Abstract
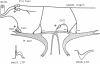
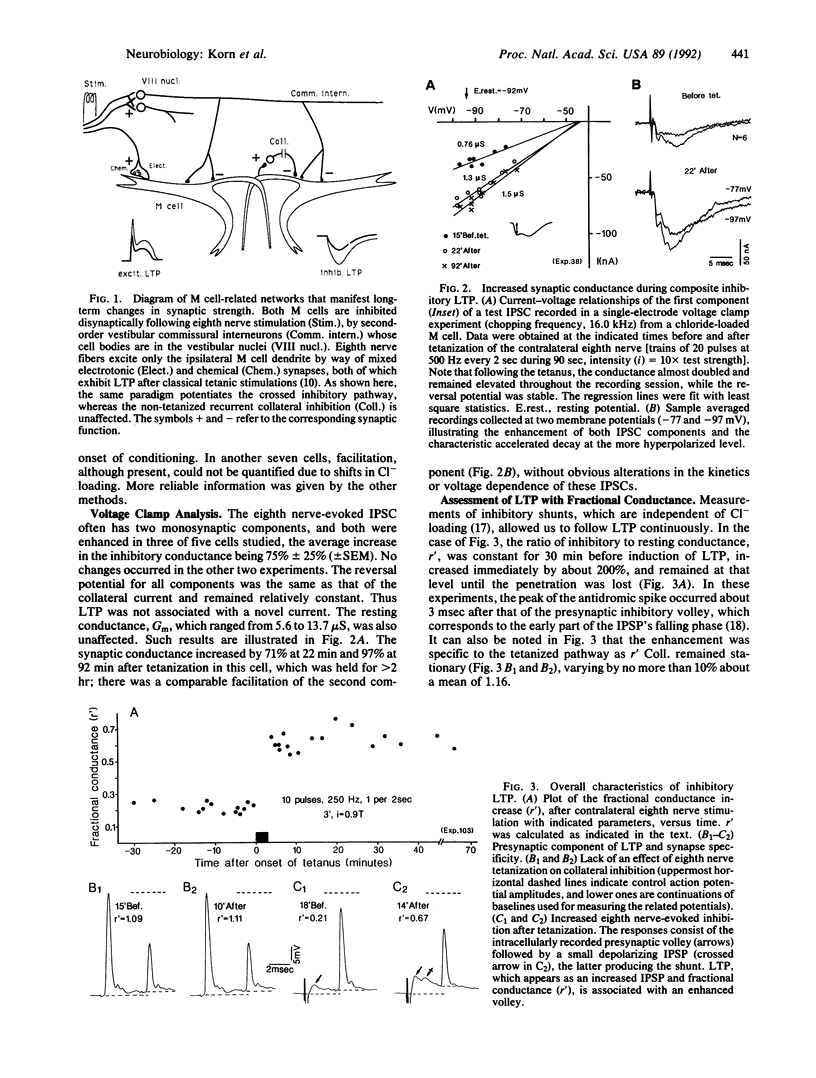
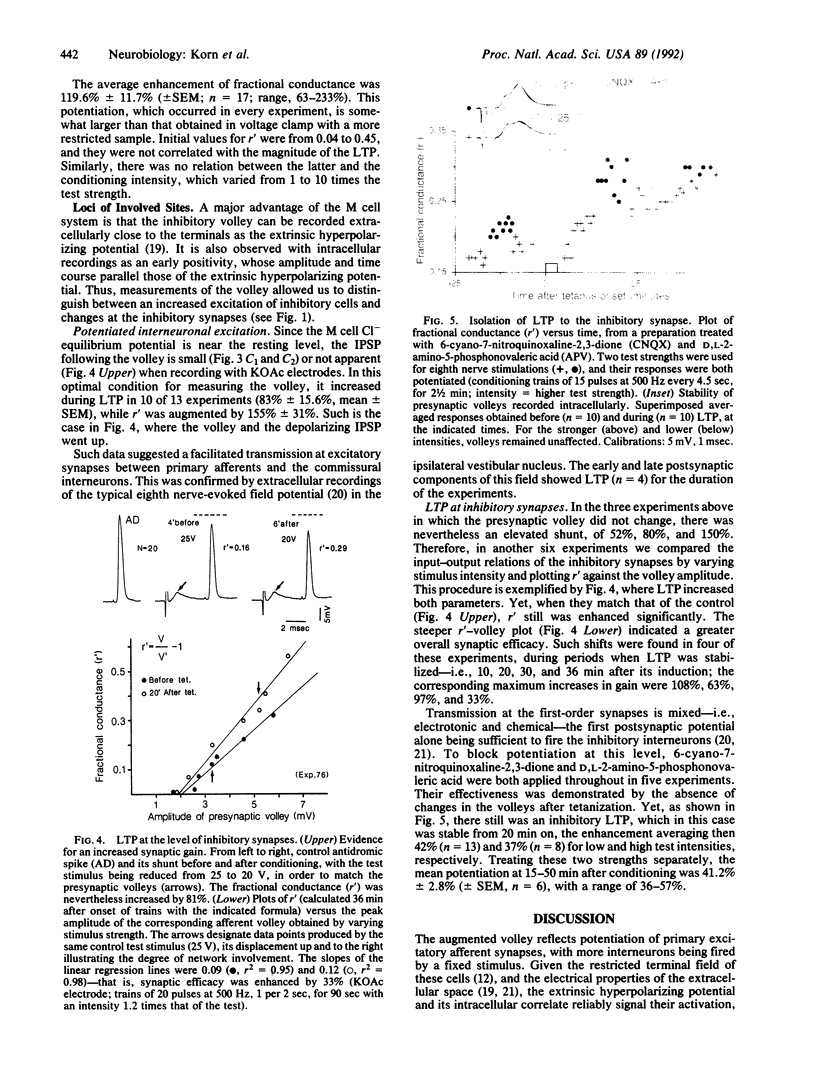
Glycinergic inhibition evoked disynaptically in the teleost Mauthner cell by stimulation of the contralateral eighth nerve exhibits long-term potentiation following classical tetanization of that pathway. This enhancement occurs at the synapses between primary afferents onto second-order interneurons and the connections between these inhibitory cells and the Mauthner neuron. The evidence for modifications of glycinergic transmission is that the slope of the relation between the presynaptic volley and the synaptic conductance can be greater after the tetanus. This increase in gain is still manifest after pharmacological block of potentiation at the excitatory synapse with glutamate antagonists. Inhibitory long-term potentiation is induced by tetani weaker than those required for enhancement of the monosynaptic excitation of the other (ipsilateral) Mauthner cell. Thus, in vivo learning can alter the balance between excitation and inhibition within a network by modifying one or both of them.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. C., Gustafsson B., Wigström H. Long-term potentiation involves enhanced synaptic excitation relative to synaptic inhibition in guinea-pig hippocampus. J Physiol. 1987 Dec;394:367–380. doi: 10.1113/jphysiol.1987.sp016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Eidelberg E. Direct afferent excitation and long-term potentiation of hippocampal interneurons. J Neurophysiol. 1982 Sep;48(3):597–607. doi: 10.1152/jn.1982.48.3.597. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega L. E., Bliss T. V., Halliwell J. V. The EPSP-spike (E-S) component of long-term potentiation in the rat hippocampal slice is modulated by GABAergic but not cholinergic mechanisms. Neurosci Lett. 1989 Sep 25;104(1-2):58–64. doi: 10.1016/0304-3940(89)90329-7. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Tseng H. Y., Hockberger P. E. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUKAWA T., FURSHPAN E. J. Two inhibitory mechanisms in the Mauthner neurons of goldfish. J Neurophysiol. 1963 Jan;26:140–176. doi: 10.1152/jn.1963.26.1.140. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Synergism at central synapses due to lateral diffusion of transmitter. