Abstract
Objectives:
Daucus carota L.(DC) commonly known as carrot, folkorically used as ethnomedicine to treat nephrosis and other urinary disorders. Hence, the present study was aimed to investigate the nephroprotective effects of ethanolic root extract of DC against gentamicin-induced nephrotoxicity in Albino Wistar rats.
Methods:
Nephrotoxicity in rats was induced by intraperitoneal administration of gentamicin (100 mg/kg/day) for 8 days. Rats of either sex were divided into four groups (n = 6). Group 1 served as control that received normal saline (i.p.) whereas Group 2 (GM) was treated with gentamicin which served as gentamicin-intoxicated group. Group 3–4 (DC200, DC 400) were pretreated with DC at doses of 200 mg/kg and 400 mg/kg (p.o.), respectively, 1 h before the gentamicin intoxication. Following treatment, the nephroprotective effects of DC were evaluated by using serum levels of urea, blood urea nitrogen (BUN), uric acid, and creatinine levels; change in body weight and wet kidney weight along with the histological observations among the experimental groups.
Results:
Gentamicin intoxication induced elevated serum urea, BUN, uric acid, and creatinine levels which was found to be significantly (P < 0.01) decreased in a dose-dependent manner in groups received DC which was also evidenced by the histological observations.
Conclusion:
DC showed a significant nephroprotective effect in a dose-dependent manner by ameliorating the gentamicin-induced nephrotoxicity and thus authenticates its ethnomedicinal use.
KEY WORDS: Carrot, ethnomedicine, gentamicin, nephroprotective, nephrosis
Introduction
Environmental pollutants, chemicals, and drugs such as antibiotics can drastically alter the anatomical and physiological functions of various organs such as kidney, heart, liver, and intestine.[1] However, drugs such as antibiotics became the major implicating factor in the acute kidney injury due to indigenous functions of kidney to excrete them. This acute renal injury often leads to renal failure which in turn associated to other pathological manifestations such as sepsis, cardiovascular disorders, and diabetes.[2]
Aminoglycoside antibiotics are clinically effective in the treatment of Gram-negative bacterial infections, but they more commonly implicate the nephrotoxicity by free radical generation, loss of brush-border integrity, acute tubular necrosis, and glomerular congestion, which ultimately leads to reduced glomerular filtration rate and acute renal dysfunction.[3,4,5,6,7] However, several pharmacological interventions such as antibiotics, calcium channel blockers, beta blockers, iNOS inhibitors, nitric oxide precursors, antianginal, hormones, antiplatelet, statins, peroxisome proliferator-activated receptor gamma agonists, tumor necrosis factor alpha synthesis inhibitors, biguanides, antioxidants, and super oxygen dismutase mimetics had the potential to halt the progression of gentamicin-induced nephrotoxicity, but their clinical setting needs to be debated.[8]
Daucus carota L.(DC) is a root vegetable commonly called as carrot, which is a biennial plant commonly cultivated in Northern India. The most abundant phytonutrients present in carrot are phenolics, polyacetylenes, carotenoids, ascorbic acid, and tocopherol.[9] It was documented that this plant had the potentials of hypotension,[10] antifertility,[11] hepatoprotective,[12] antispasmodic,[13] antibacterial,[14] monoamine oxidase inhibition,[15] and cyclooxygenase enzyme inhibition.[16] However, traditionally, it was used in the treatment of nephrosis as a nephroprotective agent.[17] However, so far, no scientific validity has been made to establish it as a nephroprotective agent. Hence, in the present study, an effort has been made to authenticate its traditional use by using ethanolic root extract of DC against gentamicin-induced nephrotoxicity in Albino Wistar rats.
Materials and Methods
Chemicals and Reagents
Gentamicin was procured as marketed formulation (Ranbiotic) from Ranbaxy laboratories (Batch no. 9758675), New Delhi, India. The diagnostic kits for the estimation of uric acid (Lot no. B12121) and blood urea nitrogen (BUN) (Lot no. B041328) were obtained from ERBA Mannheim, Transasia bio-medicals limited, Himachal Pradesh, India, and the diagnostic kits of Urea (Batch no. EUB-007) and Creatinine (Batch no. ECK-087) were obtained from Excel diagnostics, Hyderabad, India. All the other required chemicals were of analytical grade and they were obtained from Qualigen fine chemicals, Mumbai, India.
