (data not shown).
Figure 2.
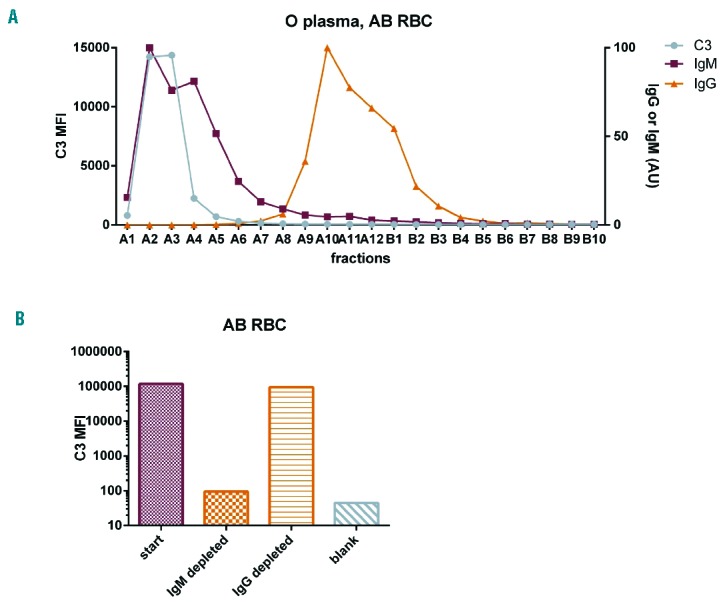
Testing methods to study complement activation by anti-RBC antibodies using ABO mismatching as a model system. (A) Group O serum was fractionated and fractions were tested for complement activation on AB RBC, and IgM/IgG concentration was measured. Complement activation can be seen to occur in the IgM-containing peak, as expected. (B) Group O serum was depleted of IgG or IgM and subsequently tested for complement activation on AB RBC. As can be seen, depleting group O serum of IgM strongly reduced the complement activation capacity, while IgG depletion left this ability intact, as expected for IgM-mediated complement activation.
In order to be able to verify our results and exclude the possibility that the result was caused by an unknown factor co-eluting with IgM or IgG, we developed a second independent technique to study complement activation by anti-RBC antibodies and tested it on the ABO mismatched model system. This second method involved depletion of IgM or IgG from group O-typed serum by IgM affinity matrix or protein G resin and then testing the starting serum and depleted samples for complement activation by a C3 deposition assay. As can be seen in Figure 2B, depleting O-typed serum of IgM abolished the serum’s ability to cause complement deposition on AB-typed RBC, while depletion of IgG hardly reduced this ability, confirming with an independent method that anti-RBC IgM is responsible for complement activation in ABO mismatch. Again, no complement activation was seen on O-typed RBC, as expected (data not shown).
Fractionation of serum from patients with autoimmune hemolytic anemia
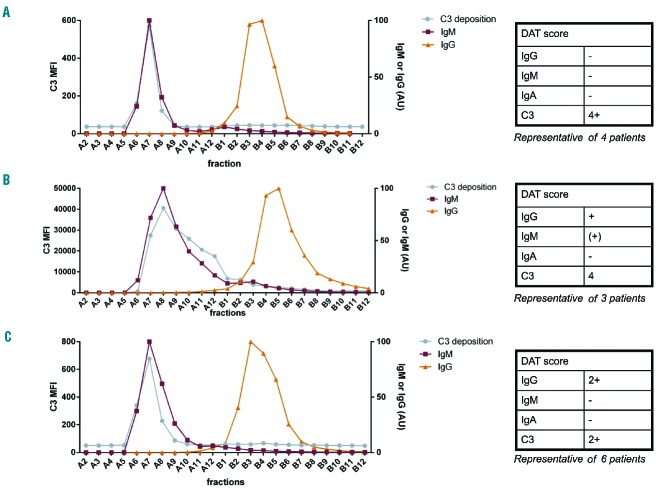
Since these methods gave the expected results in our model system of ABO mismatch, we continued to study complement activation by AIHA patients’ serum. First, we fractionated serum or recalcified plasma from four AIHA patients who had a DAT only positive for C3 and tested complement activation on RBC in the fractions to investigate which anti-RBC isotype was responsible for complement activation. It is often presumed that an anti-RBC IgM must be responsible for this, but this had not yet been directly proven. A representative result of the four tested patients is presented in Figure 3A. This figure shows that complement activation only occurs in the IgM-containing fractions, strongly suggesting that anti-RBC IgM causes complement activation on RBC, as was previously assumed.
