Abstract
Systemic AL amyloidosis, a disease with improving outcomes using novel therapies, is increasingly recognized in the elderly but treatment and outcomes have not been systematically studied in this group of patients in whom comorbidities and frailty may compound morbidity and mortality. We report the outcomes of 295 patients with systemic AL amyloidosis ≥75 years seen at the UK National Amyloidosis Centre from 2005–2012. The median age was 78.5 years. The median overall survival was 20 months. Two hundred and thirty-eight patients received chemotherapy and 57 elected for supportive care only (overall survival – 24 and 8.4 months, respectively). On intention-to-treat analysis, 44% achieved a hematologic response including a very good partial response or better in 23%. The median overall survival was 6.2 years in patients achieving very good partial response or better at the 6-month landmark analysis and 1.5 years in non-responders. Factors independently indicating a poor prognosis were: cardiac involvement, performance status ≥2; systolic blood pressure <100 mmHg and, on landmark analysis, achieving less than a very good partial response. Treatment of systemic AL amyloidosis in the elderly is challenging. Deep clonal responses are associated with excellent survival and organ responses. Achieving a response to the first-line regimen appears particularly important as outcomes of non-responders are similar to those of untreated patients. Prospective trials with lower toxicity, outpatient treatment regimens are needed.
Introduction
Systemic AL amyloidosis is a rare disorder of protein mis-folding in which extracellular accumulation of insoluble amyloid fibrils causes progressive impairment of vital organ function. Monoclonal immunoglobulin free light chains, products of an underlying B-cell/plasma cell clonal disorder, are the AL amyloid fibril precursor protein. The historically poor prognosis in systemic AL amyloidosis, with a median survival of just 13 months for patients diagnosed in the early 1990s,1 has lately improved with better understanding of the disease, improved supportive care and more effective treatments for the underlying clonal disorder, including autologous stem cell transplantation and novel therapeutic agents. The median survival in recent times is in the order of 3–4 years.2,3 However, the vital organ dysfunction in patients with AL amyloidosis continues to pose major challenges in terms of tolerability and toxicity of chemotherapy compared to those in patients receiving similar treatment for multiple myeloma. This challenge is amplified in older patients with AL amyloidosis – an increasingly recognized population for which little has been reported about treatment responses and outcomes.
Treatment of older patients with hematologic malignancies is a growing challenge in general with the aging population, a situation not helped by individuals in this age group frequently being excluded from clinical trials. Patients aged more than 70 years with multiple myeloma have significantly poorer survival than their younger counterparts and twice the risk of early death.4 This has been attributed to the presence of comorbidities and poorer tolerance of chemotherapy leading to early discontinuation of treatment and suboptimal responses. Efforts are currently being made to develop guidelines for risk stratification of older patients and use of individually tailored therapies.5 The general challenges in managing elderly patients is magnified in AL amyloidosis by amyloid-related vital organ damage, which further reduces tolerability of chemotherapy and greatly increases the risk of treatment-related toxicity. For these reasons, some older patients with AL amyloidosis may not been considered for therapy at all.
We report here on a large cohort of patients with systemic AL amyloidosis over the age of 75 years. We explored risk stratification models, and studied the impact of treatment on survival and characterized the features of patients who received greatest benefit from treatment in terms of survival and improvement in amyloidotic organ function.
Methods
Patients
All patients with AL amyloidosis of at least 75 years of age who had been evaluated at the UK National Amyloidosis Centre (NAC) between 2005 and 2012 were studied. All patients with AL amyloidosis under the age of 75 years seen during the same study period were also identified to derive the proportion of older patients as well as for overall survival outcomes. The diagnosis of amyloidosis was confirmed in all cases by a tissue biopsy demonstrating characteristic birefringence on Congo red staining. Typing of AL amyloidosis was confirmed by immunohistochemical staining with appropriate antibodies and by exclusion of hereditary amyloidosis, when necessary, by genetic sequencing of the genes implicated in hereditary amyloidosis. All patients underwent systematic review at presentation and detailed follow-up assessments at 6-monthly intervals or as clinically indicated. The assessments included clinical examination, detailed blood and urine analysis [with measurement of serum and urine monoclonal immunoglobulins and serum free light chains (FLC)], serial 123I-labeled serum amyloid P component scintigraphy to assess whole body amyloid load, an electrocardiogram and echocardiogram. Serum FLC levels were measured serially at monthly intervals using the Freelite™ assay (The Binding Site, Birmingham, UK) during treatment with chemotherapy and 1- to 2-monthly thereafter until death. Cardiac disease stage was determined according to the Mayo staging described by Dispenzieri et al.6 Amyloid organ involvement was defined according to the International Consensus Criteria (ICC) 2010.7 Renal disease was staged on the basis of the recently published renal staging criteria.8 Performance status (PS) was measured according to Eastern Cooperative Oncology Group (ECOG) criteria and heart failure symptoms were assessed using the New York Heart Association (NYHA) functional classification. Written consent to publication of anonymous material was obtained from all patients in accordance with the Declaration of Helsinki and this study was granted ethical approval.
