Abstract
Radiation therapy controls local disease but also prompts the release of tumor-associated antigens and stress-related danger signals that primes T cells to promote tumor regression at unirradiated sites known as the abscopal effect. This may be enhanced by blocking inhibitory immune signals that modulate immune activity through a variety of mechanisms. Indeed, abscopal responses have occurred in patients with lung cancer or melanoma when given anti-CTLA4 antibody and radiation. Other approaches involve expanding and reinfusing T or NK cells or engineered T cells to express receptors that target specific tumor peptides. These approaches may be useful for immunocompromised patients receiving radiation. Preclinical and clinical studies are testing both immune checkpoint–based strategies and adoptive immunotherapies with radiation.
Keywords: abscopal effect, CAR T cells, immune checkpoints, immunotherapy, ipilimumab, lung cancer, melanoma, nivolumab, OX40, radiation
Radiation therapy has traditionally been used primarily as a means of controlling local disease. Advances in radiation therapy technology have allowed the safe delivery of higher doses of radiation with ever-increasing precision, which further enhances local control of primary or even metastatic disease [1,2]. Radiation is also thought to act as a kind of in situ ‘tumor vaccine,’ in that it prompts the release of tumor-associated antigens that prime an adaptive immune system [3,4]. Previous in vivo experiments with mice have shown that irradiating tumors with five fractions of 10 Gy results in greater distant antitumor regression compared with the standard 24 Gy in 12 fractions due to elevated CD8+ T-cell response [5,6]. This idea that radiation can be used to turn a tumor into and in situ vaccine activating the immune system shifts the traditional role of radiation as being local therapy to that of systemic therapy, as antigen-primed T cells can travel to unirradiated sites of disease and promote tumor regression. This is the fundamental concept underlying the abscopal effect. Unfortunately, abscopal effects are rare, as some kinds of tumors have an escape mechanism that involves activating immunosuppressing signals that can dampen lymphocytic activity [4]. The first so-called ‘immune checkpoint’ found to have this effect was CTLA4, discovered by James Allison [7]. Allison and colleagues observed in preclinical experiments that blockade of CTLA4 promoted tumor regression. Shortly thereafter, a humanized anti-CTLA4 antibody, ipilimumab, was developed and shown to enhance T-cell responses that led to dramatic improvements in patients with melanoma [8,9].
After the discovery of CTLA4, several other immunomodulating signals were found, including PDL1, Tim-3, 4-1BB (CD137), OX40 (CD134), IDO (indoleamine-2,3-dioxygenase-1) and killer-cell immunoglobulin-like receptors (KIRs). These checkpoints target T cells through a variety of mechanisms; some signals suppress the immune system (Tim-3, IDO, PDL1, CTLA4), whereas others activate it (OX40, 4-1BB) [7,10,11]. These checkpoints also present new avenues of exploration for use with radiation. Abscopal responses have been reported by physicians treating patients with non-small-cell lung cancer (NSCLC) or melanoma with ipilimumab combined with radiation [12,13]. Moreover, not all checkpoints interact solely with T cells. For example, KIRs, which can have either activating or inhibitory activity, signal natural killer (NK) cells to destroy foreign or stressed cells [14]. Aside from stimulating endogenous T cells, another approach to improving antitumor immunity has been to administer autologous T cells or to engineer chimeric antigen receptor (CAR) T cells such that those cells target a specific tumor peptide. The adoptive immunotherapy approach may be particularly favorable for patients whose immune systems are suppressed, exhausted or both, because T cells or NK cells can be grown and expanded in the laboratory and then infused back into the patient who provided them. With these ideas in mind, preclinical and clinical studies are ongoing to test both immune checkpoint–based strategies and infused T-cell therapies in combination with radiation. Here, we review the immunotherapy approaches that we believe to have the greatest potential to enhance the efficacy of radiation over the next several years.
Immune checkpoints
PD1/PDL1
Expressed on CD8+ and CD4+ T cells, PD1 binds to either PDL1 or PDL2 (also known as B7H1 and B7H2) on either APCs or tumor cells to suppress T-cell activity (Figure 1A) [7]. Humanized antibodies that block PD1 (pembrolizumab, nivolumab) and PDL1 (MPDL3280A) have been created by various pharmaceutical companies and are currently being tested in clinical trials. In one Phase I trial, Topalian et al. tested nivolumab as monotherapy for a variety of solid tumors, including melanoma, renal cell carcinoma (RCC) and NSCLC and found objective response rates of 28% for melanoma, 27% for RCC and 18% for NSCLC. Tumors that did not express PDL1 showed no objective response [15]. In another Phase I trial, Robert and colleagues tested pembrolizumab, without radiation, for patients with ipilimumab-refractory advanced melanoma. Overall response rates were 27% for patients given 2 mg/kg doses and 32% for patients given 10 mg/kg, with similar proportions of patients showing reductions in tumor size relative to baseline (68% low dose and 73% high dose) [16]. Powles et al. investigated the use of the anti-PDL1 antibody MPDL3280A for metastatic urothelial bladder cancer in a Phase I trial. Overall response rates depended on the amount of PDL1 expressed by the tumor, as analyzed by immunohistochemical staining. Patients with high-PDL1-expressing tumors had a response rate of 43 versus 11% for patients with low-PDL1-expressing tumors [17]. Trials such as these have shown the potential of PD1/PDL1 inhibitors as a new therapeutic approach for advanced or chemoresistant tumors. Early results from the Phase III CheckMate-017 trial [18] recently led to the approval of nivolumab by the US FDA for advanced squamous NSCLC, regardless of tumor PDL1 expression.
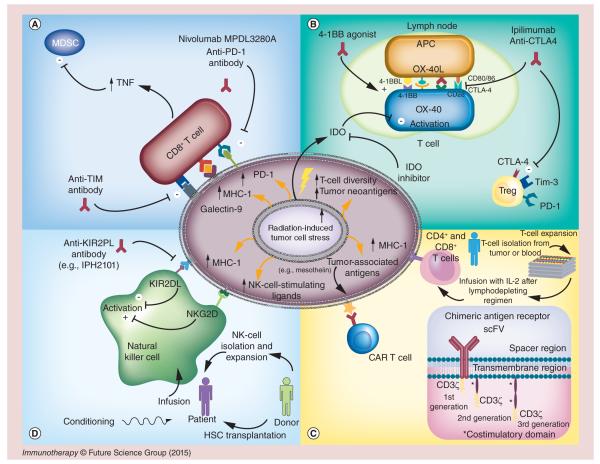
Figure 1. Radiation and immunotherapy effects on the tumor microenvironment.