Figure 3.
Identification of the antibody causing complement activation in AIHA. Sera from AIHA patients with different DAT results were fractionated and the fractions were tested for complement activation and IgG/IgM concentration. For each group of patients, complement activation was seen in the IgM-containing fractions. (A) DAT only positive for C3; (B) DAT positive for IgG, IgM and C3; (C) DAT positive for IgG and C3.
Next, we continued to test complement activation in patients with anti-RBC IgG and IgM autoantibodies detected in the DAT besides C3 to see whether the anti-RBC IgG or IgM or both are involved in complement activation in these patients. Figure 3B shows a representative result of the three patients tested from this group. As can be seen, complement deposition on RBC again occurs in the IgM-containing peak, demonstrating that also in these patients IgM is responsible for the complement activation on RBC.
Finally, we studied a group of patients who had a DAT positive for both IgG and C3, but not for IgM. In light of the above, this is a very interesting group, since it is often assumed that in these patients, the IgG (mainly IgG1 or IgG3) is responsible for complement activation. We tested whether this is true for six patients. A representative result for this group of patients is shown in Figure 3C. Strikingly, in all these patients the observed complement activation was again found to take place in the IgM-containing fractions. This strongly suggests that these patients have an undetected anti-RBC IgM that causes the complement activation, in contrast to what was previously believed. For one patient’s sample, a very small amount of complement activation was seen in the IgG peak besides the IgM peak (IgG complement activation 0.1 % of the height of the IgM peak).
We were surprised to find that anti-RBC IgM was responsible for complement activation in all tested serum samples and that IgG never was, even in our samples with a DAT positive for IgG and C3. To verify that anti-RBC IgG is not in any way negatively influenced by our fractionation strategy, we performed an IAT by FACS on four fractionated AIHA patients’ samples with known anti-RBC IgG. Anti-RBC IgG was found to be still present and able to bind to RBC in the expected fractions after size exclusion (Online Supplementary Figure S3), indicating that our previous results were not caused by any deactivation of anti-RBC IgG during our procedure.
Depletion of IgG and IgM from serum of patients with autoimmune hemolytic anemia
Next, the finding that anti-RBC IgM causes complement activation in AIHA irrespective of the presence of RBC-specific IgG was verified using the depletion setup described above on two patients’ samples positive for only C3 in the DAT and two samples positive for IgG and C3 in the DAT. As can be seen in Figure 4, in both pairs of samples IgM depletion abolished complement activation and IgG depletion left this activity mostly intact, confirming that IgM is indeed responsible for complement activation in AIHA and that our previous results were not caused by an unknown factor co-eluting with IgG or IgM.
Figure 4.
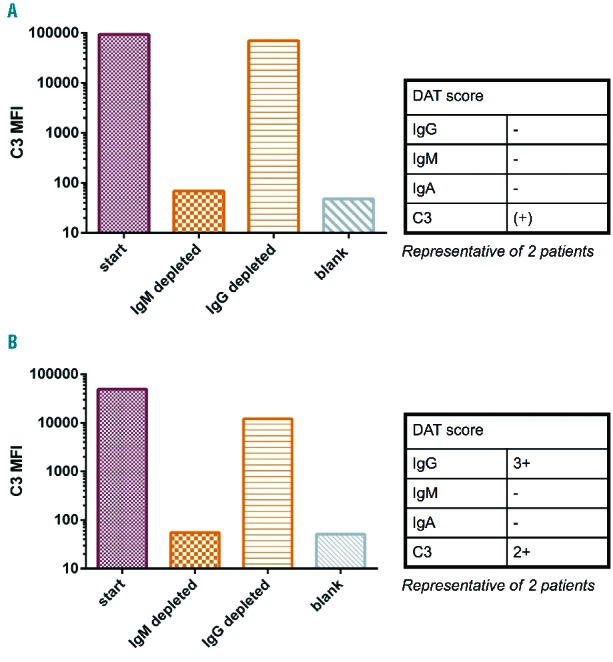
Verification with an independent method that IgM is responsible for complement activation in AIHA. AIHA patients’ serum was depleted of IgG and IgM followed by a complement activation assay. As can be seen, IgM depletion strongly reduced complement activation whereas IgG depletion left this activity mostly intact. This verifies the fractionation results that anti-RBC IgM causes complement activation in AIHA. Diagnostic DAT results are presented in the tables.
