Invasive fungal disease (IFD) in the immunocompromised host is associated with high mortality,1 prolonged stays in hospital and significant healthcare costs.2 The epidemiology of IFD within the heterogeneous group of patients with lymphoproliferative disorders is not well defined and antifungal prophylaxis practices vary. In the current era of a myriad of novel therapeutic agents, we aim to describe the epidemiology of IFD and reflect upon prevention of IFD in this cohort of patients. To this end, we conducted a retrospective cohort study at the Peter MacCallum Cancer Centre (PMCC) to determine the epidemiology of IFD in patients with lymphoproliferative disorders receiving cytotoxic chemotherapy according to disease type and chemotherapy exposure.
For the period March 2009 to December 2011, all patients with lymphoproliferative neoplasms who received mold-active antifungal therapy were retrospectively identified from the antimicrobial stewardship system (Guidance MS, Melbourne Health) and pharmacy dispensing system. Mold-active therapy was defined as treatment with a polyene, echinocandin or mold-active triazole (i.e. posaconazole or voriconazole). Clinical, microbiological and radiological records were reviewed to capture patients’ demographics, underlying lymphoproliferative disorder by type and stage, chemotherapy type and schedule, antifungal prophylaxis status, type and site of IFD, antifungal treatment received and clinical outcomes. In order to identify all patients undergoing treatment for lymphoproliferative neoplasms during the study period, diagnoses and treatment duration were extracted from the chemotherapy administration system (CHARM). Oral cytotoxic agents such as fludarabine and oral immunomodulatory drugs (lenalidomide, thalidomide) and/or proteasome inhibitors (bortezomib) were identified from the pharmacy dispensing system.
Patients received antifungal prophylaxis in accordance with the Australian national consensus guidelines for antifungal prophylaxis.3 In patients deemed at high risk of IFD without an approved indication, antifungal prophylaxis was used at the discretion of the treating clinician in consultation with the infectious diseases department. In patients suspected of having an IFD because of clinical symptoms or persistent fever, the diagnostic work-up typically included imaging with high-resolution computed tomography (CT) of the chest and sinuses (if symptoms) or fluorine-18 fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT), followed by directed tissue sampling for microscopy and fungal culture. Molecular testing with Aspergillus polymerase chain reaction (PCR) and galactomannan testing on serum and bronchoalveolar lavage (BAL) fluid were routinely performed.4 The optical index cutoff for a positive galactomannan test was 0.5 on serum and 1.0 on BAL.4,5
IFD was defined and classified according to the European Organisation for Research and Treatment of Cancer (EORTC)/Mycoses Study Group (MSG) criteria.6 Lymphoproliferative disorders were classified according to consensus definitions7 into seven categories: precursor lymphoid neoplasms; mature B-cell neoplasms - chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), diffuse large B-cell lymphoma (DLBCL), plasma cell neoplasms, other B-cell non-Hodgkin lymphoma; mature T- and NK-cell neoplasms; and Hodgkin lymphoma. Prevalence of infections was defined as the number of patients with IFD expressed as a proportion of the total number of treated patients for each category during the study period. As a novel means of determining the effect of treatment intensity on IFD risk within each category, the rate of IFD was expressed as number of IFD cases per 10,000 treatment days. Treatment days were calculated from the first to the last day of chemotherapy administered to patients within each category during the study period. Outcomes of IFD treatment were evaluated at 30 days. The study was approved by the PMCC Human Research Ethics Committee.
During the study period, 773 patients fulfilled the inclusion criteria. Overall, 29 episodes of IFD were identified in 29 patients, corresponding to an IFD prevalence of 3.8% [95% confidence interval (CI) 2.5–5.4%]. Patients with IFD had a mean age (range) of 62 years (18–88 years) and a male predominance (65%). IFD were classified as proven in ten cases, probable in eight, and possible in 11. Fluconazole and mold-active antifungal prophylaxis were administered to 287/773 (37.1%) and 38/773 (4.9%) patients, respectively.