(A, top left) T-cell and tumor interaction. PDL1 and galectin-9 expressed on the tumor-cell surface bind to PD1 and Tim-3 on CD8+ T cells to blunt the immunologic response of those T cells. Tim-3 also stimulates T-regulatory (Treg) cells. PDL1 expression can be increased after irradiation. Blocking the PDL1 axis reinvigorates CD8+ T-cell activation. Concurrent use of PDL1 blockade with radiation results in increased TNF-α, which contributes to killing of myeloid-derived suppressor cells (MDSCs), further improving T-cell function. Antibodies blocking PD1 (nivolumab and pembrolizumab) and Tim-3 reduce the population of dysfunctional CD8+ T cells, resulting in improved tumor control. (B, top right) T-cell activation at a lymph node. Antigen-presenting cells (APCs) travel to the lymph node from the tumor and activate T cells by presenting tumor antigens to the T cells. CTLA4 or CD28 can bind to CD80 (or CD86) on the T cell, serving as a regulator to balance T-cell activity. Ipilimumab and tremelimumab work at the lymph node to block CTLA4 binding with CD80 (CD86), which causes Treg cell death and CD8+ T-cell expansion. Radiation promotes T-cell receptor diversity when added to anti-CTLA4 therapy. Meanwhile, OX40 and 4-1BB agonists both contribute to T-cell stimulation in the lymph node, and IDO, secreted by tumor cells, depletes T cells of tryptophan to metabolically induce T-cell death. (C, bottom right) T-cell therapy at the tumor site. (1) Tumor-associated antigens, upregulated after irradiation, are recognized by the T-cell receptor of infused T cells. Adoptive T-cell therapy is initiated by extracting T cells from blood or tumor samples and then isolating and expanding them in tissue culture with IL-2. When enough cells have accumulated, they are tested for viability, tumor specificity and contamination, and the patient is given cyclophosphamide with other lymphodepleting agents followed by infusion of the prepared T cells and IL-2. (2) Chimeric antigen receptor (CAR) T-cell therapy involves isolating T cells from peripheral blood and genetically modifying those cells with the CAR by using a viral vector or electroporation. The CAR domain consists of the scFV that recognizes the antigen, a spacer region, a transmembrane domain and a signaling domain. First-generation CARs contain only CD3ζ domain, whereas second- and third-generation CARs include additional costimulatory domains such as CD28, OX40, ICOS, 4-1BB and others to promote T-cell effector functions. (D, bottom left) NK cell and tumor interaction. Radiation increases expression of MHC-1 and stress-induced NK cell-stimulating ligands by tumor cells. The activating ligands bind to NKG2D on the NK cells to promote NK cell-mediated lysis of tumor cells; the MHC-1 on tumor cells binds KIR2DL on the NK cells to decrease NK-cell activity. Anti-inhibitory KIR antibodies (e.g., IPH2101) prevent MHC-1 signaling from silencing NK cells. Alternatively, NK cells can be delivered via donor hematopoietic stem cell transplantation or by isolation and ex vivo expansion.
Preclinical experiments with antibodies to PD1 or PDL1 have provided insight into the role of PD1 during radiation therapy. Deng and colleagues noted an increase in PDL1 expression after irradiation of tumors in mice. The addition of anti-PDL1 IgG antibody led to rapid decreases in tumor volume compared with radiation alone or PDL1 blockade alone. Repeating the same combined immunoradiation in CD8+-depleted mice led to increases in tumor volume. This group further found that PDL1 blockade, given with radiation, promoted the secretion of TNF-α by CD8+ T cells, which suppressed the numbers of tumor-infiltrating myeloid-derived suppressor cells (MDSCs), which are known to be important in tumor immunosuppresion [19]. Another group noted that dual anti-PD1 antibody therapy with stereotactic radiosurgery (SRS) (10 Gy) in mice with implanted gliomas produced significant differences in overall survival; further, biopsy of the gliomas after this combination treatment showed higher numbers of tumor-infiltrating lymphocytes and lower numbers of T-regulatory cells (Tregs) [20]. Herbst et al. analyzed PDL1 expression in specimens of various types of tumors before and after treatment with the anti-PDL1 agent MPDL3280A. They found that levels of pretreatment tumor PDL1 did not correlate with radiographic response; rather, PDL1 expression by tumor-infiltrating immune cells increased during treatment [21]. Approximately 20 clinical trials testing radiation and PD1/PDL1 inhibitors are underway, but preliminary results are still pending. We have initiated a Phase I/II trial investigating concurrent pembrolizumab (MK-3475) and radiation for patients with NSCLC (identifier: NCT02444741 [22]) or small cell lung carcinoma (identifier: NCT02402920 [22]).
CTLA4
Produced by CD8+ T cells, CTLA4 was the first molecule found to inhibit T-cell stimulation. CTLA4 competes with CD28, a T-cell costimulatory signal, to bind either CD80 or CD86 on antigen-presenting cells (APCs) to prevent deleterious hyper T-cell activation (Figure 1B). Once bound to its ligand, CTLA4 signaling suppresses CD4+ helper T-cell activity while promoting immune-suppressing Treg function [7]. Shortly after this discovery, Allison et al. proved that using antibodies to block CTLA4 in vivo promoted tumor rejection [23]. These findings led to the development of humanized antibodies against CTLA4 (ipilimumab, tremelimumab), the first checkpoint inhibitors to be approved by the FDA. In a Phase III trial, Hodi and colleagues found that patients with advanced-stage melanoma receiving ipilimumab had longer overall survival (10.1 months) than did those treated with gp-100 vaccine (6.4 months) (p = 0.003) [8]. Other trials have shown similar results.
One of the first preclinical experiments to combine radiation and CTLA4 blockade was done by Demaria et al. That group, investigating use of this therapy in mice implanted with the poorly immunogenic 4T1 breast cancer cell line, found that combining a single 12-Gy fraction with anti-CTLA4 immunoglobulin G (IgG) not only produced significantly higher rates of overall survival and local control but also reduced the number of pulmonary metastases compared with radiation alone or anti-CTLA4 IgG alone [24]. In another series of experiments involving mice implanted with Lewis lung carcinoma cells, Yoshimoto and colleagues found that radiation (30 Gy) combined with CTLA4 blockade extended both tumor growth delay and median survival time (56 days) compared with radiation alone (46 days) or anti-CTLA4 IgG alone (<40 days). They also noted that administering anti-CD8 antibody with radiation further extended median survival time and tumor growth inhibition [25]. In other studies using tumor-infiltrating lymphocytes after anti-CTLA4 plus radiation in a melanoma mouse model, Victor and colleagues noted that anti-CTLA4 antibody decreased the number of Tregs, whereas radiation increased T-cell receptor (TCR) diversity. However, that increased diversity depended on concurrent anti-CTLA4 therapy, as it did not occur with radiation alone. That group further found that lower ratios of CD8+ T cells to Tregs (CD8/Treg) correlated with treatment resistance [26]. These results and those of similar preclinical experiments emphasizing the role of CD8+ T cells in promoting an abscopal effect during immunoradiation have led to the development of several clinical trials.