Outcome measures
Primary outcome measures studied were hematologic responses to treatment and overall survival. Hematologic responses were defined according to the amyloidosis consensus guidelines.9 FLC values were considered evaluable for assessing response if the pretreatment difference between the involved and uninvolved FLC (dFLC) was >50 mg/L with an abnormal FLC ratio. Hematologic responses were assessed as per the consensus criteria published by Palladini et al.10 The response was assessed as the best achieved response after starting chemotherapy and before any further therapy was given.
Statistics
Statistical analysis was undertaken using the SPSS 21 software package (SPSS, Chicago, IL, USA). Survival was assessed by the method of Kaplan and Meier and compared by the log-rank test. Categorical variables were compared with a chi-square or Fisher exact test as appropriate. All P values are two-sided with a significance level of 0.05. Multivariate analysis was by Cox or binary logistic regression as appropriate. All analyses were on an intent-to-treat (ITT) basis. Two landmark analyses were conducted: at 6 months, to assess the impact of presenting factors on early survival, and at 2 years. Amyloidotic organ responses are often delayed and only patients who survive long enough after treatment would benefit from these responses. The latter time-point was chosen as this was close to the median survival of the whole cohort and was a time by which patients would have had organ responses. In an elderly population, identifying patients who would genuinely benefit from a response to treatment is important.
Results
A total of 295 patients with AL amyloidosis older than 75 of age years were identified, accounting for 16% of all 1,870 AL amyloidosis patients assessed during the study period. The proportion of patients >75 years increased from 13% in 2005–2006, to 14% in 2007–2008, 17% in 2009–2010 and 18.5% in 2011–2012.
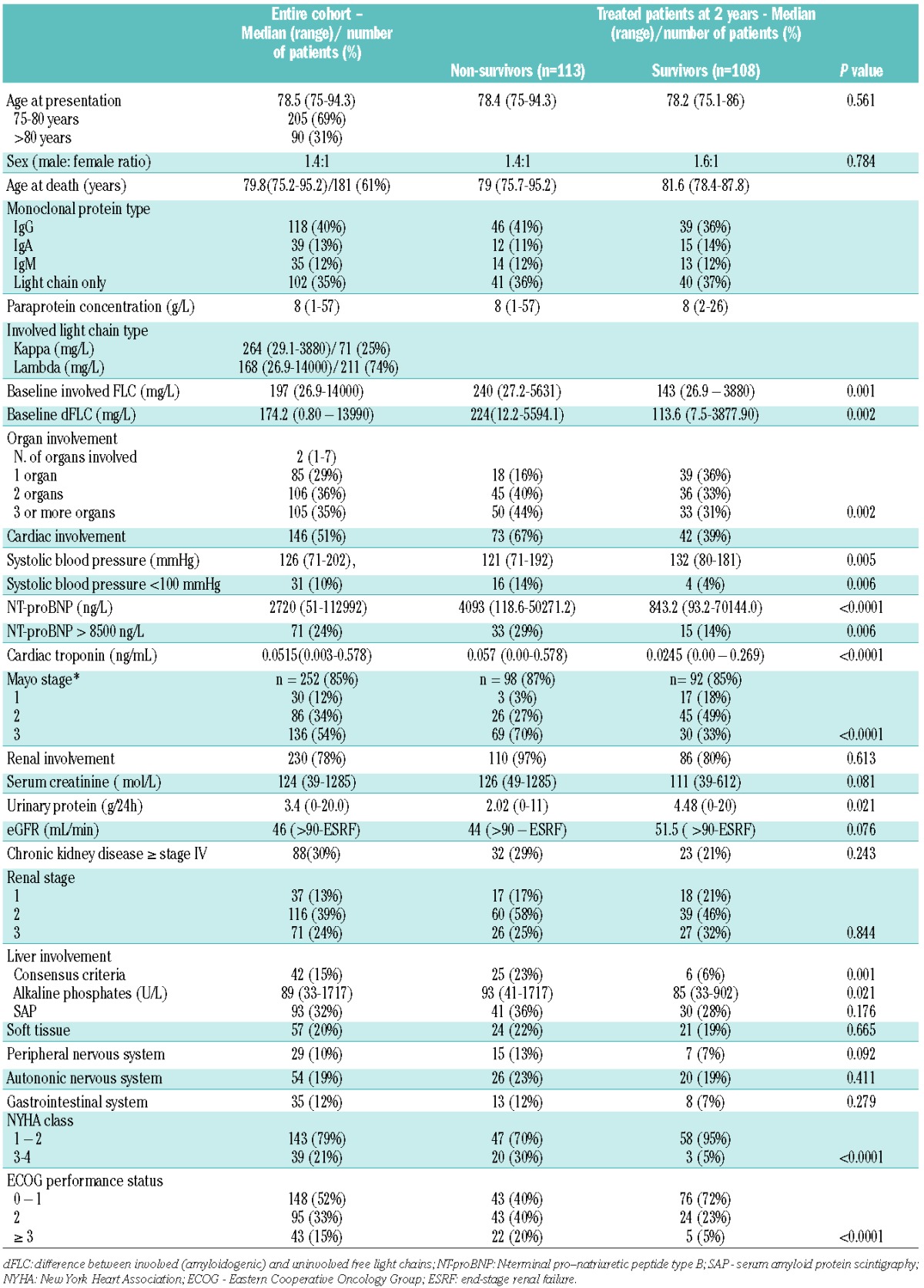
Table 1 details the presenting characteristics of the patients. The median number of organs involved was two (range, 1–7). Renal function was normal in only 9% and severe renal impairment (≥ stage IV chronic kidney disease) was seen in 30% of patients. Of the 252 patients (85%) with full baseline biomarkers [both N-terminal pro-natriuretic peptide type B (NT-proBNP) and cardiac troponin-T] available for staging, 54% had Mayo stage 3 disease at presentation. Among the remaining 43 patients with an incomplete set of biomarkers at baseline, 88% had abnormal NT-proBNP and 52% had cardiac involvement as defined by echocardiographic criteria. Among the entire group, there was echocardiographic evidence of cardiac involvement in 51% of cases. Ten percent of the cohort had a median systolic blood <100 mmHg and 24% of the cohort had an NT-proBNP concentration >8500 ng/L, both cut-offs associated with a particularly poor prognosis.11