Experimental Animals
The experimental protocol was carried out by using Albino Wistar rats of both sex weighing about 180–220 g procured from Sri Venkateshwara Enterprises, Bengaluru, India, and 1-week acclimatization was done as per CPCSEA guidelines before the study was carried out. Animals were housed in clean polypropylene cages and they were fed with standard pellet diet and water ad libitum during the study. This experimental study was approved by Institutional Animal Ethical Committee of Sree Vidyanikethan College of Pharmacy, Tirupati, India, with the approval no. SVCP/IAEC/I-006/2013-14.
Plant Material
The fresh roots of DC were obtained from the local market of Tirupati, Andhra Pradesh, India, in the month of May, 2014, and they were authenticated by Prof. P. Jayaraman at the Plant Anatomy Research Centre (PARC), Chennai, India. A voucher specimen of this plant material was deposited in the herbarium of PARC with voucher specimen no. PARC/2014/2290.
Extraction
The roots were shade dried, powdered, and sieved (mesh no. 40) to get coarse powder. Then, this powdered plant material was subjected to soxhelation using absolute ethanol as solvent for 72 h at a temperature of 40°C. After filtration, it was evaporated by using rotary vacuum evaporator at a temperature not exceeding 40°C to get crude extract. The yield was found to be 16.9% w/w.
Preliminary Phytochemical Analysis
A preliminary phytochemical analysis was carried out for the qualitative identification of phytoconstituents in DC.[18,19]
Acute Oral Toxicity Studies
Acute oral toxicity study was carried out in accordance of OECD guidelines No. 423 by using female Wistar rats. Four dosing levels (5, 50, 300, and 2000 mg/kg) (p.o.) were considered to carry out acute oral toxicity study. Three animals were selected for single escalating testing dose to observe the signs of toxicity and mortality for a period of 14 days.[20]
Gentamicin-Induced Nephrotoxicity
Twenty-four healthy Albino Wistar rats of either sex were weighed and grouped randomly into four groups (n = 6). Group 1 served as normal control (CON) receiving normal saline (i.p.) and 0.5% carboxymethyl cellulose (CMC) orally for 8 days whereas group 2 considered as gentamicin-intoxicated group (GM) receiving gentamicin (i.p.) at a dose of 100 mg/kg/day and 0.5% CMC (p.o.) for 8 days. Group 3 (DC200) and group 4 (DC 400) were considered as treatment groups that received DC at doses of 200 mg/kg/day and 400 mg/kg/day (p.o.), respectively, an hour before gentamicin intoxication for a period of 8 days.[1]
Evaluation of Nephroprotective Potential
Biochemical analysis
After 24 h of experimental protocol, the animals were euthanized by cervical dislocation under mild ether anesthesia and blood was collected by cardiac puncture. Then, the blood was made to coagulate by leaving them undisturbed for 1 h at 4°C and it was centrifuged (REMI, R-8°C laboratory centrifuge) at 3000 rpm for 15 min to separate serum. The serum was stored at -5°C until the analysis of serum urea, BUN, uric acid, and creatinine using diagnostic kits.
Change in body weight and wet kidney weight
At the final day of the experimental procedure, the body weights were measured to evaluate the change in the body weight from the initial body weight.[21,22] After the blood collection, the kidneys were excised, weighed, and the result was expressed as wet kidney weight/100 g of body weight to assess the change in kidney weight among the experimental groups.[23]
Histopathology
The excised kidneys from sacrificed animals were fixed in 10% neutral formalin solution immediately for a period of 24 h. Then, they were processed for dehydration using absolute ethanol, cleaned in xylene, and embedded in paraffin. The sectioning was made by using microtome apparatus at a thickness of 4 µm and stained with eosin and hematoxylin. The histopathology changes of each section were observed and photographed (×45) by using light microscope equipped with digital camera (OLYMPUS BX51TRF, China).