In one such report, a patient with previously treated stage III melanoma on the scalp was treated with radiation (8 Gy × 3) and experienced complete resolution, followed by a new brain metastasis and cervical lymph node recurrence 3 years later. That patient was then given ipilimumab with SRS, followed by surgical resection of the lymph node. Serum samples collected before and after SRS showed elevated titers of antimelanoma-associated antigen-3 (MAGEA3) antibody. That patient remained alive for 7 years after the initial radiation therapy [27]. Another patient with lung adenocarcinoma who developed progressive metastases in liver, hilar/mediastinal lymph nodes and bone during chemotherapy was given ipilimumab with radiation (6 Gy × 5) to a hepatic nodule. That patient’s blood cell counts showed increases in absolute lymphocyte counts (ALCs) during treatment, and 1 year later, imaging by positron emission tomography showed complete resolution of all sites of disease [12]. Another group led by Slovin and colleagues recently completed a Phase I/II clinical trial of ipilimumab with radiation for metastatic, castration-resistant prostate cancer. In that study, up to three bone metastases were treated with single 8-Gy radiation fractions with ipilimumab (at 3 or 10 mg/kg) given every 3 weeks for a total of four doses. Of 50 patients who received high-dose ipilimumab, one patient achieved complete response, and an estimated 15% experienced declines in prostate-specific antigen levels [28]. These studies demonstrate the ability of ipilimumab to promote systemic tumor immunity when given with radiation. With unprecedented results such as these in relatively chemoresistant tumors, multiPhase trials investigating ipilimumab with radiation for other types of solid tumors are underway. At our institution, we are conducting a Phase I/II trial in which patients with liver or lung metastasis are treated with concurrent ipilimumab and radiation (identifier: NCT02239900 [22]).
OX40
OX40 (CD134) is a costimulatory, or agonistic, molecule found on CD4+, CD8+, NK, NK T and Treg cells. OX40 was discovered in the late 1980s [29], and is a member of the TNF receptor superfamily, which includes other agonistic receptors such as CD27, 4-1BB, GITR and CD40 [11]. OX40 has many beneficial effects on T cells, including increasing the proliferation of CD4+ and CD8+ T cells and increasing the memory status of those T cells [30–34]. One possible undesirable effect of OX40 binding could be expansion of Tregs, because Tregs also constitutively express OX40 on their surface. Studies on this subject have produced conflicting results [35–42], but most studies to date have shown that Treg function is impaired after treatment with an OX40 agonist.
Preclinical studies of OX40 in combination with other therapies have yielded promising results. One such study combining OX40 with radiation in a lung cancer model reported in 2007 by Yokouchi et al. [43] showed that this combination resulted in an overall survival rate of 80% at 100 days, as opposed to 0% in mice treated with either modality alone. A similar study combining OX40 therapy with surgery and radiation [44] showed that OX40 given with radiation led to a survival rate of >50% at 70 days. These studies confirm the role of OX40 as a potent immune stimulator and suggest that this combination could translate well into clinical settings.
Clinical trials are ongoing in which OX40 agonists are used either as monotherapy or in various combinations with surgery, radiotherapy, tremelimumab, rituximab and cyclophosphamide. Because radiation enhances T-cell infiltration while increasing Treg levels, OX40 is expected to work well in combination because of its potential ability to suppress Treg function while enhancing T-cell proliferation and memory. One preclinical study in a murine model has shown increases in OX40 expression on CD4+ and CD8+ cells after radiation treatment [43]. One published Phase I clinical trial in patients with advanced melanoma showed that OX40 agonists led to dose-dependent expansion of CD4+ and CD8+ cells and tumor control in 12 of 30 patients, although no partial responses were observed according to the Response Evaluation Criteria in Solid Tumors system [45]. An abstract from a different clinical trial involving OX40 agonist in combination with radiation and cyclophosphamide for chemoresistant-prostate cancer also reported increases in CD4+, CD8+ and NK cells, but no increases in Tregs [46]. Although these findings suggest that OX40 agonists can stimulate T cells, clinical trials of such agents with radiation are needed.
Engineering T cells
The preclinical experiments described above underscore the vital role of lymphocytes in regression of both local and metastatic tumors [19–20,24]. We recently undertook a retrospective analysis of ALCs among more than 700 patients who received definitive chemoradiation for NSCLC at our institution. We found that significant drops in numbers of lymphocytes during the first 10 days of chemoradiation, with the nadir being at around 6 weeks, correlated with both gross tumor volume and the percentage of normal lung tissue receiving 5 Gy. We further found that lower lymphocyte plateaus during treatment were associated with worse overall survival and worse event-free survival, demonstrating the integral role of radiation and the immune response [47]. Thus, although enhancing patients’ immune systems through the use of checkpoint inhibitors and agonists is one approach to improving antitumor immunity, another may be to directly infuse the immune cells that are required.
One method of cancer treatment soon to be available in the clinic is adoptive immune-cell therapy. This method involves harvesting the patient’s own immune cells, engineering and expanding them ex vivo, and then reinfusing them back into the patient from whom they were obtained. This approach ensures patient specificity for each treatment. Three key strategies of adoptive immune-cell therapy currently being tested are adoptive T-cell therapy, CAR T-cell therapy and NK cell therapy.
Adoptive T-cell therapy
Adoptive T-cell therapy involves the expansion of immune cells that have been isolated from blood or tumor tissue. This method, first introduced by Steven Rosenberg at the US National Cancer Institute, has resulted in clinical response rates of 50% among patients with melanoma for whom T-cell isolation and expansion worked after lymphodepletion [48,49]. The successful evocation of clinical responses has also been supported by other studies of melanoma. In one clinical trial by Besser et al., 20 patients were treated with adoptive T-cell therapy after chemotherapy [50]. The clinical response rate in that study was also 50%, with two patients experiencing complete response and eight experiencing partial response. This method is effective because it uses large volumes of T cells that may already have been activated to target tumor cells. IL-2 is also given after T-cell re-infusion to help expand the cells in vivo.
Studies of adoptive T-cell therapy with radiotherapy have shown auspicious results. In one preclinical model, radiotherapy plus adoptive T-cell therapy led to eradication of metastatic breast cancer in mice after 60 Gy of radiation, cyclophosphamide and T-cell infusion [51]. Moreover, clinical responses were durable in four of the seven mice in that treatment group. Rosenberg also reported synergistic effects for patients with metastatic melanoma given total body irradiation and adoptive T-cell therapy [52]. In that study of 93 patients, 40% of those given a high dose of 12 Gy as total body irradiation had a durable complete response as opposed to only 12% for patients not given total body irradiation. Among the 20 patients with complete responses, the 5-year survival rate was 93%. Thus adoptive T-cell therapy, in combination with radiation therapy, was more effective than either modality alone, underscoring the beneficial interaction between radiation and other treatment modalities.
CAR–T-cell therapy
Use of CARs, an approach to adoptive immune-cell therapy, was conceptualized by Zelig Eshhar in 1989 [53]. It recently gained significant attention after its successful use to treat acute and chronic leukemias, for example, as revealed by Carl June’s laboratory at the University of Pennsylvania [54]. Normally, TCRs must bind to cognate antigens presented in the context of major histocompatibility complex (MHC) for specific T cells to become activated. T cells engineered to express CARs have an ability to directly target a particular antigen without requiring this MHC–TCR interaction, thereby granting CAR–T cells the ability to kill tumor cells more efficiently after antigen encounter [54]. The prototypical CAR has four components, each of which is required to trigger activation of and serial killing by the T-cell (Figure 1C). These are an antigen-specific domain, typically derived from the single chain variable fragment (scFv) of a monoclonal antibody; spacer region; a transmembrane domain; and a signaling domain [55].
