Abstract
GVHD-prophylaxis with post-transplant cyclophosphamide (CY) following ablative HLA-matched bone marrow (BM) transplantation has been reported to have comparable rates of acute GVHD with an apparent reduction in chronic GVHD and infections. We conducted a phase II trial of post-CY following reduced-intensity conditioning (RIC) using intravenous busulfan (AUC of 4,000 micromolar-minutes), Fludarabine (40mg/m2) for 4 days and CY 50mg/kg on days +3 and +4 following BM or peripheral blood (PB) transplants from matched related (MRD) or unrelated donors (MUD). MUD- recipients received anti-thymocyte globulin (ATG); however, a later amendment removed ATG. 49 patients were treated (AML/MDS: 82%). Median age was 62 years (range, 39–72). Fifteen patients received a MRD (9 PB/6 BM); 34 had a MUD (2 PB/32 BM). The cumulative incidence of grade II–IV, III–IV acute and chronic GVHD was 58%, 22% and 18%. A matched-cohort analysis compared outcomes to tacrolimus/methotrexate GVHD prophylaxis and indicated higher rates of acute GVHD grade II–IV (46% versus 19%, HR=2.8, p=0.02) and treatment-related mortality (HR 3.3, p=0.035) and worse overall survival (HR=1.9, p=0.04) with post-Cy. The incidence of chronic GVHD and CMV reactivation did not differ. This study suggests that post-transplant CY should not be used as sole GVHD-prophylaxis following a RIC transplant from HLA matched donors.
INTRODUCTION
Graft-versus-Host Disease (GVHD) remains a major limitation to allogeneic hematopoietic cell transplantation1. A calcineurin inhibitor (CNI), tacrolimus or cyclosporine, combined with methotrexate (MTX) is the most common GVHD prophylactic strategy; however, it is limited by toxicities and the need for prolonged exposure to its immunosuppressive effects. Additionally, animal models suggest that prolonged exposure to a CNI may paradoxically increase the rate of chronic GVHD2–5.
Recently the use of post-transplant cyclophosphamide (post-CY) given on days three and four following a matched donor bone marrow (BM) transplant has been evaluated as an alternative regimen for preventing GVHD6. CY is administered in the early period following allograft infusion to eliminate alloreactive T- cell clones and reduce GVHD. This regimen spares hematopoietic stem cells and also T-regulatory cells which express aldehyde dehydrogenase7. This approach avoids the need for additional immunosuppressive agents8. In so doing, it is hypothesized to improve immune reconstitution and reduce infections and chronic GVHD.
Luznik and colleagues published a prospective study using this strategy wherein patients with hematologic malignancies received an ablative conditioning regimen of busulfan and CY (BuCy) followed by a T-cell replete bone marrow graft from a matched related (MRD) or unrelated donor (MUD) and post-CY as sole GVHD prophylaxis6. In this report, the incidence of grades II–IV and III–IV acute GVHD were 43 and 10% respectively; rates comparable to CNI/MTX prophylaxis. Encouragingly, the rates of chronic GVHD and viral infections appeared lowered than historical reports. Similar results were reported in a multicenter study using myeloablative busulfan-fludarabine conditioning.9
We performed a prospective, phase II study to determine if post-CY could be used as sole GVHD prophylaxis following a reduced-intensity conditioning (RIC) regimen in a population for whom ablative conditioning would be inappropriate due to age or co-morbidities. We found that, unlike the experience with ablative conditioning, the rates of acute GVHD and TRM were inferior to that in matched historical controls receiving tacrolimus-methotrexate GVHD prophylaxis.
METHODS
Study design and patients
This was a prospective single-center phase II trial to examine the safety of RIC using intravenous Bu and Fludarabine (BuFlu) followed by post-CY as sole GVHD prophylaxis in recipients of MRD or MUD transplant for hematologic malignancies. The trial was registered with clinicaltrials.gov (NCT00800839) and approved by the institutional review board of The University of Texas, MD Anderson Cancer Center. All patients provided written informed consent in accordance to the Declaration of Helsinki.
The primary end-point was the incidence of grades II–IV acute GVHD. Secondary endpoints included the rate of engraftment and chronic GVHD and the probability of progression-free and overall survival (PFS and OS). The method of Thall, Simon and Estey was used for safety monitoring wherein stopping rules were based on historical rates for graft failure (GF, lack of neutrophil engraftment by day 30), day-100 treatment-related mortality (TRM, mortality not attributable to relapse and without GF within the first 100 days) and grade III/IV acute GVHD by 100 days with historical baseline rates of 5%, 20% and 20%, respectively10, 11.
Patients between the ages of 6 months and 75 years with hematological malignancies and a human leukocyte antigen (HLA)-A, B, C and DR allele matched related or unrelated donor were eligible. Eligible patients had to be deemed a poor candidate for myeloablative conditioning because of age > 60 years, a reduced performance status, prior transplantation or impaired organ function. Patients were required to have a total bilirubin ≤ 1.5 mg/dl and transaminases less than 3 times the upper limits of normal, a creatinine clearance above 50 mL/minute, a diffusing capacity for carbon monoxide >45% predicted corrected for hemoglobin and a left ventricular ejection fraction of ≥35%. Patients with uncontrolled infections, HIV seropositivity or inability to provide informed consent or assent were excluded.