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8708–8712. doi: 10.1073/pnas.85.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Transmission at a central inhibitory synapse. I. Magnitude of unitary postsynaptic conductance change and kinetics of channel activation. J Neurophysiol. 1982 Sep;48(3):654–678. doi: 10.1152/jn.1982.48.3.654. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Unitary conductance changes at teleost Mauthner cell glycinergic synapses: a voltage-clamp and pharmacologic analysis. J Neurophysiol. 1988 Dec;60(6):1982–1999. doi: 10.1152/jn.1988.60.6.1982. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Voltage-dependence of glycine-activated Cl- channels: a potentiometer for inhibition? J Neurosci. 1987 Mar;7(3):807–811. doi: 10.1523/JNEUROSCI.07-03-00807.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith W. H., Brown T. H., Johnston D. Voltage-clamp analysis of synaptic inhibition during long-term potentiation in hippocampus. J Neurophysiol. 1986 Apr;55(4):767–775. doi: 10.1152/jn.1986.55.4.767. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Rose G. Long-term potentiation of excitatory synaptic transmission in the rat hippocampus: the role of inhibitory processes. J Physiol. 1982 Aug;329:541–552. doi: 10.1113/jphysiol.1982.sp014318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Faber D. S. Vertebrate central nervous system: same neurons mediate both electrical and chemical inhibitions. Science. 1976 Dec 10;194(4270):1166–1169. doi: 10.1126/science.186868. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Mamounas L. A., Thompson R. F., Madden J., 4th Cerebellar GABAergic processes: evidence for critical involvement in a form of simple associative learning in the rabbit. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2101–2105. doi: 10.1073/pnas.84.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971 Jul 1;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Maru E., Ashida H., Tatsuno J. Long-lasting reduction of dentate paired-pulse depression following LTP-inducing tetanic stimulations of perforant path. Brain Res. 1989 Jan 23;478(1):112–120. doi: 10.1016/0006-8993(89)91482-0. [DOI] [PubMed] [Google Scholar]