Table 1.
Baseline characteristics of the whole patient cohort, and the survivors and non-survivors at the 2-year landmark analysis of treated patients.
Treatment
Two hundred and thirty-eight (81%) patients received chemotherapy, and 57 (19%) patients made an informed decision to proceed with supportive care only. Details of chemotherapy were incomplete for 19 (8.5%) patients and their data were excluded from the analysis of treated patients. Thalidomide-based combinations, mainly dose-attenuated oral cyclophosphamide, thalidomide and dexamethasone, were used in 100 cases (45%), melphalan-dexamethasone in 63 (29%), bortezomib-based regimens in 30 (13%), other alkylator-steroid combinations in 7 (3%), and various regimens for lymphoplasmacytic lymphoma in 22 (10%). Patients received a median of four cycles (range, 1–10). Thirty-two percent received fewer than three cycles of treatment and only 67 (35%) patients completed the planned course of six cycles of treatment.
Hematologic responses to treatment were analyzed on an ITT basis in 284 (96%) patients and a separate ITT analysis was conducted in those patients who actually received therapy. Five patients were excluded from analysis because their clonal markers were insufficiently elevated to enable assessment of response, and six patients did not attend follow-ups. On ITT analysis of the whole cohort, 125 (44%) patients had a hematologic response with 64 (23%) patients achieving a complete response (CR) or very good partial response (VGPR) (11% CR and 12% VGPR) and 61 (21%) achieving a partial response (PR). In the ITT analysis of 227 patients who received therapy, a hematologic response was achieved in 125 (55%) with 28% CR/VGPR and 27% PR. One hundred and ninety-seven of 238 (83%) treated patients were included in the evaluable response analysis, which excluded 30 patients who died before response assessment. Of the evaluable patients, 125 (63%) achieved a hematologic response comprising 32% with CR or VGPR (15% and 17%, respectively) and 31% with a PR. Sixty-nine percent of patients who received a bortezomib-based regimen achieved a hematologic response, 61% achieving a VGPR or better, while 53% and 61% of those on melphalan and thalidomide-based regimens achieved hematologic responses, respectively (Figure 1). Age did not appear to affect CR rates substantially: 21/197 (11%) patients in the 75- to 80-year old group and 9/87(10%) patients in the group over 80 years old achieved a CR.
Figure 1.