Fractionation of autoimmune hemolytic anemia eluates
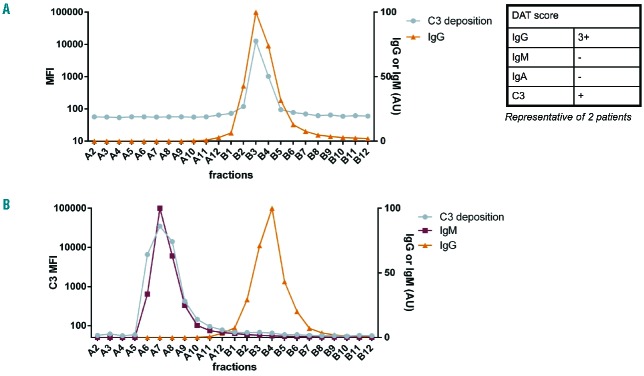
From the above results, we concluded that it is anti-RBC IgM and not IgG in AIHA patients’ plasma that is responsible for complement activation. However, autoantibodies can be present not only in plasma, but also on RBC. To test the contribution to complement activation of these autoantibodies, we eluted them from the patients’ RBC with acid elution. The resulting eluate was tested for its ability to lead to complement deposition on RBC. In three of the six tested samples with a positive DAT for at least IgG and C3, the eluate did not cause any complement deposition on RBC or only a very weak signal compared to that of serum (data not shown). In the other three, the eluate caused non-negligible complement activation.
To study the initiator of complement in eluates, two of the three complement-activating eluates were fractionated and tested in the C3 deposition assay. A representative example is shown in Figure 5A, where it can be seen that there is anti-RBC IgG present in the eluate that causes complement deposition. However, in the plasma of these patients, complement was activated by an anti-RBC IgM, as shown in Figure 5B. This means that in these samples, which constitute half the samples with a DAT positive for IgG and C3, complement was activated by both IgG and IgM. Since in this study, these samples and all other samples described above with DAT positive for at least C3 had at least an IgM activating complement detectable in the serum, the presence of C3 on patients’ RBC as detected by the routine DAT remains a strong indication for the presence of an anti-RBC IgM.
Figure 5.
Identification of the complement-activating anti-RBC antibody in eluate versus plasma. Fractionation of eluate (A) and recalcified plasma (B) of an AIHA patient and subsequent testing of complement activating ability of the different fractions. As can be seen, complement activation in the plasma was IgM-mediated and in the eluate IgG-mediated. Diagnostic DAT results are presented in the table. No IgM was measurable in the eluate.
Discussion
Identification of anti-RBC autoantibodies in AIHA is crucial for determining the treatment strategy; however, it can be challenging to detect anti-RBC IgM because of its frequently low avidity. We, therefore, explored several alternative options for detecting anti-RBC IgM. First we focused on a FACS-based adaptation of the IAT and DAT. IgG and C3 detection on RBC by FACS has been described in various previous studies,13–16 but IgM detection in serum has not and on RBC hardly. In a very recent article, concomitant detection of IgG, C3d, IgM and IgA was described.19 This study, however, does not give insight into IgM detection by FACS due to lack of samples positive for IgM.
In our hands, DAT by FACS gave very good agreement with the traditional DAT, performed using the column technique, but no true improvement in IgM detection. Since the FACS method is more laborious due to the various washing steps, we do not think it represents a substantial improvement to routine diagnostics. Only when problems are encountered in the routine techniques (e.g. with positive readings in the negative controls), might DAT by FACS be a good “escape test”.
IAT by FACS was clearly less sensitive than agglutination-based techniques. This is probably due to the fact that for the FACS method, in contrast to the agglutination method, the sample has to undergo several washing steps leading to loss of weakly binding antibodies. An improvement in detection of IgM was seen only for very few samples and we, therefore, conclude that also the IAT by FACS does not yield sufficient advantages to justify its application in routine diagnostic practices.
Since FACS-based direct detection of anti-RBC IgM did not provide a sufficient improvement, we analyzed whether the presence of C3 on RBC could be indicative of an anti-RBC IgM. We expected that if no autoantibody was detected or only low amounts of IgG, undetected anti-RBC IgM would be responsible for complement activation. This has often been assumed, but never directly proven. In this study we proved that this is indeed the case. To our surprise, not only these samples, but all 16 samples we tested with a DAT score positive for C3 turned out to have an anti-RBC IgM capable of activating complement. This was also the case when only anti-RBC IgG was detected besides the C3 on the RBC, even if there was relatively more IgG compared to C3 as measured by the DAT score; this is in contrast to what was generally assumed. Anti-RBC IgG, of which a large part is bound to the RBC, is also capable of activating complement, as could be seen in our fractionation experiments with RBC eluates. It is therefore theoretically possible that a patient only has complement-activating antibodies in the eluate. However, all samples we had access to with a complement-activating eluate also had an otherwise undetected complement-activating anti-RBC IgM in the plasma or serum. We, therefore, think that the presence of C3 might be taken as a very indicative sign of the presence of an anti-RBC IgM that could be of too low avidity to be detected itself, but that can bind with sufficient affinity in vivo to initiate the complement cascade and hence leave this covalent footprint. The presence of C3 on the patients’ RBC is proof that this complement activation occurs in vivo, making these anti-RBC IgM clinically relevant.