The scFv imparts recognition of target antigens and subsequently binds the T-cell to those antigens. Target-specific destruction also depends on the spacer region, with results in in vitro studies varying depending on the extracellular domain [56,57]. Efficient tumor lysis in vivo, however, depends on the spacer being modified to a certain length to meet the needs of a particular antigen and to avoid deleterious uptake by the Fc receptor on cells such as macrophages [58,59]. Currently, several signaling domains are in clinical use and there is ongoing debate about the merits of the components of the intracellular domain that can sustain T-cell effector function for a particular antigen. The original first-generation CARs deployed in clinical trials contained a CD3-zeta chain, as derived from the TCR signaling complex, whereas the more advanced second- or third-generation CARs contain additional costimulatory domains such as CD28, OX40 or 4-1BB [60]. These additional domains impart improved effector function such as enhanced T-cell activation for cytokine production [61]. Furthermore, other cytokines such as IL-12 can aid CAR–T cells via the recruitment of innate immune cells to attack tumor cells [62,63].
Several clinical trials using CAR–T cells targeted to various tumor-associated antigens are currently underway (i.e., identifiers: NCT02331693, NCT02349724, NCT01583686 [22]). Although this approach has been most successful to date against hematologic malignancies, there is urgency to harness this technology for solid tumors such as by targeting GD2 (osteosarcoma), EGFR (glioma), c-Met (breast cancer, lung cancer) and FAP (mesothelioma). Perhaps one of the greatest obstacles for the successful implementation of CAR–T cells for solid tumors is the inhibitory influence of the stroma, which repels and blunts T-cell functions. Combining radiation with CAR–T-cell infusion remains to be explored and could yield promising results because radiation could favorably modulate the tumor microenvironment enabling infiltration and activation of infused T cells within solid tumors.
Preclinical studies provide justification for combining radiation with CAR–T-cell infusion for cancer therapy. In one such study, irradiation of the A431-K5 carcinoma cell line led to increased expression of extracellular mesothelin, a tumor-associated antigen [64]. Another experiment with breast cancer cell lines revealed that levels of another tumor-associated antigen, HER2, increased by a factor of three in one cell line after 5 Gy irradiation, with less dramatic effects in two other cell lines in vivo [65]. c-MET is also upregulated after irradiation in lung cancer, with two NSCLC cell lines showing significant differences in c-MET expression, assessed by western blotting, 30 min after irradiation [66]. Questions remain to be addressed in in vivo experiments regarding the timing of radiation (before or after CAR–T-cell therapy): should radiation be given first, to kill tumor cells that are sensitive while allowing the CAR–T cells to penetrate the tumor and target-resistant T cells (which typically overexpress certain tumor-associated antigens)? Or would it be more beneficial to use CAR–T cells to kill tumor cells that might be radioresistant and then use radiation afterward to ‘clean up’ the tumor site? These questions will need to be answered before CAR–T-cell therapy and radiation are used in clinical settings.
NK-cell therapy
Although CD8+ T cells have a vital role in antitumor immunity, other immune cells are also important, among them NK cells. NK cells are large granular lymphocytes of the innate immune system that participate in the immediate cytotoxic destruction of malignant or virus-infected cells. NK cells express several activating and inhibitory receptors that determine their activity when encountering other cells. In the human body, healthy cells express MHC class I molecules, which bind to inhibitory KIRs such as KIR2DL1 (CD158a) expressed on NK cells, thereby inactivating the NKs and preventing them from destroying normal tissues (Figure 1D). When unopposed by inhibitory signals, stimulatory KIRs (e.g., KIR2DS1 [CD158h]) and other activating receptors (e.g., NKG2D, CD16) will activate NK cells to eliminate the target T cell via Fas ligand-induced apoptosis, exocytotic granule release or inflammatory signaling (IFN-γ). In an attempt to avoid recognition by T-cell receptors and subsequent destruction by T cells, tumor cells typically downregulate MHC-I. This tumor cell adaptation makes NK cell therapy appealing, as it then becomes susceptible to NK cell-mediated death [67,68]. Tumor cells, stressed from either chemotherapy or radiation, tend to increase levels of NK cell stimulatory ligands and surface MHC-I production, helping to facilitate both innate and adaptive immunity [67].
Analysis of resected tumor samples from patients with a variety of types of cancer has confirmed that NK cells participate in tumor regression. Sconocchia and colleagues analyzed more than 1000 colorectal tumors and found that clinical outcomes correlated with levels of infiltrating CD8+ T cells and NK cells. From this, they proposed that cross-talk between both types of cells is required [69]. Another group used the PCI-13 head and neck cancer cell line to test antitumor activity in peripheral blood samples from 51 patients with head and neck cancer and from healthy individuals. They found significantly greater antitumor activity in the blood of healthy individuals. However, once the dendritic and NK cells were removed from the peripheral blood of the healthy group, antitumor activity was significantly diminished. They also noted that surgical removal of the primary head and neck tumor in the cancer group also enhanced antitumor activity in the peripheral blood samples [70]. An analogous study of peripheral blood samples from patients with breast cancer showed similar results [71]. This indicates that NK cells are not only involved in the direct lysis of tumor cells but they also communicate with other cytotoxic cells to enhance tumor cell death.
Clinical trials have been undertaken to test NK cell infusion for patients with solid tumors. Two commonly used methods for administering donor NK cells are haploidentical (MHC matched) hematopoietic stem cell transplantation (SCT) and direct NK cell infusion. In the haploidentical transplantation, patients are given a conditioning regimen followed by donated hematopoietic stem cells sharing only half of the human leukocyte antigen subtype, along with immunosuppression to prevent graft-versus-host disease. If the engrafted NK cells do not recognize the patient’s MHC-I on the tumor, the inhibitory signal is lost leading to their activation. Adoptive immunotherapy with NK cells may achieve the same effect without SCT by isolating donor (or patient) NK cells, expanding them in vitro, and then infusing them (back) into the patient. In one early study, Iliopoulou et al. gave in vitro expanded NK cells to 16 patients who were receiving first-line or second-line chemotherapy for locally advanced lung cancer. Two patients had a partial clinical response, six had disease stabilization and seven experienced disease progression. The mean progression-free survival time in that study was 5.5 months (range 1–22 months), and the median overall survival time was 15 months (range 2–26 months) [72]. Other trials treating patients with melanoma, RCC, or other types of cancer have produced mixed clinical outcomes [73,74]. These results have prompted exploration of combining radiation therapy with NK cells, which could promote tumor cell stress and NK-cell stimulation [68]. The effects of radiation on NK cells are not well understood. In one study, Heo and colleagues studied the effects of irradiation on matrix metalloproteinases, which reduce the expression of NKGD2L on tumor cell surfaces and thus diminish NK cell activity, in the NCI-H23 NSCLC cell line. They found that irradiation increased the concentration of two matrix metalloproteinases, and this increase was reversed by using matrix metalloproteinase inhibitors [75]. Lim et al. measured NK-cell counts before and after chemoradiation in a small number of patients with locally advanced (T3–4) rectal cancer and found no statistical differences [76]. These results neither favor nor dissuade the use of radiation with NK cells, but further preclinical experimentation and clinical trials with NK cells and radiation are needed to better understand the interactions between these two therapeutic modalities.