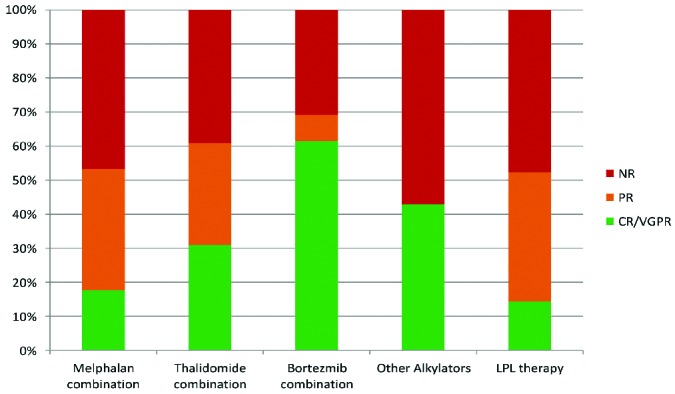
Hematologic response achieved with various chemotherapy combinations on an ITT basis. The response rates for thalidomide-based combinations were: CR/VGPR 30 (31%), PR 29 (30%); for melphalan-dexamethasone: CR/VGPR 11 (18%), PR 22 (35%); for bortezomib-based regimens: CR/VGPR 16 (61%), PR 2 (8%); for other alkylators: CR/VGPR 3(43%), PR 0 (0%); and for regimens for lymphoplasmacytic lymphoma (LPL): CR/VGPR 3 (14%), PR 8 (38%).
After appropriate counselling, 57 patients chose not to receive chemotherapy and had supportive care only. Their median age at diagnosis was 79 years [22 (39%) were over 80 years of age]. Their age at death was 80 years and the median survival without treatment was 8 months. Twenty-five (46%), 37 (65%) and 19 (33%) had cardiac, renal and liver involvement, respectively. Sixty-seven percent had Mayo stage III disease with a median NTproBNP of 6695 ng/L and 42% had NT-proBNP >8500 ng/L. Thirty patients (53%) had an estimated glomerular filtration rate <30 mL/min at presentation and 11 (20%) had systolic blood pressure <100 mmHg. Twelve (32%) had NYHA class 3 or above and 37 (66%) had ECOG PS ≥ 2.
Toxicity data were recorded in detail from 2009 as a part of our prospective observational ALChemy study, and were available for 147 patients of whom 113 (77%) experienced grade 3 or greater toxicity. Fluid retention in 32% of patients was the most commonly reported adverse event followed by infection/sepsis in 17%. Thalidomide-based regimens were associated with greatest toxicity (84%) and bortezomib-regimens the least (70%) (Fisher exact test, P=0.141).
Survival
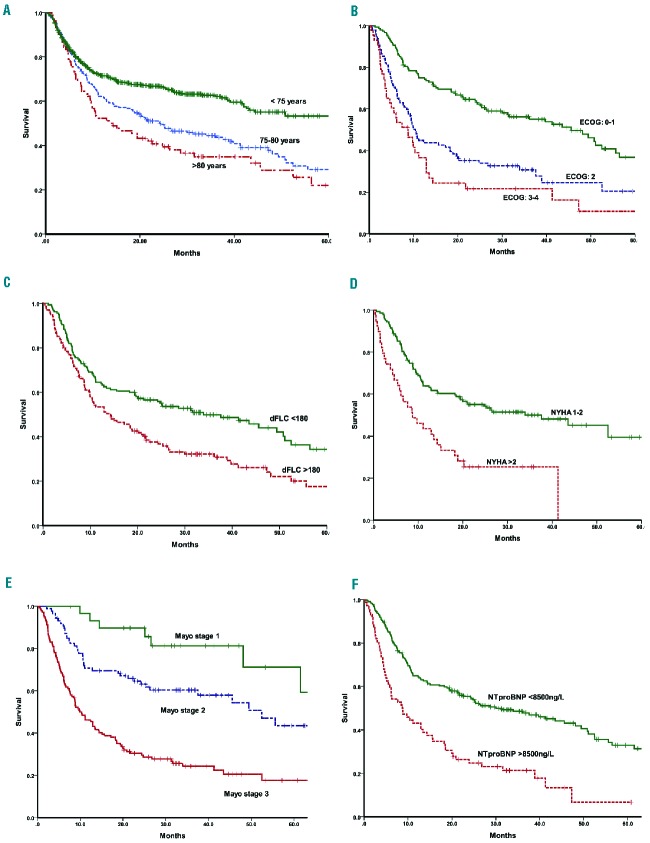
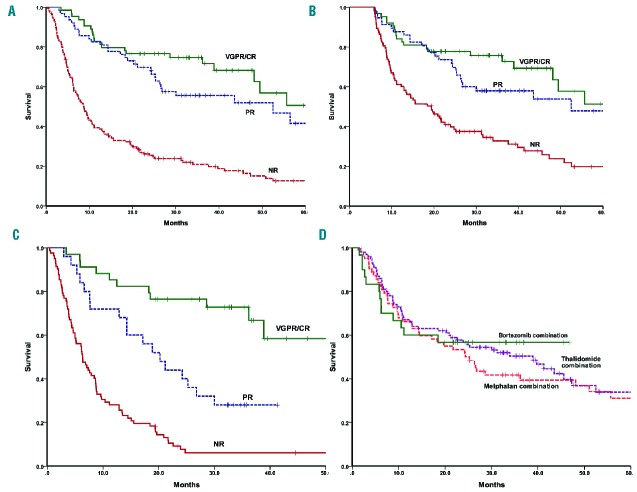
The median overall survival (OS) of the whole cohort was 20.9 months and OS rates at 1, 2 and 5 years were 59%, 47% and 26%, respectively, which are worse than the outcome for AL amyloidosis patients aged <75 years seen at the NAC during the same period (median OS, 6.1 years) (Figure 2). Early deaths at 2, 3 and 6 months were seen in 4%, 9% and 22% of patients, respectively. The OS of patients aged between 75–80 years was 24.2 months and that of patients aged >80 years was 13.5 months (Figure 2). The median OS over 2005–2006, 2007–2008, 2009–2010 and 2011–2012 were 19.6, 18.5, 14.4 and 52.5 months respectively; indicating an improvement in survival since 2011, perhaps due to the novel therapeutic agents (36% of patients were treated with bortezomib-based therapy during the period 2011–2012 compared to only 6% in 2009–2010 and none prior to 2009) and better supportive care. Patients who received treatment had a median OS of 24.7 months compared to 8.4 months for those who opted for supportive care only (P<0.0001). A CR/VGPR was associated with better OS than PR (74.7 months versus 52.5 months, respectively; P=0.037 both on an ITT analysis and of the evaluable patients) (Figure 3). The estimated 5-year survival rates of patients who achieved a CR were 68% and 89% in those aged up to 80 years and over 