- Mintz I., Gotow T., Triller A., Korn H. Effect of serotonergic afferents on quantal release at central inhibitory synapses. Science. 1989 Jul 14;245(4914):190–192. doi: 10.1126/science.2749257. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Sarvey J. M., Klee M. R. Heterosynaptic postactivation potentiation in hippocampal CA 3 neurons: long-term changes of the postsynaptic potentials. Exp Brain Res. 1979 Oct;37(2):217–229. doi: 10.1007/BF00237709. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Sarvey J. M. gamma-Aminobutyrate sensitivity does not change during long-term potentiation in rat hippocampal slices. Neuroscience. 1985 Jul;15(3):695–702. doi: 10.1016/0306-4522(85)90071-5. [DOI] [PubMed] [Google Scholar]
- Song Y. M., Huang L. Y. Modulation of glycine receptor chloride channels by cAMP-dependent protein kinase in spinal trigeminal neurons. Nature. 1990 Nov 15;348(6298):242–245. doi: 10.1038/348242a0. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Slater N. T., ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987 Apr 16;326(6114):698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Augmentation by glycine and blockade by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) of responses to excitatory amino acids in slices of rat neocortex. Neuroscience. 1990;39(1):69–79. doi: 10.1016/0306-4522(90)90222-p. [DOI] [PubMed] [Google Scholar]
- Triller A., Korn H. Variability of axonal arborizations hides simple rules of construction: a topological study from HRP intracellular injections. J Comp Neurol. 1986 Nov 22;253(4):500–513. doi: 10.1002/cne.902530407. [DOI] [PubMed] [Google Scholar]
- Wolszon L. R., Faber D. S. The effects of postsynaptic levels of cyclic AMP on excitatory and inhibitory responses of an identified central neuron. J Neurosci. 1989 Mar;9(3):784–797. doi: 10.1523/JNEUROSCI.09-03-00784.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto C., Chujo T. Long-term potentiation in thin hippocampal sections studied by intracellular and extracellular recordings. Exp Neurol. 1978 Jan 15;58(2):242–250. doi: 10.1016/0014-4886(78)90137-1. [DOI] [PubMed] [Google Scholar]
- Yang X. D., Korn H., Faber D. S. Long-term potentiation of electrotonic coupling at mixed synapses. Nature. 1990 Dec 6;348(6301):542–545. doi: 10.1038/348542a0. [DOI] [PubMed] [Google Scholar]