Statistical Analysis
The results were expressed as mean ± standard error of mean (n = 6) and the statistical analysis was made by one-way ANOVA followed by Dunnett's comparison test using computerized Graphpad prism (version 5.0, trial version, Graphpad soft ware, USA) software at a level of significance ofP < 0.01.
Results
Preliminary Phytochemical Analysis
The preliminary qualitative phytochemical analysis revealed the presence of carbohydrates, aminoacids, polyphenols, terpenoids, and phytosterols.
Acute Oral Toxicity Study
It was observed that there were no clinical signs of toxicity and mortality for a period of 14 days at a testing dose of 2000 mg/kg. As a result of this, one-tenth of the maximum tolerated dose of DC was selected as therapeutic low dose (200 mg/kg, DC200) and double of this low dose was considered as highest dose (400 mg/kg, DC400) for this study.[1]
Biochemical Analysis
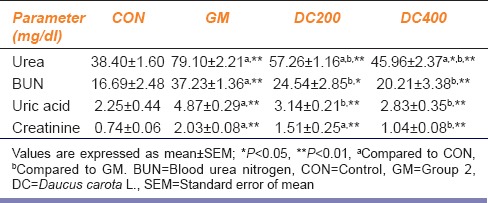
Gentamicin intoxication in GM group significantly (P < 0.01) increased the serum levels of urea, BUN, uric acid, and creatinine compared to CON group. However, with the co-administration of DC at doses of 200 mg/kg/day and 400 mg/kg/day, the elevated serum urea, BUN, uric acid, and creatinine levels were curtailed to a great extent in DC200 and DC400 groups in a dose-dependent fashion [Table 1].
Table 1.
Effect of Daucus carota L. on serum levels of urea, blood urea nitrogen, uric acid, and creatinine in gentamicin-induced nephrotoxicity in Wistar rats
Effect on Change in Body Weight and Wet Kidney Weight
At the end of the study, gentamicin intoxication made a significant (P < 0.01) weight loss as compared with CON group and the concurrent treatment of DC ablated the gentamicin-induced weight loss significantly in DC200 and DC400 [Figure 1]. On the other hand, the wet kidney weight of GM group significantly increased (P < 0.01) compared with CON group [Figure 2]. However, the treatment curtails the gentamicin-induced renal weight gain significantly in DC200 and DC400 groups.
Figure 1.
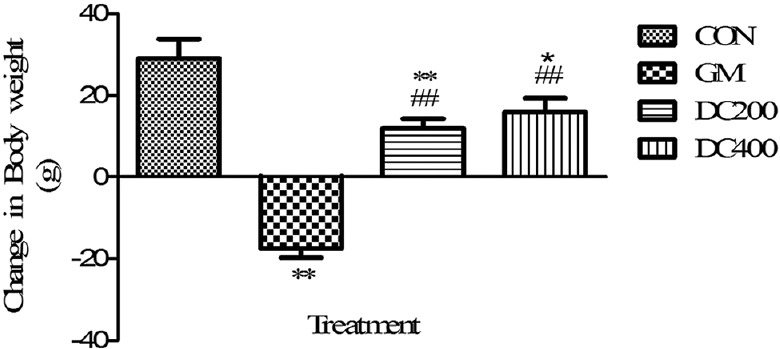
Effect of Daucus carota L. on body weight of gentamicin-intoxicated rats; data were expressed as mean ± standard error of mean (n = 6); **P < 0.01 versus control, *P < 0.05 versus control, ##P < 0.01 versus GM
Figure 2.