80 years, respectively. For patients with cardiac involvement, those achieving a VGPR/CR had a median OS of 55.6 months compared to 20.2 months for those achieving a PR (P=0.002) or 6.4 months for non-responders (P<0.0001) (Figure 3). A survival advantage for responders was also demonstrable within the very poor prognostic group with NT-proBNP >8500 ng/L as the responders had a significantly better survival with a median OS 26.8 months compared to only 5 months among the non-responders (P<0.001). The median OS of those who were treated and those who refused chemotherapy was 12.9 months and 4.8 months, respectively (P=0.009) within this subgroup. There was suggestion of better survival for patients treated with bortezomib (median OS not reached) compared to melphalan (median OS 25.2 months) or thalidomide-based regimens (median OS 38.9 months) (Figure 3). However, it is difficult to make direct comparisons between the various regimens as both the reason for choice of chemotherapy and the inevitable variability in the supportive care provided could easily have influenced survival.
Figure 2.
Kaplan-Meir analysis of overall survival (OS) of the whole cohort and risk factors adversely affecting survival. (A) OS of patients with AL amyloidosis by age group at presentation (75–80 years and >80 years compared with age <75 years). The median OS of the two older age cohorts was 24.2 months and 13.5 months, respectively, compared to 73 months in the younger cohort; (B) OS of patients presenting with ECOG PS 0–1 vs. ECOG 2 vs. ECOG 3–4 - median OS 45.6, 10.4 and 8.7 months, respectively (log rank P<0.0001); (C) OS stratified by presenting dFLC <180 mg/L vs. >180mg/L - median OS 33.9 and 14.3 months, respectively (log rank P=0.001); (D) OS by presenting NYHA class 1–2 vs. NYHA >2 - median OS 37.6 and 8.7 months, respectively (log rank P<0.0001); (E) OS by Mayo stage 1, 2 and 3 - median OS 64, 52.5 and 9.9 months, respectively (log rank P<0.0001); (F) OS by presenting NTproBNP >8500ng/L vs. <8500ng/L - median OS 30 and 8.7 months, respectively (log rank P<0.0001).
Figure 3.
Kaplan-Meir analysis of overall survival (OS) based on hematologic response to treatment (A) OS on an ITT basis by hematologic response: the median OS was 74.7 months in those achieving CR/VGPR and 52.5 months in those achieving a PR compared to 8.8 months in non-responders (log rank P<0.0001); (B) OS for patients at the 6-month landmark analysis based on hematologic response: the median OS was 74.7 months in those achieving CR/VGPR and 52.5 months in those achieving a PR compared to 19.4 months for non-responders (log rank P<0.0001); (C) OS by response for patients with cardiac involvement in the ITT cohort: the median OS was 55.6 months in those with CR/VGPR and 20.2 months in those with PR and 6.4 months in the non-responders (log rank P<0.0001); (D) OS by treatment regime: the median OS was not reached in the bortezomib group compared to 25.2 months and 38.9 in the melphalan and thalidomide groups, respectively (log rank P=0.062).