Conclusion & future perspective
Cancers often develop resistance to chemotherapy and targeted antibodies by undergoing somatic mutations that allow the tumor cells to avoid being recognized by the immune system. Immunotherapy capitalizes on this, as the immune system has the potential to recognize all foreign antigens. Recent genetic computational analysis revealed that NSCLC and melanoma have high rates of somatic mutations [77], which could explain why ipilimumab and radiation have produced abscopal effects in these diseases [12,27,78]. Cancers such as these have been classified as ‘immunogenic’ based on their response to immunotherapy. Synder et al. recently analyzed samples from 64 patients with melanoma treated with CTLA4-blocking agents and found that higher numbers of somatic mutations (the ‘mutational load’) in the tumors correlated with greater clinical benefit in terms of overall survival. Even more interesting is that certain neoepitopes of tumor antigens that are associated with better outcomes share sequence homology with bacterial and viral antigens [79]. These results suggest a novel area of investigation to determine whether radiation, which can promote antitumor immunity, strengthens the correlation between mutational load and clinical outcomes. An algorithm is also needed to predict which patients would respond well to immunotherapy and radiation; such algorithms may well include factors such as mutational load. Biomarkers would also be greatly useful for this purpose.
One particularly interesting biomarker is miRNAs within tumors. Once processed, mature miRNAs can act as oncogenes or tumor suppressors, as they bind to various complementary mRNAs to activate or suppress gene expression [80]. Several miRNAs have been implicated in activating or suppressing oncogenes in various types of cancer, including prostate cancer, RCC and NSCLC [81–83]. However, few studies have examined how radiation affects miRNA expression. Skinner et al. analyzed large discovery, model and validation cohorts to investigate differences in miRNA in 118 patients with esophageal cancer treated with chemoradiation. They found that four miRNAs (miR505, miR99-b, miR451 and miR145) were expressed at lower levels in tumors that showed complete pathological response to chemoradiation compared with tumors that did not respond completely [84]. The effects of immunotherapy on miRNA expression are also not well known. Some have hypothesized that miRNA-21 would be a useful marker for predicting immunotherapy response because it regulates STAT-3 expression, an important regulator of immune infiltration of tumors and tumor progression [85]. Studies investigating miRNA expression profiles after immunotherapy and radiation would help to further understand which genes are involved in treatment resistance. Moreover, miRNAs can serve not only as biomarkers but also as targets for therapy, because RNA that is complementary to tumor-promoting miRNAs can be synthesized [86]. miRNA profiling during immunoradiation could also help to predict which patients will have an abscopal response; clinical studies are currently ongoing to shed light on these issues.
Even with the remarkable effects of immunotherapy on cancer treatment, substantial proportions of patients do not respond to anti-PD1 or anti-CTLA4 treatment [8,15]. One cause for this could be the development of resistance. A solution to this problem that has yet to be explored is to combine immunotherapies with radiation. Victor et al. noted that a low CD8+/Treg ratio predicted resistance of melanoma to anti-CTLA4 therapy and radiation. Transcriptive profiling of these resistant tumors revealed high levels of tumor PDL1. Genetic silencing of PDL1 restored the sensitivity of melanomas to dual therapy, and the addition of anti-PDL1 IgG also increased the number of active CD8+ T cells [26]. Wolchok and colleagues demonstrated improved overall response rates after concurrent ipilimumab and nivolumab without radiation for advanced melanoma compared with previous studies investigating either antibody as monotherapy [9]. However, the optimal sequencing of immunotherapy and radiation has yet to be defined. Young and colleagues explored the use of an OX40 agonist or an anti-CTLA4 antibody against CT26 colorectal adenocarcinoma cells in mice before and after a single 20-Gy fraction of radiation. They observed longer survival and higher CD8+ T-cell counts in the mice given the anti-CTLA4 antibody before radiation or the OX40 agonist 1 day after radiation [87]. One potential explanation for this finding is that the anti-CTLA4 antibody primed the CD8+ T cells before the radiation induced antigen stimulation, whereas the OX40 agonist promoted CD8+ T-cell-mediated tumor destruction. Future experiments testing other immune modulators, including anti-PD1 antibody, during radiation are needed to optimize the sequence and timing of treatment components.
In addition to PD1, CTLA4 and OX40, other immunomodulating signaling pathways remain to be explored in combination with radiation. One example is 4-1BB (also called CD137), which is located on activated cytotoxic T cells and binds to 4-1BBL on APCs to serve as a costimulatory molecule for T-cell proliferation [88]. Mouse experiments with 4-1BB antibody agonists have shown increased antitumor CD8+ T-cell activity against sarcomas and gliomas [88]. Belcaid et al. were the first group to test 4-1BB activation with CTLA4 blockade and radiation in a glioma mouse model. In that study, mice were given single-fraction 10-Gy SRS at 10 days after intracranial implantation of glioma cells, followed by CTLA4 and 4-1BB therapy in 6-day intervals. They found that the mice given this triple therapy had the longest median survival times relative to mice given dual immunotherapy or radiation alone. However, they did note that the antitumor activity depended on CD4+ T cells rather than CD8+ T cells. They also tested the timing of CTLA4 blockade with SRS and noted that the treatment was most effective when given 2 days before the SRS, similar to the findings of Young and colleagues. Finally, the surviving mice developed long-term immunity after being rechallenged with the same glioma cell line [89].
Another potential target is Tim-3, a novel checkpoint inhibitor that has garnered attention over the past 5 years. Tim-3 expressed on T cells binds to galectin-9, which is expressed by various types of cancer (Figure 1A) [7]. Like PD1, Tim-3 is thought to induce T-cell exhaustion, based on findings that Tim-3+ CD8+ T cells are less effective at secreting cytokines that promote tumor cell death (IL-2, TNF-α, IFN-γ) [90,91]. Dual inhibition of both Tim-3 and PD1 in CT26 colon carcinoma cells was also noted to reduce tumor growth significantly compared with either therapy alone [90]. Tim-3 also activates Tregs concurrently with CD8+ T cells [92], and is a negative regulator of NK cell function [93]. Because of its synergistic inhibitory effect with PD1 on T-cell populations, Tim-3 is an appealing target to study in combination with radiation [3]. Another category of immunostimulating agents being investigated for solid tumor therapy is Toll-like receptors (TLRs). TLRs recognize pathogen associated molecular patterns (PAMPs) that are expressed by foreign cells and activate several inflammatory cytokines including TNF-α, IL-6 and INF-α [94]. Preclinical models have demonstrated an increased tumor growth inhibition in mice bearing breast cancer after radiation and Imiquimod, a TLR-7 agonist, compared with either therapy alone ([95]). Currently, a trial investigating Imiquoimoid with radiation in breast cancer patients with skin metastasis is underway (identifier: NCT01421017 [22]).