Preparative Regimen and Supportive Care
A reduced intensity preparative regimen of busulfan (Bu) and fludarabine (Flu) was utilized as has been reported by Popat et al12. A similar regimen has been reported by Mohty et al in a large, multicenter, phase 2 trial13. A “test dose” of Bu, 32mg/m2, was given on day-8 before transplant. Based on the pharmacokinetic (PK) data generated from the test dose, an adjusted dose of Bu was administered at a dose calculated to achieve a systemic exposure of 4,000 micromolar-minute. Patients received Flu 40 mg/m2 intravenously over 1 hour followed by Bu, infused over 3 hours, daily on days-6 to -3. Patients received either a peripheral blood (PB) or bone marrow (BM) graft on day 0 followed by CY 50 mg/kg intravenously on days +3 and +4. Mesna 10mg/kg IVPB was given prior to the first dose of CY and repeated every 4 hours for 10 doses. In patients for whom PK monitoring was not feasible, Bu 100mg/m2/day was administered for four days. Recipients of MUD’s received anti-thymocyte globulin (ATG) on days -3 to -1 (total dose of 4mg/kg). ATG was included in the preparative regimen based on institutional practice for recipients of unrelated donors and to allow matching with control patients receiving traditional prophylaxis. The protocol was amended removing ATG after the 34th enrolled patient to evaluate whether ATG was negatively influencing outcomes as the original reports from Luznik et al. did not include this agent6.
Patients received anti-seizure prophylaxis with phenytoin prior to the test dose of Bu and continued until its completion. Granulocyte-colony stimulating factor (G-CSF, 5mcg/kg/day) was given starting on day +5 and continued until the absolute neutrophil count (ANC) was greater than 500 × 109/L for 3 consecutive days. All supportive care measures were given per institutional practice and included routine anti-microbial prophylaxis against bacterial, fungal, Pneumocystis jirovecii, and herpes simplex infections. Patients were monitored weekly for CMV- reactivation via CMV pp65 antigen testing or polymerase chain reaction with preemptive therapy started in those with evidence of CMV- reactivation.
Regimen-related toxicity and diagnosis and treatment of acute GVHD
Toxicities were graded according to the National Cancer Institute criteria (Common Toxicity Criteria version 3). Patients suspected of having acute GVHD underwent histologic confirmation; patients with clinical grade II or higher GVHD were promptly started on systemic corticosteroids and a CNI. Acute GVHD was graded according to the Modified Consensus Criteria while chronic GVHD was classified according to the National Institute of Health’s guidelines14, 15.
Engraftment and donor chimerism
Neutrophil and platelet engraftment was defined as the first of 3 consecutive days with an ANC ≥0.5 × 109/L and a platelet count ≥ 20,000 without transfusion in the preceding 7 days. Primary GF was defined as a lack of neutrophil recovery not attributable to recurrent marrow malignancy. Secondary GF was defined as a donor chimerism of <5% not attributable to recurrent marrow malignancy in a previously engrafted patient. Donor chimerism was determined on days 30 and 100 post-transplant.
Statistical analysis
The rate of GVHD and TRM were estimated from the date of transplant using the cumulative incidence method to account for competing risks. Death or disease progression before diagnosis of GVHD were considered competing risks in the estimation of the incidence of GVHD; disease progression or relapse death were considered competing risk for TRM. OS and PFS were estimated using Kaplan-Meier method. Cox’s proportional hazards regression analyses was used to assess the impact of the following risk factors: patient age, diagnosis, donor type, disease status at time of transplant, sex and donor/recipient sex mismatch and conditioning regimens on primary and secondary endpoints. The impact of ATG was examined separately for recipients of MUD’s and the impact of graft source was only examined in MRD recipients as there were too few recipients of PB in the MUD cohort. Statistical significance was determined at the 0.05 level. Statistical analyses were performed using STATA 11.0 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
Matched cohort analysis design
A computer generated algorithm was used to identify a comparable control group who received GVHD prophylaxis with tacrolimus and short-course MTX (5mg/m2 on days +1, +3, +6, +11) and a RIC transplant during the same time period. RIC consisted of the Bu/Flu regimen employed in the trial patients or Flu (total dose of 125mg/m2) in combination with Melphalan (total dose 140mg/m2). Supportive care was otherwise identical. Patients were identified from the departmental database matching in order of priority for age, diagnosis (type of malignancy), disease status at transplant, donor type, graft source and the use of ATG.
RESULTS
Patient Characteristics
Forty-nine (49) patients were enrolled between 11/2008 and 7/2011. Acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) were the most common indication for transplant occurring in 82% (40) of the enrolled patients with 24% (12 patients) in first or second complete remission. The median age was 62 years (range, 39–72) with a median co-morbidity index of 3 (range, 0–10). Fifteen (15) patients received a transplant from a matched sibling of which 9 had a PB graft and 6 had BM; whereas, the majority of the 34 MUD recipients received BM (32 patients), Table I. The median follow-up for surviving patients was 32 months (range, 24–43).
Table I.