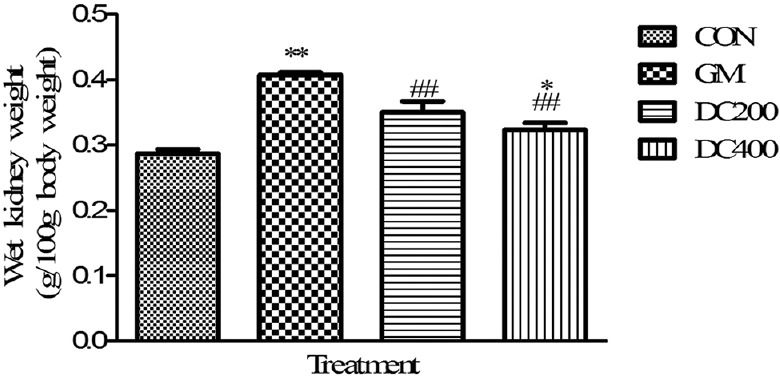
Effect of Daucus carota L. on wet kidney weight of gentamicin-intoxicated rats; data were expressed as mean ± standard error of mean (n = 6); **P < 0.01 versus control, *P < 0.05 versus control, ##P < 0.01 versus GM
Histopathology
The histopathology changes of each kidney section were interpreted in terms of presence of hyaline casts, glomerular congestion, mononuclear cells infiltration, tubular necrosis and degeneration, and intertubular hemorrhage microscopically and they were depicted in Table 2. The microscopical investigation of CON group revealed the normal renal tubular morphology with intact glomerulus [Figure 3], whereas the GM showed the majority of the histopathological events of gentamicin intoxication [Figure 4]. It was observed that treatment groups of DC remarkably ameliorate the gentamicin-induced acute renal damage manifestations in histology [Figures 5 and 6]. Indeed, DC400-treated group showed normal renal morphology even comparable to that of CON group with slight intertubular hemorrhage and sporadic hyaline casts.
Table 2.
Effect of Daucus carota L. on kidney histopathological changes induced by gentamicin-induced nephrotoxicity in Wistar rats
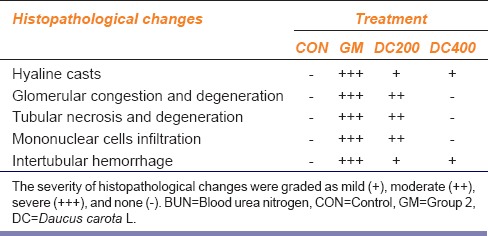
Figure 3.
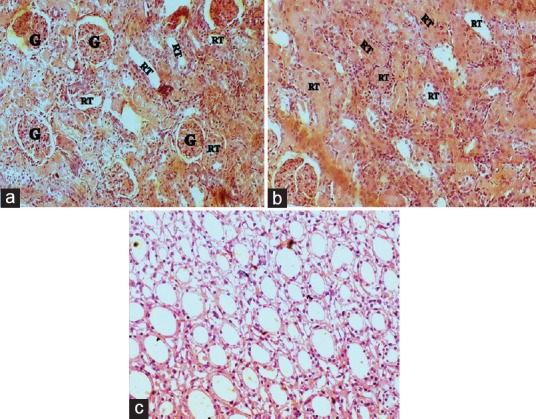
Kidney histopathology of control group (x45): A, B-cortex showing intact glomerulus (g)and normal renal tubular (rt) morphology; C-medulla showing normal renal morphology of collecting ducts
Figure 4.
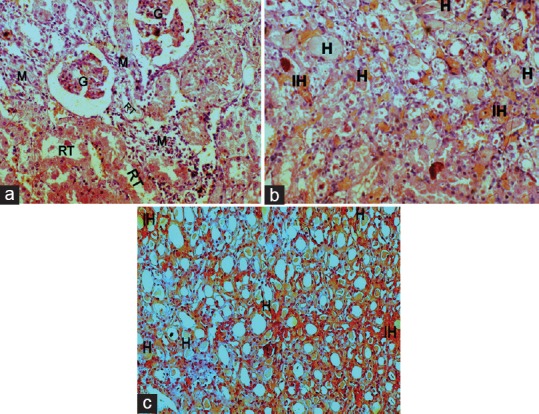
Kidney histopathology of gentamicin-intoxicated group (x45): A, B-cortex showing glomerular degeneration and atrophy (g), mononuclear cells infiltration (m), renal tubular desquamation (rt), presence of hyaline casts (h)and intertubular hemorrhage (ih); C-medulla showing hyaline casts (h) along with intertubular hemorrhage (ih)
Figure 5.
