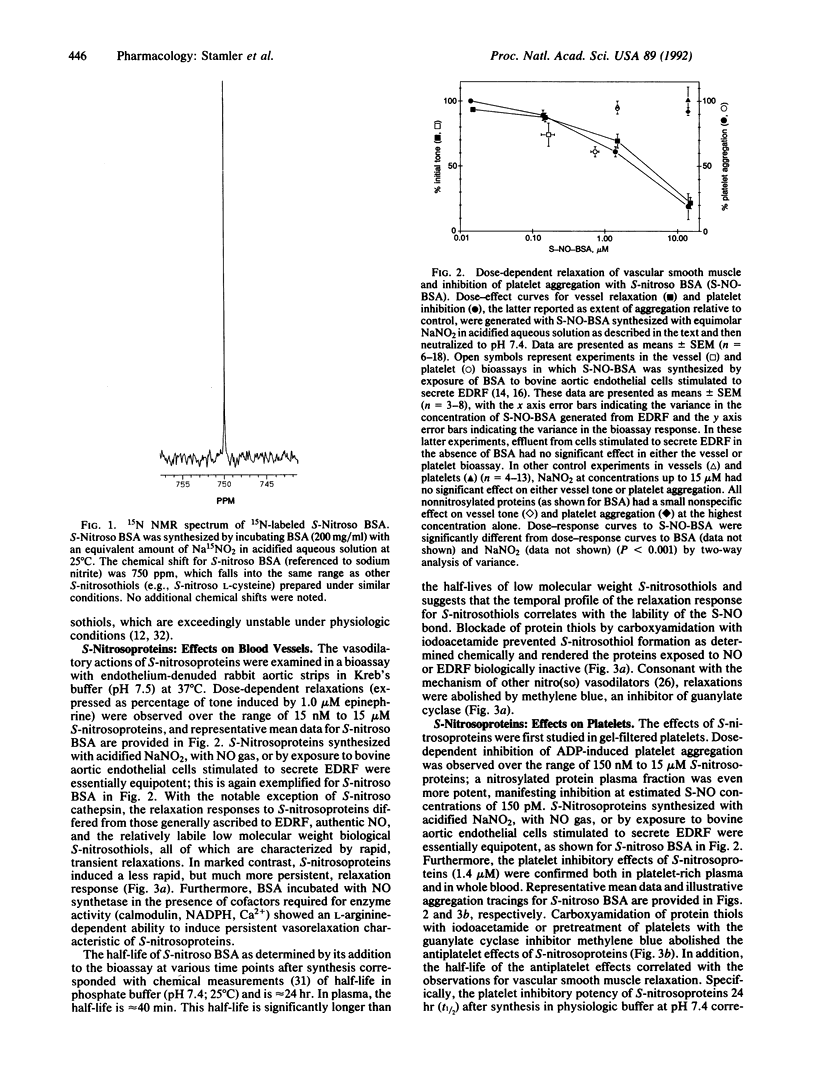
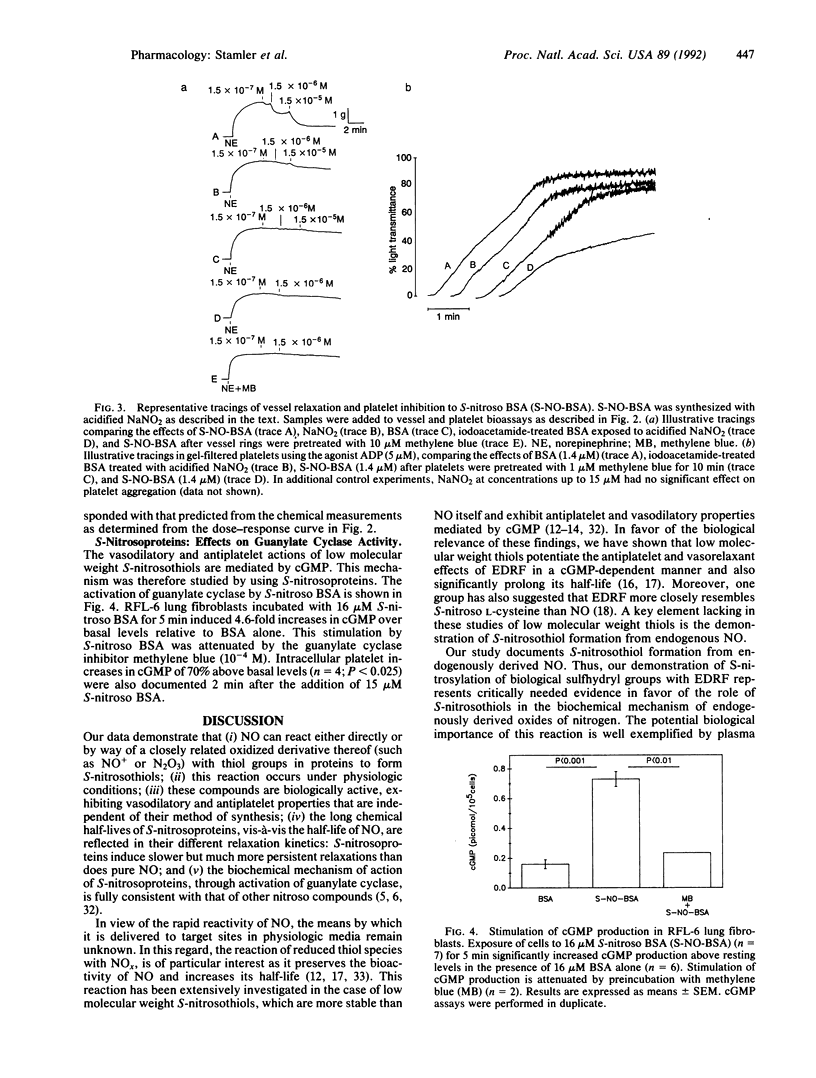
Abstract
Endothelium-derived relaxing factor (EDRF) activity has been attributed to the highly labile nitric oxide radical (NO). In view of the fact that the plasma and cellular milieux contain reactive species that can rapidly inactivate NO, it has been postulated that NO is stabilized by a carrier molecule that preserves its biological activity. Reduced thiol species are candidates for this role, reacting readily in the presence of NO to yield biologically active S-nitrosothiols that are more stable than NO itself. Because sulfhydryl groups in proteins represent an abundant source of reduced thiol in biologic systems, we examined the reaction of several sulfhydryl-containing proteins of diverse nature and function upon exposure to authentic NO and EDRF. We demonstrate that S-nitroso proteins form readily under physiologic conditions and possess EDRF-like effects of vasodilation and platelet inhibition. These observations suggest that S-nitrosothiol groups in proteins may serve as intermediates in the cellular metabolism of NO and raise the possibility of an additional type of cellular regulatory mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett B. M., Kobus S. M., Brien J. F., Nakatsu K., Marks G. S. Requirement for reduced, unliganded hemoprotein for the hemoglobin- and myoglobin-mediated biotransformation of glyceryl trinitrate. J Pharmacol Exp Ther. 1986 May;237(2):629–635. [PubMed] [Google Scholar]
- Bonnett R., Holleyhead R., Johnson B. L., Randall E. W. Reaction of acidified nitrite solutions with peptide derivatives: evidence for nitrosamine and thionitrite formation from 15N N.m.r. studies. J Chem Soc Perkin 1. 1975;(22):2261–2241. doi: 10.1039/p19750002261. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S., Fung H. L. Thiol-mediated catalysis of nitroglycerin degradation by serum proteins. Increase in metabolism was not accompanied by S-nitrosothiol production. Drug Metab Dispos. 1990 Jan-Feb;18(1):61–67. [PubMed] [Google Scholar]