Patient Characteristics
| Post-Cy All Enrolled Patients | Post-Cy Matched subset | Tacrolimus + MTX Matched Control | * P-value | |
|---|---|---|---|---|
|
| ||||
| N= 49 | N= 37 | N=37 | ||
|
| ||||
| Male/Female | 28/21 | 21/16 | 22/15 | 0.8 |
|
| ||||
| Median Age (range) | 61 years (39–72) | 61 years (39–72) | 62 years (37–72) | 0.8 |
|
| ||||
| Median Co-Morbidity Index (range) | 3 (0–10) | 3 (0–10) | 3 (0–8) | 0.4 |
|
| ||||
| Diagnosis (%) | ||||
| AML/MDS | 40 | 30 (81%) | 30 (81%) | 1.0 |
| ALL | 1 | 1 (3) | 1 (3) | |
| CLL | 5 | 4 (11) | 0 (0) | |
| NHL | 3 | 2 (5) | 6 (16) | |
|
| ||||
| Stage at Transplant (%) | ||||
| CR1 | 9 | 5 | 5 | 1.0 |
| CR2 | 3 | 3 | 4 | |
| Primary Induction Failure | 19 | 12 | 12 | |
| Untreated | 3 | 3 | 4 | |
| >CR2 | 15 | 14 | 12 | |
|
| ||||
| Donor and Stem Cell Source | ||||
| MRD BM | 6 | 6 | 1 | 0.04 |
| MRD PB | 9 | 9 | 14 | |
| MUD BM | 32 | 20 | 16 | 0.1 |
| MUD PB | 2 | 2 | 6 | |
|
| ||||
| ATG (MUD’s only) | 22 | 22 | 22 | 1.0 |
|
| ||||
| RIC Regimen | ||||
| BU/Flu | 49 | 37 | 15 | |
| Flu/Mel | 22 | |||
|
| ||||
| Female D/Male R | 9 | 8 | 3 | 0.09 |
|
| ||||
| CMV Status D/R | ||||
| D + or −/R+ | 39 | 31 | 27 | 0.3 |
| D+/R− | 6 | 3 | 5 | |
| D−/R− | 4 | 3 | 5 | |
P value for comparison of Post-Cy matched cohort and Tacrolimus+ MTX matched control
Engraftment
The median time to neutrophil engraftment was 16 and 20 days for recipients of PB and BM grafts, respectively, while the median time to platelet engraftment was 25 and 26 days. Stopping criteria were met when 5 patients failed to engraft by day +28. Three patients had primary graft failure (GF) (6%) while 2 patients experienced delayed engraftment. These rates are similar to graft failure rates seen in other studies9, 16. All three patients with primary GF received BM, 2 from MUDs and one from a sibling; none of these patients received ATG. Two of these 3 patients died from infectious complications, and the third patient is alive and in remission following a second transplant. The infused total nucleated cell (TNC) dose did not differ between the three patients with primary GF (median TNC/kg 2.97 × 108) and engrafting patients (median TNC/kg 2.98 × 108) following BM infusions. Of the two patients with delayed engraftment, both receive bone marrow grafts from a MRD. One engrafted on day +38 but died from infectious complications on day +48 post-transplant; the second patient engrafted on day +30. The median TNC/kg was lower in these two patients (0.73 × 108) when compared to engrafted patients (2.98 × 108, p=0.05). The median day 30 donor chimerism for evaluable engrafting patients was T-cell 100% (range, 0–100) and myeloid 100% (range, 0–100), and the median day 100 chimerism was T-cell 100% (range, 0–100) and myeloid 100% (range, 34–100). Mixed T-cell and myeloid chimerism was present at 30 days in 28% (n=13) of engrafting patients.
Acute and Chronic GVHD, Treatment-Related Mortality (TRM) and Survival
The cumulative incidence of day-100 grade II–IV and III–IV acute GVHD was 53% (95% confidence interval [C.I.], 40–70) and 22% (95% C.I., 13–38). The cumulative incidence of chronic GVHD at 1 year was 18% (95% C.I., 9–33%). The day- 100 and 2-year TRM was 14% (95% C.I., 7–28) and 39% (95% C.I., 27–55). Table II.
Table II.
Outcomes for Post-CY following RIC for patients with Hematologic Malignancies (N=49)
| % Cumulative Incidence | 95% C.I. | |
|---|---|---|
|
| ||
| Acute GVHD Grade II–IV at Day 100 | 53% | (40–70) |
|
| ||
| Acute GVHD Grade III–IV at day 100 | 22% | (13–38) |
|
| ||
| Chronic GVHD at 1 year | 18% | (9–33) |
|
| ||
| Day 100 TRM | 14% | (7–28) |
|
| ||
| 1-year TRM | 31% | (20–47) |
|
| ||
| CMV reactivation (patients at risk) | 54% | (40–72) |
|
| ||
| Progression at 2 years | ||
| Overall | 26% | (17–42) |
| AML in CR1 or CR2 | 18% | (5–64) |
| AML > CR2 | 31% | (18–53) |
|
| ||
| PFS at 2 years | ||
| Overall | 26% | (15–39) |
| AML in CR1 or CR2 | 36% | (11–63) |
| AML > CR2 | 24% | (11–40) |
|
| ||
| OS at 2 years | ||
| Overall | 33% | (20–46) |
| AML in CR1 or CR2 | 36% | (11–63) |
| AML > CR2 | 31% | (16–48) |
Risk factors analysis for the rate of acute GVHD and TRM were assessed in 46 patients who engrafted. Patient age, sex, donor/recipient sex mismatch, diagnosis, disease status at transplant, and conditioning regimen were found not to impact the rate of acute GVHD II–IV and III/IV, OS, progression, or TRM (Supplemental table 1). The impact of graft source on the rate of acute GVHD was examined in the MRD cohort. Excluding patients with early deaths and delayed engraftment, our data showed a higher incidence of acute GVHD grade II–IV following receipt of PB, however, this did not reach statistical significance (56% vs. 40%, HR= 1.1, p=0.9, 95% C.I. 0.2–5.9), Table III. The impact of ATG was examined in the MUD BM cohort (n=30 patients at risk; 20 ATG/10 no ATG). The cumulative incidence of acute GVHD grade II–IV was higher for those patients who did not receive ATG (80% vs. 36%, HR=2.8, p=0.03, 95% CI 1.1–7.5). Table III. There was a trend toward decreased 2-year NRM in the ATG group (25% vs 40%, HR=0.4) though this was not statistically significant (p=0.25). Table III.