The use of monoclonal antibodies to block inhibitory KIRs, thereby activating NK cells, has also been explored. One preclinical experiment revealed that blocking KIR2DL’s interaction with the human leukocyte antigen class I receptor promoted IL-2-activated, NK-cell–mediated lysis of acute myeloid leukemia [96]. One humanized anti-KIR2DL antibody, IPH2101, is being tested in Phase I trials (Figure 1D). In one such trial, Benson and colleagues found that IPH2101 did not produce any objective clinical response in 32 patients with refractory multiple myeloma, but it was found to activate NK cells [97]. Another Phase I study found that higher doses of IPH2101 for acute myeloid leukemia led to improved overall survival and perhaps better progression-free survival [98]. Preclinical experiments are needed to clarify the mechanisms of action of monoclonal anti-inhibitory KIRs combined with radiation for solid tumors, as infusion of large numbers of NK cells has produced inconsistent objective response rates [72–74].
Another new target for immune modulation is IDO, an enzyme involved in tryptophan catabolism, and its homologs. IDO is expressed by both tumor cells and regulatory APCs; it degrades tryptophan into kynurenines outside the cells, within the tumor cell microenvironment, depleting extracellular tryptophan stores and leading to T-cell tryptophan starvation, which in turn causes cell death (Figure 1B). IDO also affects several other immune processes, including complement inhibition [99], NK-cell dysfunction [100], MDSC immunosuppression, Treg infiltration and activation [101], and macrophage M2 polarization [102]. Overall, IDO has been recognized as a target molecule in the tumor microenvironment to prevent T cells from becoming deactivated. In one clinical trial involving 15 patients with stage III/IV metastatic NSCLC, treatment with an IDO vaccine produced a partial response in one patient and stable disease in six [103]. Because drugs targeting IDO seem to be effective as monotherapy, the potential exists to overcome tumor microenviron-mental immunosuppression by combining such drugs with other therapies such as radiation. Radiation-resistant cancers show increased expression of TGF-β and higher numbers of Tregs and MDSCs, all of which inhibit the targeting of tumor cells by T cells, a scenario IDO inhibition could improve [104–106]. One study has already shown that IDO inhibition can aid in prolonging survival after chemoradiation therapy [99], and more studies are needed in which IDO is used in combination with other forms of treatment.
In summary, immunotherapy presents many new avenues to explore regarding the role of radiation in priming a systemic T-cell response in the treatment of solid tumors. So far, combinations of radiation with inhibitors of the CTLA4 and PD1 checkpoints have shown promise for synergistic improvement. The anti-tumor effectiveness of other promising new immune modulators (e.g., OX40, 4-1BB, IDO, TIM-3) may also be enhanced by combining them with radiation. Another approach to stimulating antitumor immunity is adoptive cell therapies (e.g., adoptive T-cell therapy, CAR–T cells, NK cells), which could also be enhanced by radiation. With several possible therapeutic combinations, our mission is to discover an algorithm to predict which patients will experience an abscopal response to widespread metastatic disease. However, caution is essential because many of these agents can also cause auto-immunity and inflammation, such as pulmonary pneumonitis, that could be exacerbated by radiation. As such, Phase I trials must be conducted carefully to avoid harming patients. We are now on the verge of a profound change in oncology, in that we can now harness a patient’s own immune system to battle their cancer. In combination with radiation, one of the oldest and most effective forms of local control, immunotherapy has the potential to transform oncology through the induction of durable systemic control.
Executive summary.
CTLA4 blockade with radiation promotes antitumor immunity
Anti-CTLA4 therapy delays tumor growth by stimulating cytotoxic CD8+ T cells and reducing T-regulatory cell (Treg) function.
Radiation given with anti-CTLA4 antibody has produced abscopal responses in patients with advanced melanoma or lung cancer.
PD1/PDL1 inhibition & radiation effects the tumor microenvironment
Anti-PDL1 therapy reinvigorates certain populations of T cells.
Clinical trials of anti-PD1 antibodies for previously treated chemoresistant tumors have shown improvement in objective response rates relative to chemotherapy.
Anti-PDL1 therapy with radiation has reduced local tumor volume and reduced the numbers of immune-suppressing cells in murine models of lung cancer.
OX40 agonists
OX40 agonists suppress Tregs and activate cytotoxic CD8+ T cells.
Adding radiation to OX40 activators increases overall survival and local control in mice implanted with various solid tumors.
Further validation in clinical trials is needed.
T-cell therapies can increase lymphocyte infiltration into tumors
Adoptive T-cell therapy involves obtaining tumor-specific lymphocytes, expanding them and re-infusing them back into the same patient.
Combining adoptive T-cell therapy with radiation led to clinical benefit (relative to monotherapy) in metastatic melanoma.
T cells engineered to express chimeric antigen receptors can target specific solid tumor antigens that are upregulated after irradiation (e.g., mesothelin) without having to interact with major histocompatibility complex (MHC) proteins.
Natural killer (NK) cell receptors bind to MHC-1 and other antigens on tumors that can either inhibit or stimulate NK-cell activity.
NK cells can recognize cells with abnormally low MHC-1 expression.
Irradiation of tumor cells seems to promote activation of NK cells, but further clarification of this method by which it does so is needed.
Future perspective
Biomarkers such as tumor mutational load and miRNAs may help to predict which patients will experience abscopal events after immunotherapy and radiation.
Novel combinations and optimal sequencing of immune checkpoint inhibitors with radiation require further investigation.
Preclinical models of sequential 4-1BB simulation and CTLA4 inhibition with radiation promoted CD4+ T cell-induced destruction of glioma.
Tim-3 works with PD1 to promote Treg activation and CD8+ T cell-induced cell death, making it an ideal immunotherapy to combine with radiation.
Anti-inhibitory KIR antibodies block NK cell binding to MHC-1 and increase NK cell killing, but outcomes are inconsistent in hematologic and solid malignancies.
IDO, a catabolic T-cell deactivator, is an appealing therapeutic target as it also affects several types of immune cells (myeloid-derived suppressor cells, Tregs, NK cells) in the tumor microenvironment.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional Phase I/II trial of stereotactic body radiation therapy for lung metastases. J. Clin. Oncol. 2009;27(10):1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 2.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional Phase I/II trial of stereotactic body radiation therapy for liver metastases. J. Clin. Oncol. 2009;27(10):1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 3.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J. Natl Cancer Inst. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol. Res. 2014;2(9):831–838. doi: 10.1158/2326-6066.CIR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camphausen K, Moses MA, Menard C, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63(8):1990–1993. [PubMed] [Google Scholar]
- 6.Chakravarty PK, Guha C, Alfieri A, et al. Flt3l therapy following localized tumor irradiation generates long-term protective immune response in metastatic lung cancer: its implication in designing a vaccination strategy. Oncology. 2006;70(4):245–254. doi: 10.1159/000096288. [DOI] [PubMed] [Google Scholar]
- 7.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. • Ipilimumab increases overall survival in patients with melanoma.