In the ITT cohort, on univariate analysis, factors adversely affecting survival were: cardiac involvement, advanced Mayo disease stage, high NT-proBNP levels, systolic blood pressure <100 mmHg, peripheral neuropathy, liver involvement according to the ICC or on serum amyloid P component scintigraphy, three or more organs involved, dFLC >180 mg/L, higher NYHA dyspnea grade and poor ECOG PS. In particular, patients presenting with NT-proBNP >8500 ng/L (n=72) had a significantly worse outcome than those with NT-proBNP <8500 ng/L (Figure 2 and Table 2). On multivariate analysis, independent factors adversely affecting survival were: achieving less than VGPR, dFLC >180 mg/L, poor ECOG PS and cardiac involvement (separate models run for each: cardiac involvement by either of the criteria: ICC, biomarker/echocardiographic criteria, advanced Mayo disease stage and NT-proBNP >8500 ng/L) (Table 2).
Table 2.
Median survival (Kaplan-Meier analysis) of patients in relation to various baseline characteristics: Multivariate analyses at baseline, and at the 6-month and 2-year landmark analyses.
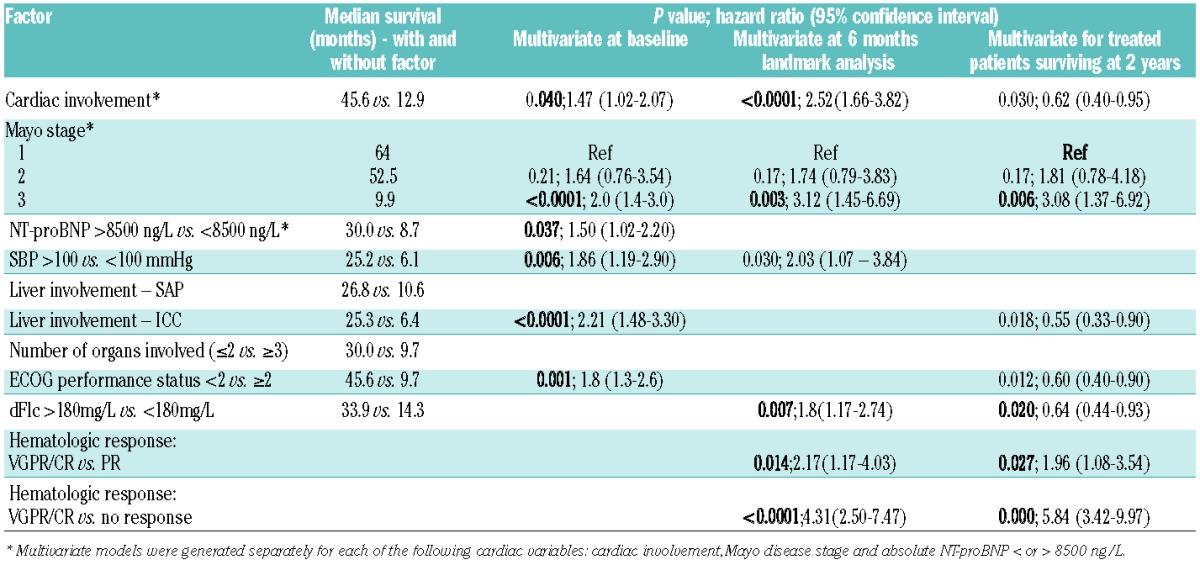
A landmark analysis was performed for patients alive at 6 months (233 of the 295 patients). The median OS of these 233 patients was 38.9 months. The landmark analysis confirmed that those achieving a VGPR/CR had better outcome compared to those with PR or non-responders (median OS of patients achieving a ≥VGPR was 74.7 months compared to 52.5 months for those with lesser degrees of response; log rank P<0.001) (Figure 3). Factors adversely affecting survival were similar to those identified at presentation.
One hundred and eight of the treated patients survived at least 2 years from diagnosis. When compared to patients who died before 2 years (n=113), the surviving group, unsurprisingly, had better prognostic factors. The characteristics of patients surviving >2 years are detailed in Table 1.
Organ responses
According to the ICC and renal response criteria, at 6 months, on an ITT basis of patients who received therapy, 31/193(16%) had a renal response, 14/121 (12%) had a cardiac response and 5/31 (16%) had a liver response. Considering assessable patients (i.e. excluding those who died before response assessment), 31/104 (30%) assessable patients achieved a renal response, 14/55 (25%) had a cardiac response and 5/13 (38%) had a liver response. Assessing impact of depth of hematologic response on organ response, 58% of the renal responders, 71% of the cardiac responders and 38% of the liver responders had attained a VGPR/CR. At 24 months, 25/193 (13%) had a renal response (of whom 50% attained VGPR/CR), 15/121 (12%) had a cardiac response (of whom 73% attained VGPR/CR) and 5/31 (16%) achieved a liver response (of whom 60% attained VGPR/CR).