Table III.
Cumulative Incidence of Grade II–IV and III–IV acute GVHD at Day 100, and 1-Year NRM in Matched Siblings who received PB versus BM and Matched Unrelated Donors who did or did not receive ATG*.
| N=44* | %Cumulative Incidence | Hazard Ratio (95% CI) | p-value |
|---|---|---|---|
|
| |||
| Acute GVHD II–IV | |||
| MRD PB (n=9) | 56% (31–100) | 1.1 (0.2–5.9) | 0.9 |
| MRD BM (n=5) | 40% (14–100) | Ref. | |
|
| |||
| Acute GVHD III–IV | |||
| MRD PB | 11% (2–70) | N/E | 0.5 |
| MRD BM | 0% | ||
|
| |||
| Acute GVHD II–IV | |||
| MUD BM ATG (n=20) | 36% (21–68) | Ref. | |
| MUD BM No ATG (n=10) | 80% (59–100) | 2.8 (1.1–7.5) | 0.03 |
|
| |||
| Acute GVHD III–IV | |||
| MUD BM ATG (n=20) | 10% (3–41) | Ref. | |
| MUD BM No ATG (n=10) | 50% (27–93) | 3.1 (0.8–11) | 0.1 |
|
| |||
| NRM 1-year | |||
| MUD BM ATG (n=20) | 25% (12–53) | 0.4 | 0.25 |
| MUD BM No ATG (n=10) | 40% (19–85) | Ref. | |
3 patients who experienced primary graft failure were removed from the analysis, 2 MUD BM recipients and 1 MRD BM recipient. 2 MUD patients who received PB and ATG were removed from the analysis of the effect of ATG.
For the entire cohort, the 2-year PFS and OS probabilities were 26% (95% C.I., 15–39) and 33% (95% C.I., 20–46), respectively. The PFS for patients with AML in complete remission 1 or 2 was 36% (95% C.I., 11–63). Table II.
Results of Matched-Control Analysis
A total of 133 patients received a RIC regimen with standard GVHD prophylaxis of tacrolimus and mini-dose MTX over the study period. Comparison of outcomes between this unmatched-cohort and the patients enrolled onto the phase 2 trial with post-transplant CY was performed prior to the matched cohort analysis. There was an increased risk of grades II–IV and III/IV acute GVHD and trends for worst NRM and OS in those who received post-transplant CY. Supplemental Table 2.
Matching then was performed for which matched controls were successfully identified for 37 of the 49 patients enrolled onto the protocol based on the matching algorithm detailed in the methods section. The exclusion of ATG in a portion of the MUD recipients enrolled onto the study resulted in the inability to identify a historical match in 11 of the 12 unmatched cases. The patient characteristics for the remaining 37 enrolled patients and 37 matched controls are shown in Table II, with the two groups having similar characteristics with the exception that PB grafts were more common in MRD’s receiving tacrolimus/MTX (14/15 patients) versus post-CY (9/15 patients). Among the matched controls, the cumulative incidence of Grade II–IV acute GVHD was comparable for recipients of RIC with the Bu/Flu and Flu/Melphalan regimens (20% versus 18%. HR=1.2, p=0.8, 95% CI 0.3–5.3). Furthermore, within the matched, control group, BuFlu and FluMel conditioning resulted in statistically comparable rates of PFS, progression, TRM, aGVHD III/IV, and cGVHD justifying the use of both conditioning regimens within the matched-control cohort. Supplemental Table 3.
The incidences of grade II–IV and III–IV acute GVHD were significantly higher in recipients of Post-CY when compared to matched patients who received a tacrolimus/MTX prophylaxis (Grade II–IV 46% vs. 19%, HR=2.8, p=0.02, 95% CI 1.1–6.8). This resulted in higher TRM at 2 years post-transplant (HR 3.3, 95% C.I., 1.1–8.5, p=0.035) and worse OS (HR 1.9; 95% CI 1.02–3.5, p=0.04) for patients receiving Post-Cy. Table IV; figure 1. The cumulative incidence of chronic GVHD and the rate of CMV reactivation did not differ while the median time to neutrophil and platelet engraftment was statistically higher in patients who received Post-CY as compared to matched-controls.
Table IV.