Patients with precursor lymphoid neoplasms had the highest prevalence of IFD (5/17; 29.4%, 95% CI 9.5–68.6%), followed by patients with mature B-cell neoplasms-CLL/SLL (4/51; 7.8%, 95% CI 2.1–20.1%), DLBCL (8/186; 4.3%, 95% CI 1.9–8.5%) and plasma cell neoplasms (7/251; 2.8%, 95% CI 1.1–5.7%). IFD events per treatment days demonstrated a similarly larger relative burden of disease in patients with precursor lymphoid neoplasms (10.7 IFD per 10,000 treatment days) (Table 1). Mold-active antifungal prophylaxis was used in 52.9% of patients with precursor lymphoid neoplasms. Of the five patients with precursor lymphoid neoplasms who developed IFD, three had received fluconazole prophylaxis, one had received posaconazole prophylaxis and one received no antifungal prophylaxis. IFD was observed to occur at different treatment stages of disease, and these are summarized in Table 2.
Table 1.
Characteristics of studied patients with lymphoproliferative disorders (2009–2011).
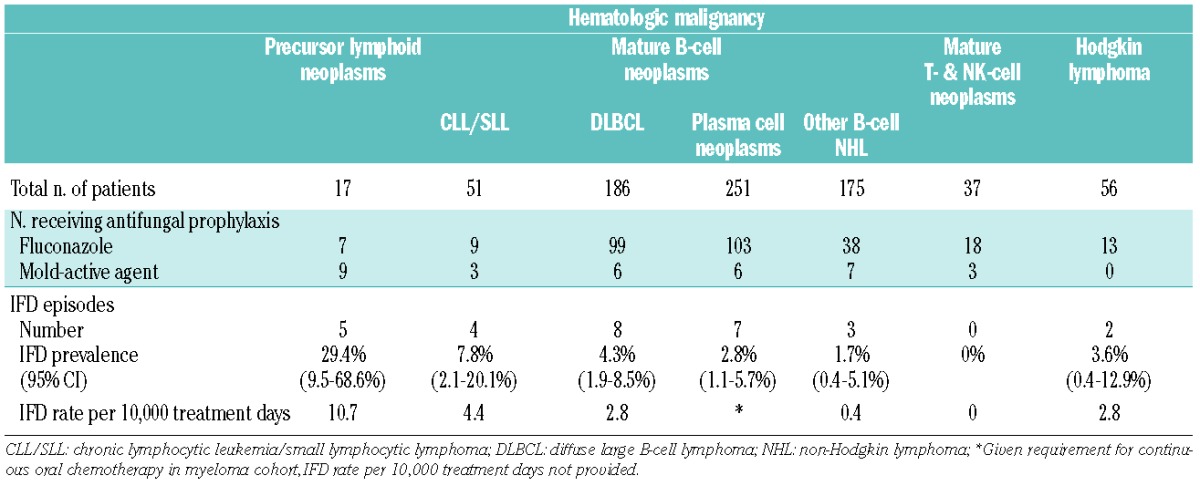
Table 2.
Characteristics of patients with lymphoproliferative disorders with invasive fungal disease (2009–2011).