- 9.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. • Nivolumab demonstrates improvement in patients with advanced melanoma.
- 10.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2014;2(5):393–398. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 11.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The tnfrs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr. Opin. Immunol. 2013;25(2):230–237. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 2013;1(6):365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiniker SM, Chen DS, Reddy S, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl. Oncol. 2012;5(6):404–407. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purdy AK, Campbell KS. Natural killer cells and cancer: regulation by the killer cell Ig-like receptors (KIR) Cancer Biol. Ther. 2009;8(23):2211–2220. doi: 10.4161/cbt.8.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a Phase 1 trial. Lancet. 2014;384(9948):1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 17.Powles T, Eder JP, Fine GD, et al. Mpdl3280a (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 18.Spigel DR, Reckamp KL, Rizvi NA, et al. A Phase III study (CheckMate 017) of nivolumab (NIVO; anti-programmed death-1 [PD-1]) vs docetaxel (DOC) in previously treated advanced or metastatic squamous (SQ) cell non-small cell lung cancer (NSCLC) J. Clin. Oncol. 2015;33(Suppl.) abstr 8009. [Google Scholar]
- 19.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-l1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 2014;124(2):687–695. doi: 10.1172/JCI67313. •• Radiation synergistically works with anti-PD-L1 therapy to promote antitumor immunity.
- 20.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013;86(2):343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-l1 antibody MPDL3280a in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinicaltrials.gov. https://clinicaltrials.gov/
- 23.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 24.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005;11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 25.Yoshimoto Y, Suzuki Y, Mimura K, et al. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PLoS ONE. 2014;9(3):e92572. doi: 10.1371/journal.pone.0092572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. • CD8+ T cell/Treg ratio predicts response to radiation and anti-CTLA-4 therapy.
- 27.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(2):293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter Phase I/II study. Ann. Oncol. 2013;24(7):1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paterson DJ, Jefferies WA, Green JR, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 MR detected only on CD4 positive T blasts. Mol. Immunol. 1987;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 30.Hirschhorn-Cymerman D, Budhu S, Kitano S, et al. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J. Exp. Med. 2012;209(11):2113–2126. doi: 10.1084/jem.20120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J. Immunol. 2008;180(4):2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 32.So T, Choi H, Croft M. OX40 complexes with phosphoinositide 3-kinase and protein kinase B (PKB) to augment TCR-dependent PKB signaling. J. Immunol. 2011;186(6):3547–3555. doi: 10.4049/jimmunol.1003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaki S, Ine S, Kawabe T, et al. Ox40 and IL-7 play synergistic roles in the homeostatic proliferation of effector memory CD4(+) T cells. Eur. J. Immunol. 2014;44(10):3015–3025. doi: 10.1002/eji.201444701. [DOI] [PubMed] [Google Scholar]
- 34.Byun M, Ma CS, Akcay A, et al. Inherited human OX40 deficiency underlying classic kaposi sarcoma of childhood. J. Exp. Med. 2013;210(9):1743–1759. doi: 10.1084/jem.20130592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baeyens A, Saadoun D, Billiard F, et al. Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context. J. Immunol. 2015;194(3):999–1010. doi: 10.4049/jimmunol.1400504. [DOI] [PubMed] [Google Scholar]
- 36.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating fcgammars, leading to antitumor efficacy. Immunol. Cell Biol. 2014;92(6):475–480. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 37.Mahmud SA, Manlove LS, Schmitz HM, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 2014;15(5):473–481. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piconese S, Timperi E, Pacella I, et al. Human OX40 tunes the function of regulatory T cells in tumor and nontumor areas of hepatitis c virus-infected liver tissue. Hepatology. 2014;60(5):1494–1507. doi: 10.1002/hep.27188. [DOI] [PubMed] [Google Scholar]
- 39.Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol. Res. 2014;2(2):142–153. doi: 10.1158/2326-6066.CIR-13-0031-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voo KS, Bover L, Harline ML, et al. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J. Immunol. 2013;191(7):3641–3650. doi: 10.4049/jimmunol.1202752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voo KS, Foglietta M, Percivalle E, et al. Selective targeting of Toll-like receptors and OX40 inhibit regulatory T-cell function in follicular lymphoma. Int. J. Cancer. 2014;135(12):2834–2846. doi: 10.1002/ijc.28937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golovina TN, Mikheeva T, Suhoski MM, et al. CD28 costimulation is essential for human T regulatory expansion and function. J. Immunol. 2008;181(4):2855–2868. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokouchi H, Yamazaki K, Chamoto K, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci. 2008;99(2):361–367. doi: 10.1111/j.1349-7006.2007.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gough MJ, Crittenden MR, Sarff M, et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J. Immunother. 2010;33(8):798–809. doi: 10.1097/CJI.0b013e3181ee7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curti BD, Kovacsovics-Bankowski M, Morris N, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73(24):7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magdalena Kovacsovics-Bankowski LC, Vercellini J, Crittenden M, Lary S, Curti B, Weinberg A. Phase i/ii clinical trial of anti-OX40, radiation and cyclophosphamide in patients with prostate cancer: immunological analysis. J. Immunother. Cancer. 2013;1:255. [Google Scholar]
- 47.Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung v5 and its effects on non-small cell lung cancer patient outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2014;89(5):1084–1091. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 2014;192(12):5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu R, Forget MA, Chacon J, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;18(2):160–175. doi: 10.1097/PPO.0b013e31824d4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a Phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 2010;16(9):2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 51.Filatenkov A, Baker J, Muller AM, et al. Treatment of 4T1 metastatic breast cancer with combined hypofractionated irradiation and autologous T-cell infusion. Radiat. Res. 2014;182(2):163–169. doi: 10.1667/RR13471.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gross G, Waks T, Eshhar Z. Expression of immunoglobulint-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA. 1989;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen MC, Riddell SR. Designing chimeric antigen receptors to effectively and safely target tumors. Curr. Opin. Immunol. 2015;33C:9–15. doi: 10.1016/j.coi.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guest RD, Hawkins RE, Kirillova N, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scfvs and antigens. J. Immunother. 2005;28(3):203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 57.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ror1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 2013;19(12):3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IGG1 FC ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17(10):1206–1213. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- 59.Hudecek M, Sommermeyer D, Kosasih PL, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol. Res. 2015;3(2):125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/Akt/Bcl-xL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 2010;18(2):413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71(17):5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 63.Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hassan R, Williams-Gould J, Steinberg SM, et al. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin. Cancer Res. 2006;12(16):4983–4988. doi: 10.1158/1078-0432.CCR-06-0441. [DOI] [PubMed] [Google Scholar]