Outcomes of Matched-Cohort Analysis: Post-CY versus Tacrolimus/MTX
| Hazard Ratio (95% CI) | p-value | ||
|---|---|---|---|
|
| |||
| Median Follow-up (range) | |||
| Post-Cy | 37 months (24–43) | ||
| Tacrolimus/MTX | 20 months (12–63) | ||
|
| |||
| Acute GVHD II–IV | |||
| Post-CY | 46% (32–65) | 2.8 (1.1–6.8) | |
| Tacrolimus/MTX | 19% (10–37) | Ref. | 0.02 |
|
| |||
| Acute GVHD III–IV | |||
| Post-CY | 14% (6–32) | ||
| Tacrolimus/MTX | 0% (2–70) | N/E | 0.02 |
|
| |||
| Chronic GVHD at 1 year | |||
| Post-CY | 20% (10–39) | 1.0 (0.4–2.8) | |
| Tacrolimus/MTX | 22% (12–40) | Ref. | 0.9 |
|
| |||
| Day-100 TRM | |||
| Post-CY | 11% (4–27) | 2.1 (0.4–11.6) | |
| Tacrolimus/MTX | 5% (1–21) | Ref. | 0.4 |
| 2-Year TRM | |||
| Post-CY | 35% (23–54) | 3.3 (1.1–8.5) | |
| Tacrolimus/MTX | 13% (6–30) | Ref. | 0.035 |
|
| |||
| Day-100 CMV-Reactivation | |||
| Post-CY | 1.3 (0.6–2.6) | ||
| Tacrolimus/MTX | Ref. | 0.5 | |
|
| |||
| Progression at 2-years | |||
| Post-CY | 35% (23–54) | 0.9 (0.4–1.9) | |
| Tacrolimus/MTX | 44% (30–63) | Ref. | 0.8 |
|
| |||
| PFS at 2 years | |||
| Post-CY | 22% (10–36) | 1.6 (0.9–2.8) | |
| Tacrolimus/MTX | 43% (27–58) | Ref. | 0.1 |
|
| |||
| OS at 2 years | |||
| Post-CY | 30% (16–45) | 1.9 (1.02–3.2) | |
| Tacrolimus/MTX | 52% (35–67) | Ref. | 0.04 |
Figure 1.