Discussion
Improved awareness of AL amyloidosis, general longevity and availability of non-invasive investigative modalities suggest that AL will be increasingly recognized in older individuals especially as the prevalence of monoclonal gammopathy of undetermined significance, a usual precursor of AL, rises with age. There are no studies, to the best of our knowledge, focusing specifically on AL amyloidosis among older patients. Hence little is known about its true natural history or its potential to respond (or not) to chemotherapy. Chemotherapy in AL is challenging in all age groups due to multisystem vital organ dysfunction, which reduces its tolerability and increases the likelihood of treatment-related toxicities. These challenges have historically led to many older patients being denied therapy. A UK Department of Health document suggested that clinicians may place too much emphasis on chronological age as a proxy for other factors which are often but not necessarily associated with age, such as comorbidities and frailty.12 The issue of frailty due to comorbidities that are likely to worsen with treatment, and frailty caused by the illness for which treatment is being given, and which may be partly reversible, remains a difficult area to navigate.
The median age at diagnosis of patients with AL amyloidosis is about 60 years2,13 and nearly a fifth of all patients seen at the UK NAC were aged >75 years. The presenting features of patients aged >75 years were, in general, similar to those of younger AL patients. Nearly two-thirds of all patients had Mayo stage III disease at presentation compared to approximately 40% in younger patients14 raising a serious concern that there may be a greater delay in diagnosis of amyloidosis in elderly patients in whom symptoms may well have been attributed to other co-morbidities. Although, in the UK, a substantial majority of patients with amyloidosis are seen at our national referral center, we acknowledge that patients who are very elderly with poor performance status may not be either referred or are too unfit to travel – a possible bias in this study. Cardiac amyloidosis in the elderly is an area of increasing interest not only in relation to AL type, but also because of the availability of treatment options for those with wild-type transthyretin. Early use of non-invasive radionuclide imaging with magnetic resonance imaging, 99mTc-DPD15 or 18Fflorbetapir16 should be considered to avoid diagnostic delay.
The decisions to treat and the type of chemotherapy to use in elderly patients may be influenced by factors including social situations that may not be an issue in younger individuals. One-fifth of patients in our cohort made an informed decision not to receive chemotherapy and chose supportive care only. This was a frail group, with poor ECOG PS and advanced cardiac involvement. Perhaps due to the limitations of cardiac biomarkers in older patients, biomarker-based staging was not always helpful in identifying the patients with poorest prognoses – a fifth of Mayo stage III patients survived for more than 2 years. By contrast, functional markers such as NYHA class and ECOG PS, which reflect patients’ overall physiological state, appeared to have better discriminatory capacity.
The chemotherapy regimens used in the study reflected those in practice in the UK during the study period. Treatment was evidently challenging with only a third of all patients completing the planned six cycles of chemotherapy. There was a suggestion of better tolerance and higher responses with bortezomib-based regimens in this cohort but further studies are needed before any firm conclusions can be reached.
The overall hematologic response rate of only 44% based on this standard ITT analysis of the whole cohort may appear disappointingly low, but when the analysis was performed excluding patients who opted not be treated, 63% achieved a clonal response including one third who achieved a VGPR or better. This response rate compares well to responses reported by our group and others using chemotherapy combinations with AL amyloidosis in general.14,17 Whereas, the median OS of the whole cohort of just over 2 years is shorter than the 3- to 4-year survival of the AL population in general,3 deeper clonal responses translated into an excellent survival advantage (median OS 6.2 years for those achieving a ≥VGPR and estimated 5-year survival for those with CR was 76% in concordance with AL amyloidosis in general).18,19 Considering the UK population in general, the Office for National Statistics in England and Wales has projected that the life expectancies for 75-year old males and females are 11 and 13 years, and those for 80-year old males and females are 8.2 and 9.6 years, respectively.20 With the outcome of patients in CR approaching these figures, our data support treating older patients with high efficacy regimens aimed at achieving deep clonal responses.
Cardiac involvement is the most important determinant of clinical outcome in patients with AL amyloidosis21 in general, and was also associated with poor outcome in this cohort of older patients.21 On multivariate analysis, independent factors adversely affecting survival were NTproBNP >332 ng/L or advanced Mayo stage or cardiac involvement (independently analyzed), liver involvement according to the ICC, systolic blood pressure <100 mmgHg, dFLC >180 mg/L and ECOG PS ≥2. These factors remained significant in the landmark analysis of the 233 (79%) patients surviving 6 months. At both the 6-month and 2-year landmark analyses, an additional factor which independently affected survival was achieving a hematologic response to treatment. In AL amyloidosis, the final aim is for the hematologic response to eventually translate into organ responses, but the latter are often much delayed and organ function may continue to improve for a long period in association with a sustained clonal response. At 2 years, on an ITT basis of patients who received treatment, 13% achieved a renal response, 12% had a cardiac response and 16% had a liver response. A high proportion of organ responders had achieved a ≥VGPR to chemotherapy. This gratifyingly confirms that striving for an excellent hematologic response is crucial since such responses translate into organ responses even in elderly patients.