Based on our findings we propose the following work-flow for AIHA diagnostics (Figure 6). When a patient presents with clinical hemolysis and a positive DAT, it is advisable to carry out a monospecific DAT. If this turns out to be positive for IgG and not C3 (left arm of diagram), the result is clear: an IgG-mediated AIHA. In the case that the DAT is positive for C3 and possibly IgM (right arm), the result is also clear: IgM-mediated AIHA. It is advisable to test for the thermal amplitude of the IgM to best predict the scope of the disease. A DAT positive for C3 and IgG (and possibly IgM) was until now more challenging. Samples can be tested for complement-activating ability of the serum, for example, by a complement deposition test as described by Meulenbroek et al.18 If this result is positive, complement-activating activity in the serum is proven and, based on the results presented in our study, we propose that this group of patients should be considered as having an IgG/IgM-mediated AIHA. If the serum is negative in the hemolysin test, it is theoretically possible that the only complement activator of the sample is an IgG in the eluate, although we have never encountered such a sample. We, therefore, expect that these patients also have an anti-RBC IgM in their serum, as did all the samples in our study, and that these patients should be considered as having an IgG/IgM-mediated AIHA. Certainty could be obtained by performing fractionation or deletion experiments as described in this study. When deciding treatment strategy and probable course of the disease, clinicians should bear in mind that the IgG and C3 DAT-positive patients most probably also have an anti-RBC IgM, which means that these patients do not have solely IgG-mediated AIHA, as currently often considered. However, it should be kept in mind that the proposed workflow is based on a laboratory study and not yet on clinical data, so although it can help a physician in thinking, it cannot be used to fully base treatment on.
Figure 6.
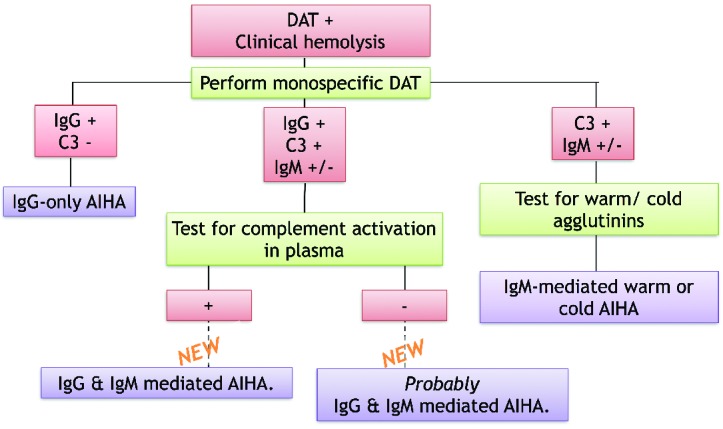
Proposed diagnostic workflow for AIHA. The conclusion of the middle arm is based on the results presented in this paper.
Furthermore, these results have implications for transfusion. When donor RBC are transfused into a patient, they will immediately come into contact with the anti-RBC antibodies in the plasma. For AIHA patients who have at least C3 on their RBC, this means that the donor RBC will come into contact with complement-activating anti-RBC IgM even if these have not been detected otherwise and even if the patient has other antibodies bound to their RBC (e.g. RBC-bound IgG). Administration of a complement inhibitor, e.g. C1-inhibitor20 or TNT003,21 in conjunction with the transfusion might therefore be beneficial for the yield of the transfusion.