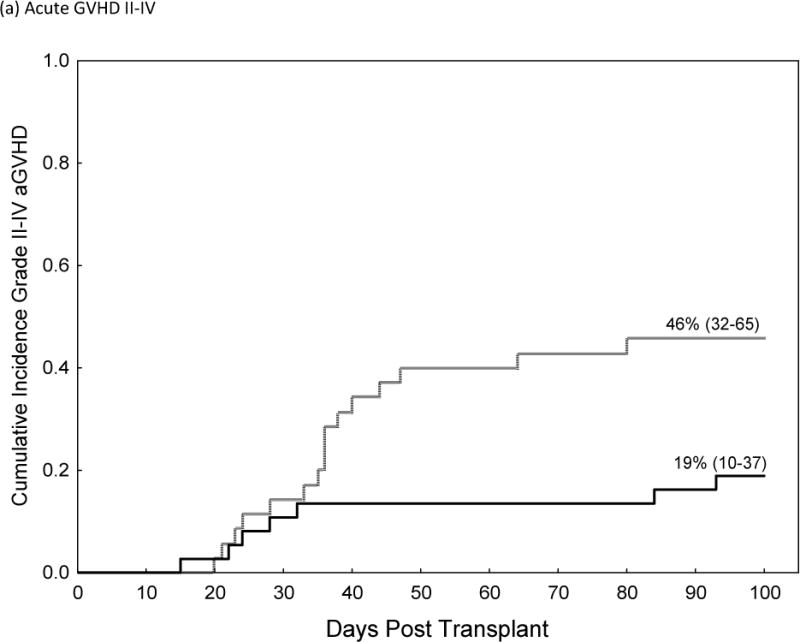
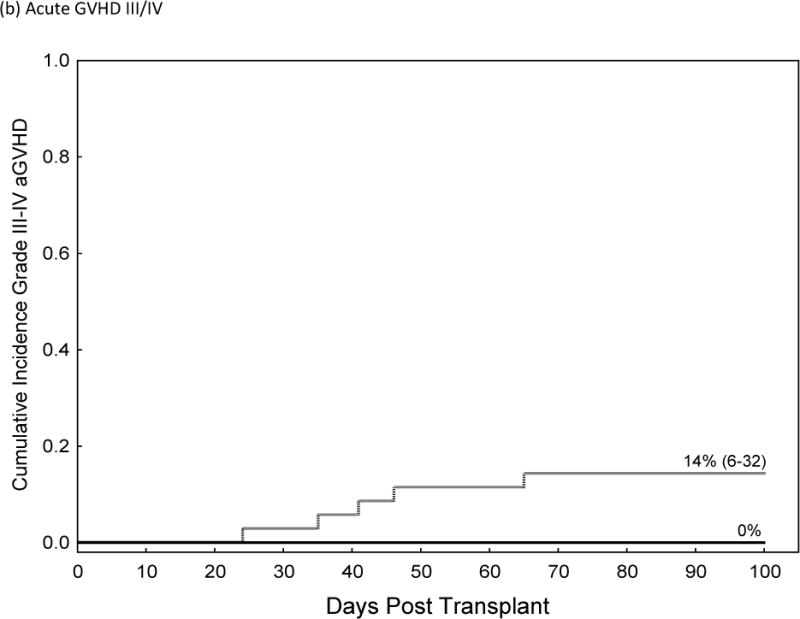
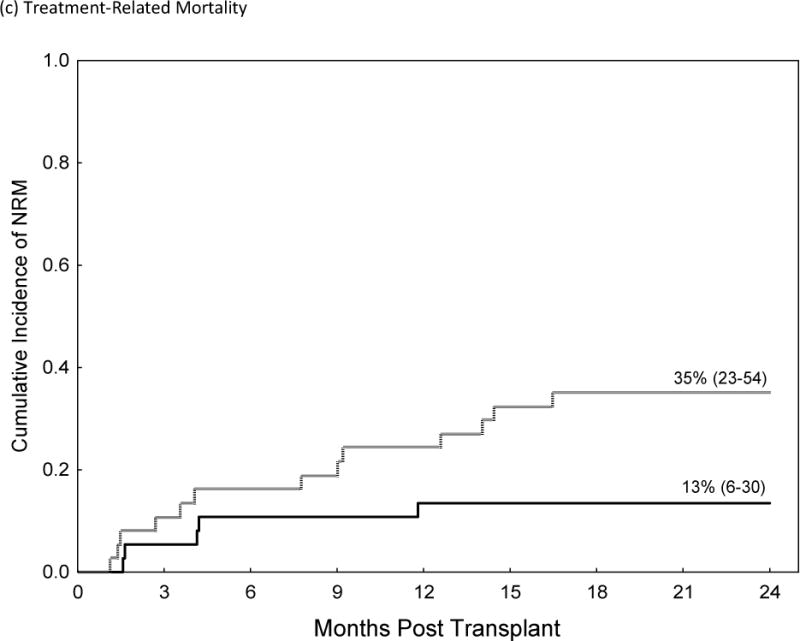
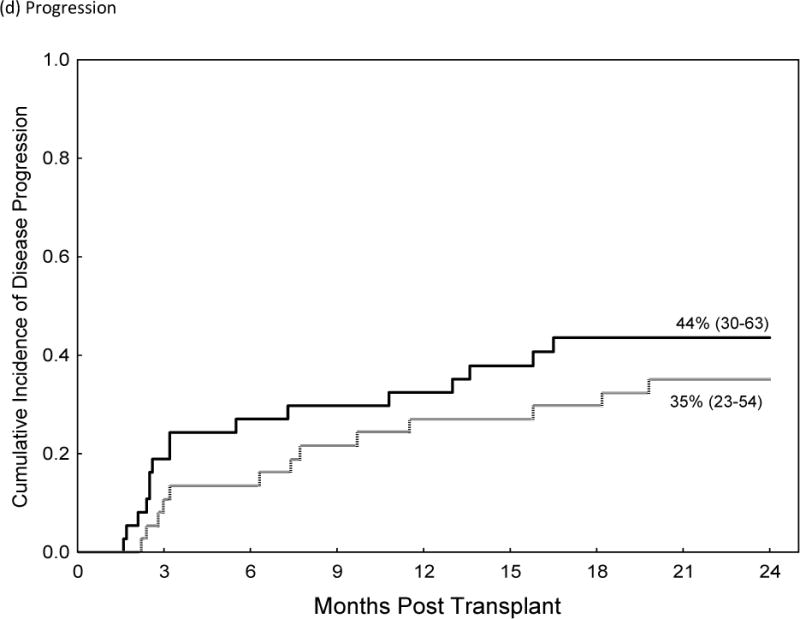
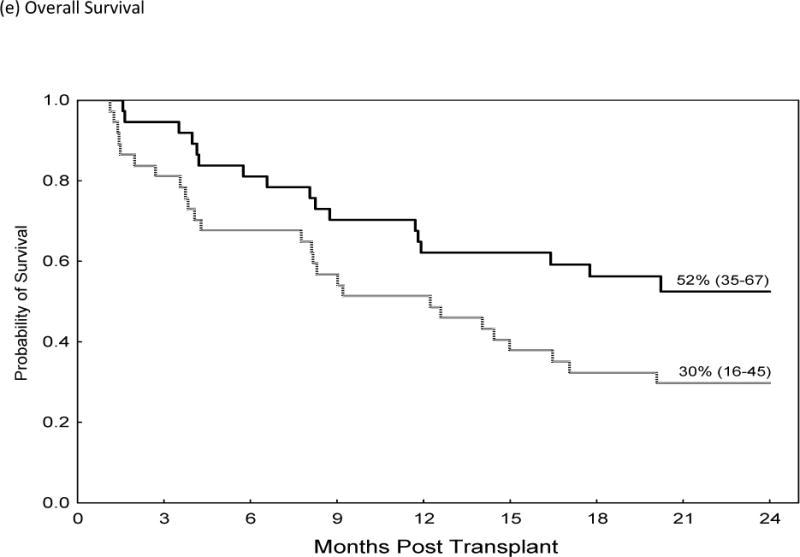
Comparison of Acute GVHD II–IV (a), Acute GVHD III/IV (b), Non-Relapse Mortality (c), Relapse (d) and Over-all Survival (e)in Recipients of GVHD Prophylaxis with Post-Cy (hashed line) and Tacrolimus/Methotrexate (solid line) following RIC allogeneic transplants from MRD and MUD’s
DISCUSSION
Improvement in GVHD prophylaxis can be realized by either a reduction in the incidence and severity of acute GVHD or a reduction in complications associated with immunosuppressive therapy. Ultimately the goal is to achieve long-term disease-free survival with full immune reconstitution without acute or chronic GVHD. Risks for GVHD related complications differ based on patient factors including age and sex-match as well as treatment factors such as conditioning regimen and donor/graft source. In this trial, we sought to determine whether post-CY would be effective in patients receiving RIC and have any advantages compared to use of standard CNI- based GVHD prophylaxis. We found that in the RIC setting, the post- CY regimen was associated with a higher rate of acute GVHD, TRM and inferior PFS and OS compared to matched controls.