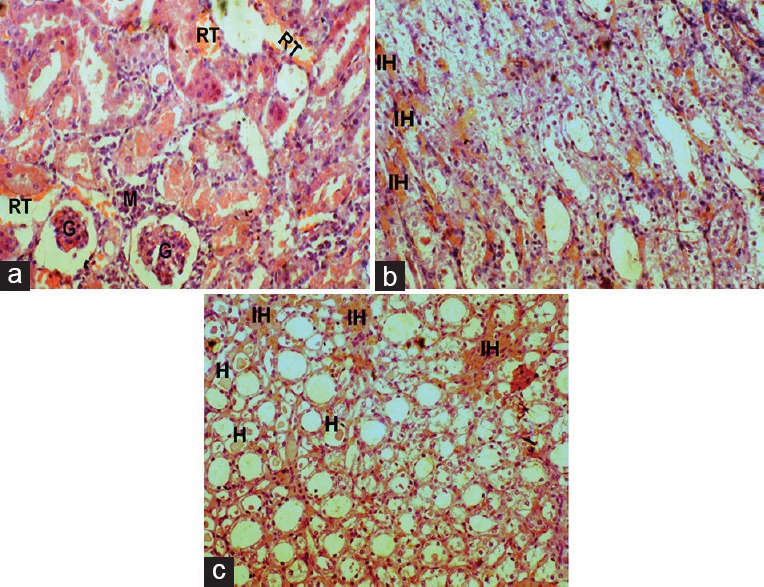
Effect of DC on Kidney histopathology of gentamicin-intoxicated rats in DC200 group (x45): A, B-cortex showing moderate glomerular atrophy (g), renal tubular desquamation (rt), and mononuclear cells infiltration (m); C-medulla showing mild intertubular hemorrhage (ih) with hyaline casts (h)
Figure 6.
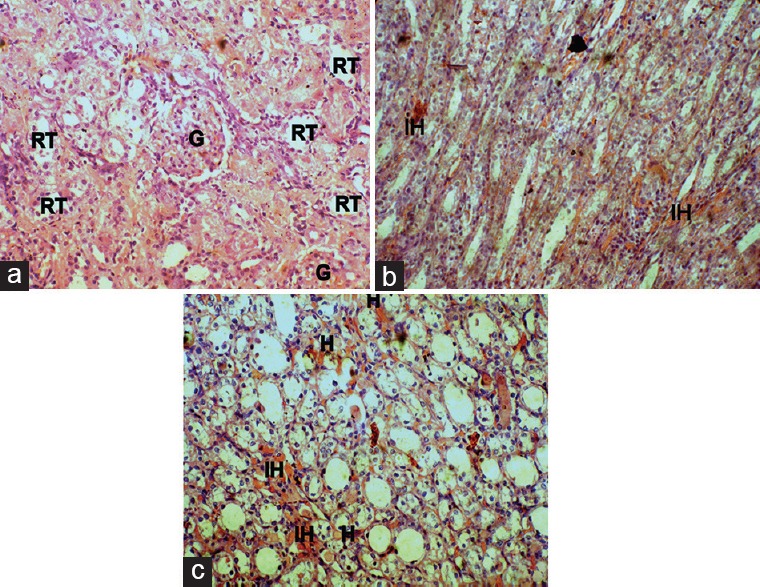
Effect of DC on Kidney histopathology of gentamicin-intoxicated rats in DC400 group (x45): A, B-cortex showing normal renal tubular morphology (rt) with intact glomerulus (g); C-medulla showing sporadic hyaline casts (h) with mild intertubular hemorrhage (ih)
Discussion
Despite the use of gentamicin was limited with nephrotoxicity, it becomes a promising therapeutic antibiotic due to its potent bactericidal and less bacterial resistance properties. Accumulation of drug tends to be a primary key pathological event in gentamicin-inducing nephrotoxicity and subsequent renal dysfunction.[24] Being as cationic in nature, gentamicin has a strong affinity toward negatively charged brush-border membrane components of proximal tubule where it forms drug receptor complex with megalin, a cationic drug receptor. Then, pinocytosis translocates the drug to lysosomes, where phospholipidosis takes place to interrupt various intracellular renal functions leading to renal injury.[25,26] This renal injury in turn manifests the migration of monocytes and macrophages to the site of injury by stimulating intercellular adhesion molecule-1 and monocyte chemoattractant protein[27,28] while several other studies reported the role of reactive oxygen species in implicating the pathogenesis of gentamicin-induced nephrotoxicity.[29]
Gentamicin-intoxicated nephrotoxicity is functionally evident by the elevated serum levels of urea, BUN, uric acid, and creatinine; structurally characterized by tubular necrosis, glomerular atrophy, mononuclear cell infiltration, intertubular hemorrhage, and hyaline casts. Similar sort of alterations were observed with the gentamicin treatment in GM and treatment groups. As an indicative of decrease in glomerular filtration rate, there was an increase in the serum creatinine levels in the GM group,[30] whereas the serum urea and BUN were found to be increased as an indicative of parenchyma tissue injury after tubular necrosis.[31] The serum uric acid levels were found to be increased because of accumulation by the decrease in glomerular filtration rate in gentamicin-intoxicated rats, but with the supplementation of DC, it dose dependently ameliorated the gentamicin-induced elevated serum levels of urea, BUN, uric acid, and creatinine in DC200 and DC400 groups. However, in DC400 group, the nephroprotective property was found to be very prominent with high dose when compared with DC200 group. These results notified the improved renal function by the effective clearance of urea, BUN, creatinine, and uric acid.