- Cooke J. P., Stamler J., Andon N., Davies P. F., McKinley G., Loscalzo J. Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am J Physiol. 1990 Sep;259(3 Pt 2):H804–H812. doi: 10.1152/ajpheart.1990.259.3.H804. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Restoration of the responsiveness of purified guanylate cyclase to nitrosoguanidine, nitric oxide, and related activators by heme and hemeproteins. Evidence for involvement of the paramagnetic nitrosyl-heme complex in enzyme activation. J Biol Chem. 1978 Dec 10;253(23):8433–8443. [PubMed] [Google Scholar]
- Davies P. F., Truskey G. A., Warren H. B., O'Connor S. E., Eisenhaure B. H. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J Cell Biol. 1985 Sep;101(3):871–879. doi: 10.1083/jcb.101.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Feinman R. D., Lubowsky J., Charo I., Zabinski M. P. The lumi-aggregometer: a new instrument for simultaneous measurement of secretion and aggregation by platelets. J Lab Clin Med. 1977 Jul;90(1):125–129. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Gorsky L. D., Pollock J. S., Ishii K., Schmidt H. H., Heller M., Murad F. Hormone-induced biosynthesis of endothelium-derived relaxing factor/nitric oxide-like material in N1E-115 neuroblastoma cells requires calcium and calmodulin. Mol Pharmacol. 1990 Jul;38(1):7–13. [PubMed] [Google Scholar]
- Hawiger J., Parkinson S., Timmons S. Prostacyclin inhibits mobilisation of fibrinogen-binding sites on human ADP- and thrombin-treated platelets. Nature. 1980 Jan 10;283(5743):195–197. doi: 10.1038/283195a0. [DOI] [PubMed] [Google Scholar]
- Hu L. Y., Abeles R. H. Inhibition of cathepsin B and papain by peptidyl alpha-keto esters, alpha-keto amides, alpha-diketones, and alpha-keto acids. Arch Biochem Biophys. 1990 Sep;281(2):271–274. doi: 10.1016/0003-9861(90)90443-3. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Keen J. H., Habig W. H., Jakoby W. B. Mechanism for the several activities of the glutathione S-transferases. J Biol Chem. 1976 Oct 25;251(20):6183–6188. [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo J. N-Acetylcysteine potentiates inhibition of platelet aggregation by nitroglycerin. J Clin Invest. 1985 Aug;76(2):703–708. doi: 10.1172/JCI112024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo J. Structural and kinetic comparison of recombinant human single- and two-chain tissue plasminogen activator. J Clin Invest. 1988 Oct;82(4):1391–1397. doi: 10.1172/JCI113743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane D. E., Mills D. C. Inhibition by ADP of prostaglandin induced accumulation of cyclic AMP in intact human platelets. J Cyclic Nucleotide Res. 1981;7(1):1–11. [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide: biosynthesis and biological significance. Trends Biochem Sci. 1989 Dec;14(12):488–492. doi: 10.1016/0968-0004(89)90181-3. [DOI] [PubMed] [Google Scholar]