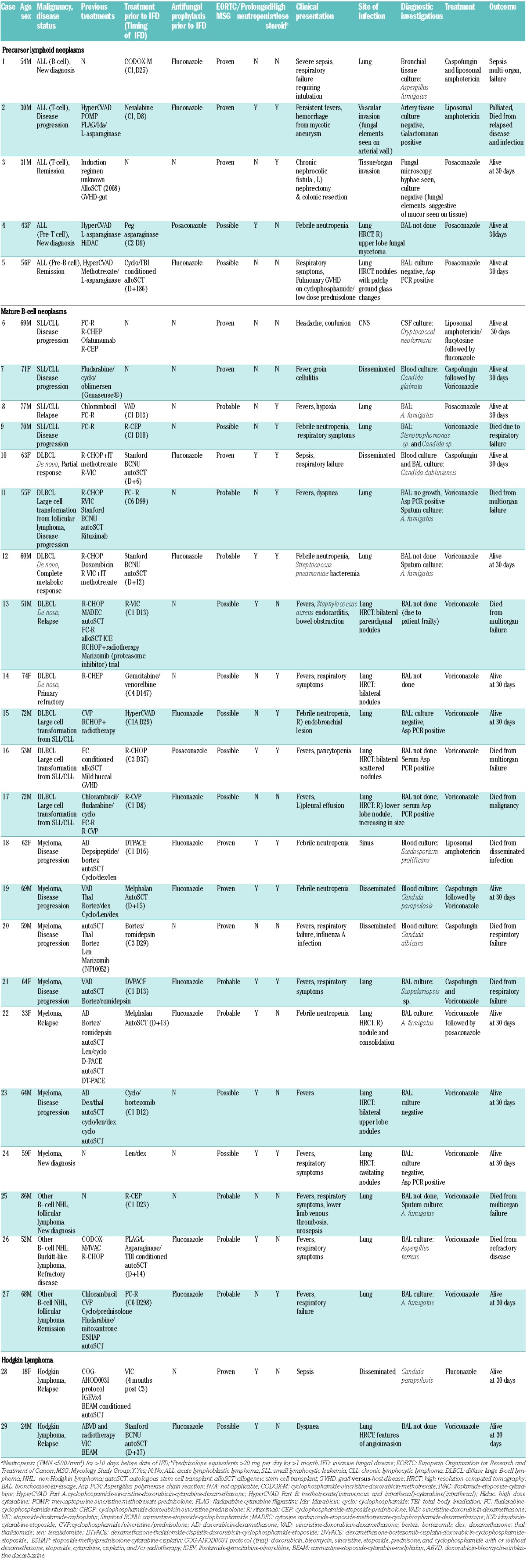
Aspergillus species was the most frequently identified fungal pathogen (13 cases) (cultured in 7 cases; detected on PCR in 8 cases). Other fungal pathogens, in order of reducing frequency included Candida (5 cases), Scedosporium (1 case), Scopulariopsis (1 case) and Cryptococcus (1 case) species. The pulmonary system (19 cases) was the most common site of IFD, followed by blood (5 cases), sinus (2 cases), soft tissue/viscera (2 cases) and central nervous system (1 case). Of the 29 patients treated for IFD, 12 (41.4%) required admission to the intensive care unit and 30-day all-cause mortality was 31.0% (9/29).
IFD is an important and potentially modifiable cause of morbidity and mortality in patients with lymphoproliferative disorders receiving chemotherapy. Few studies have described the epidemiology and treatment outcome of IFD in these patients and none has incorporated all of the current diagnostic strategies available (e.g. Aspergillus PCR, galactomannan and FDG PET/CT diagnostics). Our study identified an overall IFD prevalence of 3.8% with cases occurring in all disease subsets except mature T-and NK-cell lymphoma. The prevalence of IFD was highest in patients with precursor lymphoid neoplasms (29.4%). This occurred despite 52.9% of patients receiving mold-active prophylaxis. This finding is consistent with a 28% incidence reported at another Australian center8 and may be attributed to the increasing intensity of induction chemotherapy protocols for lymphoblastic lymphoma comprising high corticosteroid exposure and prolonged periods of neutropenia. Use of antifungal prophylaxis in this cohort is challenging given the potential for drug interactions with vinca alkaloids.8 Triazole antifungal drugs potentiate vincristine-related neuropathy and although antifungal prophylaxis is sometimes administered intermittently or withheld during vincristine-containing treatment, this approach is complicated by the variable half-lives of these agents.9
The observed higher frequency of IFD in patients with lymphoblastic lymphoma argues for new approaches to the prevention of IFD in this group of patients, including a reappraisal of polyene and echinocandin prophylaxis. An alternative approach to mitigating the clinical consequence of IFD would be routine enhanced surveillance with a combination of Aspergillus PCR and galactomannan testing as has been evaluated in allogeneic stem cell recipients.10 We did not observe a well-defined high-risk period for IFD in our patients - some IFD cases were diagnosed during induction chemotherapy and others during treatment for progressive or relapsed disease - making a targeted surveillance approach more challenging.