Moreover, gentamicin induced weight loss that resulted from renal tubular injury and acidosis-impaired dehydration, and anorexia was ablated to a great extent by DC treatment.[23] The increased wet kidney weight of gentamicin-treated rats due to edema induced by acute tubular necrosis was even normalized by the DC treatment. Thus, the results notified that DC may act by antagonizing the gentamicin-implicated acute renal tubular necrosis and acidosis to attenuate the nephrotoxicity. The histopathological observations also revealed the protective role of DC in parallel to the results of biochemical analysis. After the gentamicin administration, the GM groups showed the extensive renal tubular necrosis, glomerular atrophy, mononuclear cell infiltration, hyaline casts, and intertubular hemorrhage. However, DC supplementation showed the marked attenuation of histopathological implications induced by gentamicin, especially in DC400 group with slight presence of intertubular hemorrhage and sporadic hyaline casts. Thus, the histopathology results demonstrated the ablated inflammatory events by the cellular anti-inflammatory properties of polyacetylenes present in carrot.[32]
By considering the oxidative stress in the pathophysiology of nephrotoxicity, antioxidants therapy opted to be an alternative choice in its management. Polyphenolic compounds reported to possess nephroprotective property by promoting antioxidant enzyme system, thereby attenuating ROS generation and lipid peroxidation.[33] In evidence of this, the polyphenolic compounds of DC can contribute to nephroprotection by its antioxidant activity. The natural antioxidants such as β-carotene,[9] a terpenoid constituent of the crude extract can ameliorate the nephrotoxicity by its free radical scavenging activity.
Thus, all together from the results of biochemical and morphologic pathology, DC had the ability to halt the gentamicin nephrotoxicity dose dependently by having rich antioxidant and cellular anti-inflammatory property.[8] However, the other mechanisms such as N-balancing properties and antagonizing principles against phospholipidosis in proximal tubules cannot be ruled out.
Conclusion
Concurrent administration of ethanolic extract of DC dose dependently attenuated the pathological implications of gentamicin nephrotoxicity to a great extent. Being as a source of rich carotenoid, polyphenolic and polyacetylene constituents in carrot, the principle mechanism to elucidate the nephroprotective potentiality possibly attributed due to its antioxidant and cellular anti-inflammatory properties. Thus, it validated the traditional use as an ethnomedicine against nephrosis. However, further studies are needed to identify and characterize the phytoconstituents from DC; and also to explore the exact mechanism to act as nephroprotective, before being establish it in clinical setting.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
The authors were greatly thankful to B. Masthan Rao, the chairman of BMR Industries Private Limited, India, for providing funding to this research work. The authors also wish to thank the principal Dr. C. K. Ashok kumar, Sree Vidyanikethan College of Pharmacy and to the management of Sree Vidyanikethan Group of Institutions for providing the necessary facilities to carry out the research work.