- Marple D. N., Cassens R. G., Topel D. G., Christian L. L. Porcine corticosteroid-binding globulin: binding properties and levels in stress-susceptible swine. J Anim Sci. 1974 Jun;38(6):1224–1228. doi: 10.2527/jas1974.3861224x. [DOI] [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Myers C. B., Ohlstein E. H., Ballot B. A., Hyman A. L., Kadowitz P. J. Inhibition of human platelet aggregation by S-nitrosothiols. Heme-dependent activation of soluble guanylate cyclase and stimulation of cyclic GMP accumulation. Mol Pharmacol. 1983 May;23(3):653–664. [PubMed] [Google Scholar]
- Mordvintsev P. I., Rudneva V. G., Vanin A. F., Shimkevich L. L., Khodorov B. I. Ingibiruiushchee vliianie na agregatsiiu trombotsitov dinitrozil'nykh kompleksov zheleza s nizkomolekuliarnymi ligandami. Biokhimiia. 1986 Nov;51(11):1851–1857. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Osborne J. A., Lento P. H., Siegfried M. R., Stahl G. L., Fusman B., Lefer A. M. Cardiovascular effects of acute hypercholesterolemia in rabbits. Reversal with lovastatin treatment. J Clin Invest. 1989 Feb;83(2):465–473. doi: 10.1172/JCI113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Stamler J., Mendelsohn M. E., Amarante P., Smick D., Andon N., Davies P. F., Cooke J. P., Loscalzo J. N-acetylcysteine potentiates platelet inhibition by endothelium-derived relaxing factor. Circ Res. 1989 Sep;65(3):789–795. doi: 10.1161/01.res.65.3.789. [DOI] [PubMed] [Google Scholar]
- Umemoto A., Monden Y., Tsuda M., Grivas S., Sugimura T. Oxidation of the 2-hydroxyamino derivative of 2-amino-6-methyl-dipyrido[1,2-a: 3',2'-d] imidazole (Glu-P-1) to its 2-nitroso form, an ultimate form reacting with hemoglobin thiol groups. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1326–1331. doi: 10.1016/s0006-291x(88)80507-2. [DOI] [PubMed] [Google Scholar]
- Yeates R. A., Laufen H., Leitold M. The reaction between organic nitrates and sulfhydryl compounds. A possible model system for the activation of organic nitrates. Mol Pharmacol. 1985 Dec;28(6):555–559. [PubMed] [Google Scholar]