IFD occurred at a lower rate in patients with CLL/SLL (7.8%), DLBCL (4.3%) and plasma cell neoplasms (2.8%). Various studies have found invasive mold infection complicating alemtuzumab treatment in patients with CLL/SLL, most likely due to the combination of humoral immunodepletion inherent to the disease and treatment-related immunosuppression.11 In patients with myeloma, IFD has been observed to occur during disease progression and following a median of five lines of prior treatment.12 While there are some reports of IFD rates in the other lymphoproliferative disorders, there are no studies to date quantifying the burden of disease and role of antifungal prophylaxis in these patients. Consistent with findings in other groups of immunocompromised patients, Aspergillus and Candida were the most frequent IFD pathogens in our cohort. Overall, we observed a 30-day all-cause mortality of 31.0% and this is consistent with previous studies.8 There is a possibility that IFD diagnoses are delayed in these patients as they lie outside traditional risk groups due to uncertainty surrounding IFD risk, the paucity of data on IFD epidemiology and absence of standardized antifungal prophylaxis recommendations amid evolving disease treatments.
Study limitations include the retrospective nature of the study, and the fact that it was undertaken in a quaternary referral center. Our IFD prevalence may be an underestimate as cases were defined on the basis of receipt of antifungal agents; however, patients at this center are more likely to be pretreated and therefore at higher risk.
In summary, we observed significant mortality in patients with IFD complicating lymphoproliferative disorders, and identified patients with precursor lymphoid neoplasms as the subgroup at highest risk. The increasing age-standardized incidence of lymphoproliferative disorders in the aging population receiving chemotherapy means that the burden of IFD is anticipated to increase over time. Larger, multicentre, prospective, surveillance studies are, therefore, required to quantify IFD risk and to test strategies for early detection and/or prevention.
Acknowledgments
We would like to acknowledge Dr. D Carney for his assistance in classifying the hematologic malignancies and reviewing the manuscript.
Footnotes
Funding: no external funding was sourced for this study.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Pagano L, Caira M, Candoni A, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica 2010;95(4):644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananda-Rajah MR, Cheng A, Morrissey CO, et al. Attributable hospital cost and antifungal treatment of invasive fungal diseases in high-risk hematology patients: an economic modeling approach. Antimicrob Agents Chemother 2011;55(5):1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slavin MA, Thursky KA, Worth LJ, et al. Introduction to the updated Australian and New Zealand consensus guidelines for the use of antifungal agents in the haematology/oncology setting, 2014. Intern Med J 2014;44(12b):1267–1276. [DOI] [PubMed] [Google Scholar]
- 4.Heng SC, Chen SC, Morrissey CO, et al. Clinical utility of Aspergillus galactomannan and PCR in bronchoalveolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis in patients with haematological malignancies. Diagn Microbiol Infect Dis 2014;79(3): 322–327. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen MH, Leather H, Clancy CJ, et al. Galactomannan testing in bronchoalveolar lavage fluid facilitates the diagnosis of invasive pulmonary aspergillosis in patients with hematologic malignancies and stem cell transplant recipients. Biol Blood Marrow Transplant 2011;17(7):1043–1050. [DOI] [PubMed] [Google Scholar]
- 6.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46(12):1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swerdlow S, Campo E, Harris N, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2008. [Google Scholar]
- 8.Henden A, Morris K, Truloff N, Nakagaki M, Kennedy GA. Incidence and outcomes of invasive fungal disease in adult patients with acute lymphoblastic leukemia treated with hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone: implications for prophylaxis. Leuk Lymphoma 2013;54(6):1329–1331. [DOI] [PubMed] [Google Scholar]
- 9.Moriyama B, Henning SA, Leung J, et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 2012;55(4):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrissey CO, Chen SC, Sorrell TC, et al. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis 2013;13(6):519–528. [DOI] [PubMed] [Google Scholar]
- 11.Thursky KA, Worth LJ, Seymour JF, Miles Prince H, Slavin MA. Spectrum of infection, risk and recommendations for prophylaxis and screening among patients with lymphoproliferative disorders treated with alemtuzumab*. Br J Haematol 2006;132(1):3–12. [DOI] [PubMed] [Google Scholar]
- 12.Teh BW, Teng JC, Urbancic K, et al. Invasive fungal infections in patients with mutiple myeloma:a multi-centre study in the era of novel myeloma therapies. Haematologica 2015;100(1):e28–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]