The results of this phase II trial have furthered our knowledge of Post-CY GVHD prophylaxis in many important ways. First, the patient population and the intensity of the conditioning regimen may be important. While original reports have suggested post-Cy GVHD prophylaxis results in similar rates of acute GVHD as CNI- based prophylaxis in patients receiving myeloablative conditioning6, results of our matched-cohort analysis suggest that Post-CY is less effective in the RIC setting involving older or medically infirm patients. The rate of grade II–IV acute GVHD was more than 2-fold higher than CNI-based prophylaxis. Higher rates of acute GVHD were associated with poorer TRM and OS with no associated improvement in rates of progression in this patient population. Better than expected outcomes in our matched-control cohort may have accounted for the inferior results seen with the post-CY group; however, we feel this is unlikely to be the case. First, an unmatched analysis (using the total 133 controls) demonstrated consistent results as those obtained in the matched-analysis. Supplemental Table II. Secondly, the rate of acute GVHD seen in our control group is similar to the published rates seen in a recent multicenter trial which employed a similar conditioning regimen and CNI/MTX in older-aged patients with AML (NCT00070135)17, a recent study by Alyea et al, and published studies from our institution18, 19.
Also differing from the earlier reports, in our study, we did not see a reduction in the rate of chronic GVHD with the post-CY regimen. It is possible that the high rate of acute GVHD and/or the resultant need for initiation of a CNI may have negated the apparent protection from chronic GVHD, which has been previously reported with the CNI-free, post-CY prophylaxis regimen2–6. Additionally, the predominant use of bone marrow grafts in the earlier reports may account for the lower rates of chronic GVHD.
Two additional areas of debate with respect to GVHD prevention include the use of ATG and bone marrow versus peripheral blood progenitor cells as the graft source. Registry data from Soiffer et al. suggest that the use of ATG may have detrimental overall effect when RIC is employed20. While the use of ATG in a subset of MUD recipients may be one explanation for differences in our outcomes when compared to the original reports of Post-CY6, we feel this is unlikely to be the case. A comparison of outcomes for MUD recipients with or without receipt of ATG found that the use of ATG resulted in a statistically lower (not higher) rate of acute GVHD (Table III). It is possible that ATG may have negatively impacted progression and OS, as suggested by Soiffer et al, but our numbers are too small to allow for an analysis accounting for disease type and remission status to examine this question. Thus, the role of ATG with post-Cy GVHD prophylaxis should be further evaluated. Additionally, bone marrow was the sole graft source for patients in the original Post-Cy publication6. We sought to determine whether PB could also be employed with Post-CY. PB remains the most common graft source used for MRD transplants making it important to determine if this source can be employed with the post-CY strategy. Our data showed a trend to a higher rate of acute GVHD grade II–IV following PB transplants, however, this did not reach statistical significance in this relatively small study. The results suggest caution should be used when using PB grafts with post-CY.
The question remains why outcomes were worse for recipients of Post-CY following our RIC with Bu/Flu when compared with matched-controls who received CNI/MTX prophylaxis. First, it is important to note that this observation was seen with the RIC Bu/Flu RIC regimen, and while the impact of post-Cy on other RIC regimens can be inferred, it cannot be definitively concluded. It is possible that a more ablative regimen is needed to result in significant alloreactivity at the time of infusion of CY on days +3 and +4. However, recent work has suggested Post- CY may not work through ablation of alloreactive T-cells (as originally suggested in mouse models) but through preferential sparing of suppressive T-cells (regulatory T-cells)7. If this is true, it is unclear why a reduction of conditioning intensity would be detrimental to this proposed mechanism.
A multi-center trial by Kanakry evaluating GVHD prophylaxis with post-CY following myeloablative conditioning with Bu (targeting an AUC of roughly 5000 micromolar-minute) and Flu has recently been reported.9 It should be noted that the rates of grade II–IV aGVHD (51%), grade III–IV aGVHD (15%), and chronic GVHD (14%) reported in the Kanakry trial were comparable to the rates of GVHD in our trial of (53%), (22%), and (18%), respectively. In contrast, an impressive PFS and OS of 62% and 67% at 2 years was seen in the Kanakry publication compared to a PFS/OS in our study of 26%, and 33% at 2 years, likely reflecting differences in the patient populations in each study. The Kanakry study, involved a younger population, with median age of 49 years, fit for a myeloablative regimen (HCT-CI was not reported). Our cohort of patients were older-aged, with median age of 62 years and had a high rate of co-morbidities (median HCT-CI of 3), necessitating only a RIC regimen. The rates of relapse were slightly lower in the Kanakry study (cumulative incidence relapse 22% vs 26% in our study), but the TRM was significantly higher in our study (31% vs 16%), suggesting that the older, medically infirm patients were less capable to tolerate the aGVHD that occurred. A study by Sorror et al demonstrated increased rates of TRM with aGVHD in older patients and those with a higher co-morbidity index21, 22. This suggests that in our study population, GVHD occurring in patients with advanced age and co-morbidities resulted in high TRM and is likely to account for the differing results from those seen in other reports of post-CY.
The Blood and Marrow Transplant Clinical Trials Network (BMTCTN) will be conducting a three-arm study of novel GVHD prophylaxis strategies following RIC, T-cell replete, matched donor transplants. Due to concerns with the use of Post-CY prophylaxis in the RIC setting, one treatment-arm will test post- Cy combined with a CNI and mycophenolate mofetil (MMF), a strategy which has been shown to be successful in RIC and NMA haploidentical hematopoietic transplantation23, 24.