More knowledge is required to enable refined patient selection, with the dual objectives of avoiding toxicity from unhelpful treatment while enabling treated patients to have the best chance of achieving a deep clonal response; thus, age alone should not be used as a surrogate of fitness for treatment. Critical questions about the choice of initial therapy and the actual schedule of the regimen remain unanswered by this retrospective analysis and toxicity data are limited. Our current, prospective ALchemy study may address some of these issues.
In summary, the presentation of elderly patients with systemic AL amyloidosis is similar to that of the AL population in general. There are a higher proportion of patients with advanced stage disease, perhaps reflecting delay in diagnosis. Outcomes in those achieving a VGPR or better are good and translate into organ responses. Choosing an appropriate, highly effective, first-line treatment appears crucial as patients may not remain fit for salvage therapies. Excluding the very frail patients with advanced organ involvement, this study strongly supports the use of rapidly effective, frontline treatment for older patients with AL amyloidosis, striving for an early deep clonal response with good prospects of long-term survival. Prospective studies in older patients with novel agents with a better toxicity profile and ease of administration, such as oral proteasome inhibitors, may allow a greater proportion of patients to benefit from treatment.
Acknowledgments
We are grateful to our radiographers, Mr David Hutt and Ms Stef Mcknight for undertaking SAP scintigraphy. We acknowledge Ms Janet Gilbertson for histology, our clinical nurses and all the hematologists who took care of the patients.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kyle RA, Gertz MA, Greipp PR, et al. Long-term survival (10 years or more) in 30 patients with primary amyloidosis. Blood 1999;93(3):1062–1066. [PubMed] [Google Scholar]
- 2.Merlini G, Stone MJ. Dangerous small B-cell clones. Blood 2006;108(8):2520–2530. [DOI] [PubMed] [Google Scholar]
- 3.Wechalekar AD, Hawkins PN, Gillmore JD. Perspectives in treatment of AL amyloidosis. Br J Haematol 2008;140(4):365–377. [DOI] [PubMed] [Google Scholar]
- 4.Anagnostopoulos A, Gika D, Symeonidis A, et al. Multiple myeloma in elderly patients: prognostic factors and outcome. Eur J Haematol 2005;75(5):370–375. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood 2011;118(17):4519–4529. [DOI] [PubMed] [Google Scholar]
- 6.Dispenzieri A, Gertz M, Kyle R, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol 2004;22(18):3751–3757. [DOI] [PubMed] [Google Scholar]
- 7.Gertz MA, Merlini G. Definition of organ involvement and response to treatment in AL amyloidosis: an updated consensus opinion. Amyloid 2010;17:48–49. [Google Scholar]
- 8.Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 2014;124(15): 2325–2332. [DOI] [PubMed] [Google Scholar]
- 9.Comenzo RL, Reece D, Palladini G, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain (AL) amyloidosis. Leukemia 2012;26 (11):2317–2325. [DOI] [PubMed] [Google Scholar]
- 10.Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol 2012;30(36):4541–4549. [DOI] [PubMed] [Google Scholar]
- 11.Wechalekar AD, Schonland SO, Kastritis E, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 2013;121(17):3420–3427. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health. The impact of patient age on clinical decision-making in oncology. Department of Health; 2012. [Google Scholar]
- 13.Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJ Med 1998;91(2):141–157. [DOI] [PubMed] [Google Scholar]
- 14.Wechalekar AD, Goodman HJ, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood 2007;109(2):457–464. [DOI] [PubMed] [Google Scholar]
- 15.Hutt DF, Quigley AM, Page J, et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging 2014;15(11):1289–1298. [DOI] [PubMed] [Google Scholar]
- 16.Dorbala S, Vangala D, Semer J, et al. Imaging cardiac amyloidosis: a pilot study using (18)F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging 2014;41(9):1652–1662. [DOI] [PubMed] [Google Scholar]
- 17.Palladini G, Perfetti V, Obici L, et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood 2004;103(8):2936–2938. [DOI] [PubMed] [Google Scholar]
- 18.Sanchorawala V, Seldin DC, Magnani B, Skinner M, Wright DG. Serum free light-chain responses after high-dose intravenous melphalan and autologous stem cell transplantation for AL (primary) amyloidosis. Bone Marrow Transplant 2005;36(7): 597–600. [DOI] [PubMed] [Google Scholar]
- 19.Dispenzieri A, Lacy MQ, Katzmann JA, et al. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2006;107(8)3378–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Office of Statistics. Report: Decennial Life Tables No.16 (2000–2002). www.statistics.gov.uk 2009.
- 21.Merlini G, Palladini G. Amyloidosis: is a cure possible? Ann Oncol. 2008;19 (Suppl 4)iv63–66. [DOI] [PubMed] [Google Scholar]