References
- 1.Hussain T, Gupta RK, Sweety K, Eswaran B, Vijayakumar M, Rao CV. Nephroprotective activity of Solanum xanthocarpum fruit extract against gentamicin-induced nephrotoxicity and renal dysfunction in experimental rodents. Asian Pac J Trop Med. 2012;5:686–91. doi: 10.1016/S1995-7645(12)60107-2. [DOI] [PubMed] [Google Scholar]
- 2.Ouédraogo M, Lamien-Sanou A, Ramdé N, Ouédraogo AS, Ouédraogo M, Zongo SP, et al. Protective effect of Moringa oleifera leaves against gentamicin-induced nephrotoxicity in rabbits. Exp Toxicol Pathol. 2013;65:335–9. doi: 10.1016/j.etp.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Elfarra AA, Duescher RJ, Sausen PJ, O'Hara TM, Cooley AJ. Methimazole protection of rats against gentamicin-induced nephrotoxicity. Can J Physiol Pharmacol. 1994;72:1238–44. doi: 10.1139/y94-176. [DOI] [PubMed] [Google Scholar]
- 4.Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: Nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–12. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geleilete TJ, Melo GC, Costa RS, Volpini RA, Soares TJ, Coimbra TM. Role of myofibroblasts, macrophages, transforming growth factor-beta endothelin, angiotensin-II, and fibronectin in the progression of tubulointerstitial nephritis induced by gentamicin. J Nephrol. 2002;15:633–42. [PubMed] [Google Scholar]
- 6.Martínez-Salgado C, López-Hernández FJ, López-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223:86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull. 2009;32:61–7. doi: 10.1248/bpb.32.61. [DOI] [PubMed] [Google Scholar]
- 8.Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62:179–86. doi: 10.1016/j.phrs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Sharma KD, Karki S, Thakur NS, Attri S. Chemical composition, functional properties and processing of carrot-a review. J Food Sci Technol. 2012;49:22–32. doi: 10.1007/s13197-011-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilani AH, Shaheen E, Saeed SA, Bibi S, Irfanullah, Sadiq M, et al. Hypotensive action of coumarin glycosides from Daucus carota. Phytomedicine. 2000;7:423–6. doi: 10.1016/s0944-7113(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 11.Majumder PK, Dasgupta S, Mukhopadhaya RK, Mazumdar UK, Gupta M. Anti-steroidogenic activity of the petroleum ether extract and fraction 5 (fatty acids) of carrot (Daucus carota L.) seeds in mouse ovary. J Ethnopharmacol. 1997;57:209–12. doi: 10.1016/s0378-8741(97)00056-1. [DOI] [PubMed] [Google Scholar]
- 12.Bishayee A, Sarkar A, Chatterjee M. Hepatoprotective activity of carrot (Daucus carota L.) against carbon tetrachloride intoxication in mouse liver. J Ethnopharmacol. 1995;47:69–74. doi: 10.1016/0378-8741(95)01254-b. [DOI] [PubMed] [Google Scholar]
- 13.Gambhir SS, Sen SP, Sanyal AK, Das PK. Antispasmodic activity of the tertiary base of Daucus carota, Linn.Seeds. Indian J Physiol Pharmacol. 1979;23:225–8. [PubMed] [Google Scholar]
- 14.Kumarasamy Y, Cox PJ, Jaspars M, Nahar L, Sarker SD. Screening seeds of Scottish plants for antibacterial activity. J Ethnopharmacol. 2002;83:73–7. doi: 10.1016/s0378-8741(02)00214-3. [DOI] [PubMed] [Google Scholar]
- 15.Gupta L, Garg RP, Sharma RC, Arora RB. Monoamine oxidase inhibiting activity of Daucus carota. Indian J Exp Biol. 1973;11:342–3. [PubMed] [Google Scholar]