In conclusion, use of post- CY has been proposed as a CNI-free regimen which could avoid ongoing post- transplant immunosuppressive therapy. In a matched control analysis, we found that post-CY when combined with RIC was inferior compared to matched controls receiving tacrolimus-methotrexate for prevention of grades 2–4 and 3–4 acute GVHD, resulting in poorer treatment-related and overall survival. Our trial suggests that traditional GVHD prophylaxis with a CNI/MTX should be employed rather than post- CY when performing RIC transplant from a HLA- matched related or unrelated donor. Post- CY combined with a CNI/MMF will be studied as alternative strategy in an upcoming BMTCTN trial.
Supplementary Material
Highlights.
We report the results of a phase 2 trial of GVHD prophylaxis utilizing Post-Cy
Patients were treated with the reduced-intensity Bu/Flu regimen
Outcomes were compared to a matched cohort who received standard GVHD prophylaxis
The Post-Cy group had higher acute GVHD rates and subsequent worse survival
Post-Cy should be avoided in patients ineligible for myeloablative conditioning
Acknowledgments
AA and RC designed and performed the research, enrolled and cared for patients, collected, analyzed and interpreted data and wrote the manuscript; RS analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; BA and UP designed and performed the research, enrolled and cared for patients; CH, RJ, ES, IK, MQ, YN, NS, SA, BO, GA, SC and PK enrolled and cared for patients; JB, JC and GR collected, analyzed and performed statistical analysis.
FINANCIAL DISCLOSURE STATEMENT
Borje Andersson receives Consulting for Otsuka America Research, Inc. Uday Popat, Yago Nieto and Richard Champlin received research support from Otsuka America Research, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alousi AM, Bolanos-Meade J, Lee SJ. Graft-versus-host disease: state of the science. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2013;19(1 Suppl):S102–108. doi: 10.1016/j.bbmt.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature medicine. 1999;5(11):1298–1302. doi: 10.1038/15256. e-pub ahead of print 1999/11/05. [DOI] [PubMed] [Google Scholar]
- 3.Fischer AC, Ruvolo PP, Burt R, Horwitz LR, Bright EC, Hess JM, et al. Characterization of the autoreactive T cell repertoire in cyclosporin-induced syngeneic graft-versus-host disease. A highly conserved repertoire mediates autoaggression. Journal of immunology (Baltimore, Md: 1950) 1995;154(8):3713–3725. e-pub ahead of print 1995/04/15. [PubMed] [Google Scholar]
- 4.Beschorner WE, Hess AD, Shinn CA, Santos GW. Transfer of cyclosporine-associated syngeneic graft-versus-host disease by thymocytes. Resemblance to chronic graft-versus-host disease. Transplantation. 1988;45(1):209–215. doi: 10.1097/00007890-198801000-00043. e-pub ahead of print 1988/01/01. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins MK, Schwartz RH, Pardoll DM. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988;241(4873):1655–1658. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]
- 6.Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. doi: 10.1126/scitranslmed.3006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunologic research. 2010;47(1–3):65–77. doi: 10.1007/s12026-009-8139-0. e-pub ahead of print 2010/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Statistics in medicine. 1995;14(4):357–379. doi: 10.1002/sim.4780140404. e-pub ahead of print 1995/02/28. [DOI] [PubMed] [Google Scholar]
- 11.Thall PF, Simon RM, Estey EH. New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1996;14(1):296–303. doi: 10.1200/JCO.1996.14.1.296. e-pub ahead of print 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 12.Popat URBR, Chen J, Alousi AM, Anderlini P, Ciurea SO, Hosing C, Jones RB, Kebriaei P, Khouri IF, Konoplev S, De Lima M, Nieto Y, Oran B, Qazilbash MH, Rondon G, Shpall EJ, Verstovsek S, Andersson B, Champlin RE. Allogeneic Transplantation for Myelofibrosis: Benefit of Dose Intensity. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(suppl) abstr 7011. [Google Scholar]
- 13.Mohty M, Malard F, Blaise D, Milpied N, Furst S, Tabrizi R, et al. Reduced-toxicity conditioning with fludarabine, once-daily intravenous busulfan, and antithymocyte globulins prior to allogeneic stem cell transplantation: Results of a multicenter prospective phase 2 trial. Cancer. 2014 doi: 10.1002/cncr.29087. [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 15.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. The New England journal of medicine. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devine SM, Owzar K, Blum W, DeAngelo D, Stone RM, Hsu JW, et al. A Phase II Study of Allogeneic Transplantation for Older Patients with AML in First Complete Remission Using a Reduced Intensity Conditioning Regimen: Results From CALGB 100103/BMT CTN 0502. ASH Annual Meeting Abstracts. 2012;120(21):230. [Google Scholar]
- 18.Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105(4):1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 19.de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104(3):857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 20.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. e-pub ahead of print 2011/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorror ML, Martin PJ, Storb RF, Bhatia S, Maziarz RT, Pulsipher MA, et al. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood. 2014;124(2):287–295. doi: 10.1182/blood-2014-01-550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML, Storb RF, Sandmaier BM, Maziarz RT, Pulsipher MA, Maris MB, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(29):3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. e-pub ahead of print 2008/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18(12):1835–1844. doi: 10.1016/j.bbmt.2012.07.003. e-pub ahead of print 2012/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