- 65.Cao N, Li S, Wang Z, et al. Nf-kappab-mediated HER2 overexpression in radiation-adaptive resistance. Radiat. Res. 2009;171(1):9–21. doi: 10.1667/RR1472.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhardwaj V, Zhan Y, Cortez MA, et al. c-met inhibitor mk-8003 radiosensitizes c-Met-expressing non-small-cell lung cancer cells with radiation-induced c-Met-expression. J. Thorac. Oncol. 2012;7(8):1211–1217. doi: 10.1097/JTO.0b013e318257cc89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012;12(4):239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol. Immunol. 2013;10(3):230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sconocchia G, Eppenberger S, Spagnoli GC, et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology. 2014;3(8):e952197. doi: 10.4161/21624011.2014.952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baskic D, Vujanovic L, Arsenijevic N, Whiteside TL, Myers EN, Vujanovic NL. Suppression of natural killer-cell and dendritic-cell apoptotic tumoricidal activity in patients with head and neck cancer. Head Neck. 2013;35(3):388–398. doi: 10.1002/hed.22968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piroozmand A, Hassan ZM. Evaluation of natural killer cell activity in pre and post treated breast cancer patients. J. Cancer Res. Ther. 2010;6(4):478–481. doi: 10.4103/0973-1482.77110. [DOI] [PubMed] [Google Scholar]
- 72.Iliopoulou EG, Kountourakis P, Karamouzis MV, et al. A Phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer. Immunol. Immunother. 2010;59(12):1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15(12):1563–1570. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 74.Arai S, Meagher R, Swearingen M, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a Phase I trial. Cytotherapy. 2008;10(6):625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 75.Heo W, Lee YS, Son CH, Yang K, Park YS, Bae J. Radiation-induced matrix metalloproteinases limit natural killer cell-mediated anticancer immunity in NCI-H23 lung cancer cells. Mol. Med. Rep. 2015;11(3):1800–1806. doi: 10.3892/mmr.2014.2918. [DOI] [PubMed] [Google Scholar]
- 76.Lim SH, Chua W, Cheng C, et al. Effect of neoadjuvant chemoradiation on tumor-infiltrating/associated lymphocytes in locally advanced rectal cancers. Anticancer Res. 2014;34(11):6505–6513. [PubMed] [Google Scholar]
- 77.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. • Tumor mutational and neopeptide sequence load predicts response to anti-CTLA-4 therapy in melanoma.
- 80.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat. Rev. Drug Discov. 2010;9(10):775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ueno K, Hirata H, Shahryari V, et al. Tumour suppressor microRNA-584 directly targets oncogene rock-1 and decreases invasion ability in human clear cell renal cell carcinoma. Br. J. Cancer. 2011;104(2):308–315. doi: 10.1038/sj.bjc.6606028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mishra S, Deng JJ, Gowda PS, et al. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor ii (tgfbr2) expression in prostate cancer. Oncogene. 2014;33(31):4097–4106. doi: 10.1038/onc.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamashita R, Sato M, Kakumu T, et al. Growth inhibitory effects of mir-221 and mir-222 in non-small cell lung cancer cells. Cancer Med. 2015 doi: 10.1002/cam4.412. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Skinner HD, Lee JH, Bhutani MS, et al. A validated miRNA profile predicts response to therapy in esophageal adenocarcinoma. Cancer. 2014;120(23):3635–3641. doi: 10.1002/cncr.28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z, Han J, Cui Y, Fan K, Zhou X. Circulating microRNA-21 as noninvasive predictive biomarker for response in cancer immunotherapy. Med. Hypotheses. 2013;81(1):41–43. doi: 10.1016/j.mehy.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 86.Thorsen SB, Obad S, Jensen NF, Stenvang J, Kauppinen S. The therapeutic potential of microRNAs in cancer. Cancer J. 2012;18(3):275–284. doi: 10.1097/PPO.0b013e318258b5d6. [DOI] [PubMed] [Google Scholar]
- 87.Young K, Cottam B, Baird JR, Gough MJ, Crittenden M. Ideal timing of immunotherapy with radiation in murine tumor models. Int. J. Radiat. Oncol. Biol. Phys. 2014;90((1) (Suppl.)):S58. [Google Scholar]
- 88.Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol. Cancer Ther. 2012;11(5):1062–1070. doi: 10.1158/1535-7163.MCT-11-0677. [DOI] [PubMed] [Google Scholar]
- 89.Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE. 2014;9(7):e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting TIM-3 and Pd-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of TIM-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117(17):4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gautron AS, Dominguez-Villar M, De Marcken M, Hafler DA. Enhanced suppressor function of TIM-3+ FOXP3+ regulatory T cells. Eur. J. Immunol. 2014;44(9):2703–2711. doi: 10.1002/eji.201344392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hou H, Liu W, Wu S, et al. Tim-3 negatively mediates natural killer cell function in LPS-induced endotoxic shock. PLoS ONE. 2014;9(10):e110585. doi: 10.1371/journal.pone.0110585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rich AM, Hussaini HM, Parachuru VP, Seymour GJ. Toll-like receptors and cancer, particularly oral squamous cell carcinoma. Front. Immunol. 2014;5:464. doi: 10.3389/fimmu.2014.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dewan MZ, Vanpouille-Box C, Kawashima N, et al. Synergy of topical Toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin. Cancer Res. 2012;18(24):6668–6678. doi: 10.1158/1078-0432.CCR-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romagne F, Andre P, Spee P, et al. Preclinical characterization of 1–7f9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114(13):2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benson DM, Jr, Hofmeister CC, Padmanabhan S, et al. A Phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120(22):4324–4333. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vey N, Bourhis JH, Boissel N, et al. A Phase 1 trial of the anti-inhibitory KIR MAB IPH2101 for AML in complete remission. Blood. 2012;120(22):4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 99.Li M, Bolduc AR, Hoda MN, et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J. Immunother. Cancer. 2014;2:21. doi: 10.1186/2051-1426-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng YP, Zhang JJ, Liang WB, et al. Elevation of MMP-9 and IDO induced by pancreatic cancer cells mediates natural killer cell dysfunction. BMC Cancer. 2014;14:738. doi: 10.1186/1471-2407-14-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu J, Du W, Yan F, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol. 2013;190(7):3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 102.Wang XF, Wang HS, Wang H, et al. The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: Focus on macrophage polarization of THP-1 cells. Cell Immunol. 2014;289(1–2):42–48. doi: 10.1016/j.cellimm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 103.Iversen TZ, Engell-Noerregaard L, Ellebaek E, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin. Cancer Res. 2014;20(1):221–232. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
- 104.Boothe DL, Coplowitz S, Greenwood E, et al. Transforming growth factor beta-1 (TGF-beta1) is a serum biomarker of radiation induced fibrosis in patients treated with intracavitary accelerated partial breast irradiation: preliminary results of a prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2013;87(5):1030–1036. doi: 10.1016/j.ijrobp.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu CT, Chen MF, Chen WC, Hsieh CC. The role of IL-6 in the radiation response of prostate cancer. Radiat. Oncol. 2013;8:159. doi: 10.1186/1748-717X-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kachikwu EL, Iwamoto KS, Liao YP, et al. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(4):1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]