- 16.Momin RA, De Witt DL, Nair MG. Inhibition of cyclooxygenase (COX) enzymes by compounds from Daucus carota L.Seeds. Phytother Res. 2003;17:976–9. doi: 10.1002/ptr.1296. [DOI] [PubMed] [Google Scholar]
- 17.Duke JA, Bogenschutz-Godwin MJ, Cellier J, Duke PA. 2nd ed. Washington, DC: CRC Press; 2002. Handbook of Medicinal Herbs; pp. 156–7. [Google Scholar]
- 18.Trease GE, Evans WC. 12th ed. London: Bailliere Tindall; 1989. Textbook of Pharmacognosy. [Google Scholar]
- 19.Kokate CK, Purohit AP, Gokhale SB. 2nd ed. Mumbai: Nirali Prakashan, Pune; 1994. Practical Pharmacognosy. [Google Scholar]
- 20.Vol. 423. France: OECD; 2001. OECD. Acute oral toxicity-acute toxic class method. OECD Guideline for Testing of Chemicals. [Google Scholar]
- 21.Annie S, Rajagopal PL, Malini S. Effect of Cassia auriculata Linn. Root extract on cisplatin and gentamicin-induced renal injury. Phytomedicine. 2005;12:555–60. doi: 10.1016/j.phymed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Harlalka GV, Patil CS, Patil MR. Protective effect of Kalanchoe pinnata pers. (Crassulaceae) on gentamicin-induced nephrotoxicity in rats. Indian J Pharmacol. 2007;39:201–5. [Google Scholar]
- 23.Feyissa T, Asres K, Engidawork E. Renoprotective effects of the crude extract and solvent fractions of the leaves of Eucleadivinorum Hierns against gentamicin-induced nephrotoxicity in rats. J Ethnopharmacol. 2013;145:758–66. doi: 10.1016/j.jep.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Hori R, Inui K. Cellular basis of aminoglycoside nephrotoxicity. Physiology. 1989;4:181–4. [Google Scholar]
- 25.Laurent G, Carlier MB, Rollman B, Van Hoof F, Tulkens P. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: In vitro and in vivo studies with gentamicin and amikacin. Biochem Pharmacol. 1982;31:3861–70. doi: 10.1016/0006-2952(82)90303-3. [DOI] [PubMed] [Google Scholar]
- 26.Sandhu JS, Sehgal A, Gupta O, Singh A. Aminoglycoside nephrotoxicity revisited. J Indian Acad Clin Med. 2007;8:331–3. [Google Scholar]
- 27.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987;84:5788–92. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang WW, Feng L, Mathison JC, Wilson CB. Cytokine expression, upregulation of intercellular adhesion molecule-1, and leukocyte infiltration in experimental tubulointerstitial nephritis. Lab Invest. 1994;70:631–8. [PubMed] [Google Scholar]
- 29.Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999;31:971–97. doi: 10.1081/dmr-100101947. [DOI] [PubMed] [Google Scholar]
- 30.Laskshmi BV, Sudhakar M. Protective effect of Zingiber officinale on gentamicin-induced nephrotoxicity in rats. Int J Pharmacol. 2010;6:58–62. [Google Scholar]
- 31.Gilbert DN, Wood CA, Kohlhepp SJ, Kohnen PW, Houghton DC, Finkbener HC, et al. Polyaspartic acid prevents experimental aminoglycoside nephrotoxicity. J Infect Dis. 1989;159:945–53. doi: 10.1093/infdis/159.5.945. [DOI] [PubMed] [Google Scholar]
- 32.Christensen LP, Brandt K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J Pharm Biomed Anal. 2006;41:683–93. doi: 10.1016/j.jpba.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 33.Wongmekiat O, Leelarugrayub N, Thamprasert K. Beneficial effect of shallot (Allium ascalonicum L.) extract on cyclosporine nephrotoxicity in rats. Food Chem Toxicol. 2008;46:1844–50. doi: 10.1016/j.fct.2008.01.029. [DOI] [PubMed] [Google Scholar]