Using C3 as a footprint of anti-RBC IgM, considerably more patients will be identified as having anti-RBC IgM. The clinical relevance of these additionally detected cases will now have to be determined. For this, we will continue to collect clinical data on treatment response in patients who would have been considered as having IgG-mediated AIHA previously but should now be recognized as having IgG- and IgM-mediated AIHA and compare them to other AIHA patients.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Petz LD, Garratty G, Immune hemolytic anemia’s. Chapter 3: Classification and clinical characteristics of autoimmune hemolytic anemias. Churchill Livingstone, Philadelphia, Pennsylvania, USA, 1980; 2nd edition: 61–114. [Google Scholar]
- 2.Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev 2010;24(3):195–210. [DOI] [PubMed] [Google Scholar]
- 3.Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev 2008;22(1): 1–15. [DOI] [PubMed] [Google Scholar]
- 4.Engelfriet CP, van’t Veer MB, Maas N, Ouwehand WH, Beckers DO, von den Borne AE. Autoimmune haemolytic anemias. In: Kay AB, Denman AM, Wright R, eds. Clinical Immunology and Allergy. London: Baillieres Tindall; 1987:251–267. [Google Scholar]
- 5.Zeerleder S. Autoimmune haemolytic anaemia – a practical guide to cope with a diagnostic and therapeutic challenge. Neth J Med 2011;69(4):177–184. [PubMed] [Google Scholar]
- 6.Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol 2002;69 (4):258–271. [DOI] [PubMed] [Google Scholar]
- 7.King KE, Ness PM. Treatment of autoimmune hemolytic anemia. Semin Hematol 2005;42(3):131–136. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Gu W. Immunotherapy treatments of warm autoimmune hemolytic anemia. Clin Dev Immunol. 2013:561852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schollkopf C, Kjeldsen L, Bjerrum OW, et al. Rituximab in chronic cold agglutinin disease: a prospective study of 20 patients. Leuk Lymphoma 2006;47(2):253–260. [DOI] [PubMed] [Google Scholar]
- 10.Berentsen S, Ulvestad E, Gjertsen BT, et al. Rituximab for primary cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood 2004;103(8): 2925–2928. [DOI] [PubMed] [Google Scholar]
- 11.Shulman IA, Branch DR, Nelson JM, Thompson JC, Saxena A, Petz LD. Autoimmune hemolytic anemia with both cold and warm autoantibodies. JAMA 1985;253(12):1746–1748. [PubMed] [Google Scholar]
- 12.Sokol RJ, Hewitt S, Stamps BK. Autoimmune hemolysis: mixed warm and cold antibody type. Acta Haematol 1983;69(4):266–274. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhary R, Das SS, Gupta R, Khetan D. Application of flow cytometry in detection of red cell bound IgG in Coombs negative AIHA. Hematology 2006;11(4):295–300. [DOI] [PubMed] [Google Scholar]
- 14.Fayek MH, Saad AA, Eissa DG, Tawfik LM, Kamal G. Role of gel test and flow cytometry in diagnosis of Coombs’ negative autoimmune haemolytic anaemia. Int J Lab Hematol 2012;34(3):311–319. [DOI] [PubMed] [Google Scholar]
- 15.Lin JS, Hao TC, Lyou JY, Chen YJ, Liu HM, Tzeng CH, Chiou TJ. Clinical application of a flow cytometric direct antiglobulin test. Transfusion 2009;49(7):1335–1346. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Shi J, Zhou Y, Ruan C. Detection of red blood cell-bound immunoglobulin G by flow cytometry and its application in the diagnosis of autoimmune hemolytic anemia. Int J Hematol 2001;73(2):188–193. [DOI] [PubMed] [Google Scholar]
- 17.Von dem Borne AE, Engelfriet CP, Beckers D, Van der Kort-Henkes G, Van der Giessen M, Van Loghem JJ. Autoimmune haemolytic anaemias. II. Warm haemolysins – serological and immunochemical investigations and 51Cr studies. Clin Exp Immunol 1969;4(3):333–343. [PMC free article] [PubMed] [Google Scholar]
- 18.Meulenbroek EM, Wouters D, Zeerleder S. Methods for quantitative detection of antibody-induced complement activation on red blood cells. J Vis Exp. 2014;(83):e51161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alzate MA, Manrique LG, Bolanos NI, Duarte M, Coral-Alvarado P, Gonzalez JM. Simultaneous detection of IgG, IgM, IgA complexes and C3d attached to erythrocytes by flow cytometry. Int J Lab Hematol 2015;37(3):382–389. [DOI] [PubMed] [Google Scholar]
- 20.Wouters D, Stephan F, Strengers P, et al. C1-esterase inhibitor concentrate rescues erythrocytes from complement-mediated destruction in autoimmune hemolytic anemia. Blood 2013;121(7):1242–1244. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, Rose EL, Singh A, et al. TNT003, and inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinin disease patient autoantibodies. Blood 2014;123(26):4015–4022. [DOI] [PubMed] [Google Scholar]