Abstract
Objectives:
Benign prostatic hyperplasia (BPH) is a common and progressive disease affecting elderly males, often associated with lower urinary tract symptoms (LUTS). α1-blockers are the mainstay in symptomatic therapy of BPH. Because of their greater uroselectivity and minimal hemodynamic effects, alfuzosin, tamsulosin, and silodosin are generally preferred. The aim of this study was to compare the efficacy and tolerability of alfuzosin, tamsulosin, and silodosin in patients with BPH and LUTS.
Methods:
Ninety subjects with BPH and LUTS were randomized into three groups of thirty in each, to receive alfuzosin sustained release (SR) 10 mg, tamsulosin 0.4 mg, or silodosin 8 mg for 12 weeks. The primary outcome measure was a change in the International Prostate Symptom Score (IPSS), and the secondary outcome measures were changes in individual subjective symptom scores, quality of life score (QLS), and peak flow rate (Qmax) from baseline. The treatment response was monitored at 2, 4, 8, and 12 weeks.
Results:
IPSS improved by 88.18%, 72.12%, and 82.23% in alfuzosin SR, tamsulosin and silodosin groups (P < 0.001) at 12 weeks. Improvement in QLS was >75% in all the three groups (P < 0.001). A significant improvement in Qmax was seen with alfuzosin and tamsulosin (P = 0.025 and P < 0.001) but not with silodosin (P = 0.153). However, the intergroup differences in IPSS, QLS, and Qmax were not significant. Ejaculatory dysfunction was more common with silodosin and corrected QT (QTc) prolongation occurred only with alfuzosin (two subjects) and tamsulosin (three subjects).
Conclusion:
Alfuzosin, tamsulosin, and silodosin showed similar efficacy in improvement of LUTS secondary to BPH, with good tolerability, acceptability, and minimum hemodynamic adverse effects. Alfuzosin, tamsulosin, and silodosin are comparable in efficacy in symptomatic management of BPH. The occurrence of QTc prolongation in three subjects with tamsulosin in the present study is an unexpected adverse event as there are no reports of QTc prolongation with tamsulosin in any of the previous studies.
KEY WORDS: α1-blockers, alfuzosin, benign prostatic hyperplasia, clinical trial, silodosin, tamsulosin
Introduction
Benign prostatic hyperplasia (BPH) is a common and progressive disease affecting elderly males, often associated with lower urinary tract symptoms (LUTS) characterized by voiding symptoms (hesitancy, weak/slow stream, intermittency, straining, and incomplete voiding), storage symptoms (frequency, urgency, nocturia, urge incontinence), and postmicturition symptoms (postvoid dribbling), which may adversely affect the quality of life (QOL).[1] In addition, BPH can also lead to more serious complications, such as acute urinary retention, recurrent urinary tract infections, hematuria, bladder calculi, and renal dysfunction.[2]
BPH typically develops after the age of 40 years, ranging in prevalence from >50% at 60 years, to as high as ~90% by 85 years of age.[3] Histologically, BPH is characterized by a progressive increase in the number of epithelial and stromal cells that develop initially in the periurethral area of the prostate gland with increased prostatic smooth muscle tone resulting in urethral constriction and outflow obstruction.[1,2]
The management of BPH involves nonpharmacological, pharmacological, or surgical intervention and the choice of therapy typically depending on the pattern and severity of symptoms. Though surgery is the definitive treatment for BPH, the risk, complications, and the cost of surgical intervention, have prompted the need for effective and safe noninvasive modalities of treatment like drug therapy. Two major classes of drugs used in the management of BPH are α1-blockers and 5α-reductase inhibitors, the former addressing the dynamic component by relaxing the smooth muscles of prostate and prostatic urethra, providing quicker symptomatic relief and the latter affecting the static component by inducing gradual regression in gland size. The clinical benefit of α1-blockers has shown to be independent of baseline prostate volume.[2,3]
The α1 receptors are postsynaptic, present in vascular and nonvascular smooth muscles. There are several subtypes of α1 receptors which include α1A, α1B, and α1D. The α1A receptors, predominantly located in the smooth muscles of genitourinary tract, are the primary regulators of smooth muscle tone in the bladder neck and prostate; α1B receptors are present in the vascular smooth muscle and regulate the vascular tone, and α1D subtype mediates contraction of the bladder muscle.[1] The α1 antagonists generally preferred in the management of BPH are alfuzosin, tamsulosin, and silodosin because of their minimal hemodynamic adverse effects. Alfuzosin though not completely α1A selective, shows greater uroselectivity, whereas tamsulosin and silodosin have higher α1A selectivity.[1,4]
Silodosin is a new α1A antagonist approved for the treatment of BPH with LUTS, claimed to have the highest α1A selectivity, rapid onset of action, better relief of nocturia, and no risk of corrected QT (QTc) prolongation.[4] However, there is no adequate data from the Indian population to confirm the claimed advantages of silodosin over alfuzosin and tamsulosin. In the present prospective study, the comparative efficacy and tolerability of alfuzosin, tamsulosin and silodosin were assessed in subjects with symptomatic BPH in a tertiary care teaching hospital.
Methods
After obtaining approval and clearance from the Institutional Ethics Committee (ethics committee approval number: ECR/216/Inst/KAR/2013), ninety subjects with LUTS secondary to BPH were included for the present prospective, randomized, comparative, open-label study in a tertiary care hospital, between June 2013 and June 2014. Written informed consent was obtained from all the subjects after fully explaining the study procedure to their satisfaction. The study was conducted in accordance with the International Conference of Harmonization guidelines on good clinical practice and Indian Council of Medical Research's ethical guidelines for biomedical research on human participants (2006). The study was registered retrospectively with the Clinical Trials Registry-India (CTRI/2013/07/003805).
Male patients ≥45 years with symptomatic BPH with International Prostate Symptom Score (IPSS) of ≥8, quality of life score (QLS) of ≥3, and a peak flow rate (Qmax) of <15 ml/s, but >4 ml/s with a voided volume of >100 ml were included in the study. Subjects with severe hepatic or renal insufficiency, urinary tract infections, urethral stricture, neurogenic bladder, prostate specific antigen ≥5 ng/ml, history of urethral or prostatic surgery, hypotension or severe untreated hypertension, history of esophageal or intestinal obstruction, history of multiple drug abuse, significant psychiatric problems, serious disease or malignancy, increased risk of QTc prolongation (e.g., hypokalemia, concomitant use of Class Ia and III antiarrhythmics, antipsychotics, tricyclic antidepressants, etc.), concomitant use of 5α-reductase inhibitors and strong cytochrome P450 3A4 (CYP3A4) inhibitors, likelihood of requiring catheterization within next 3 months were excluded from the study.
A detailed history taking and relevant investigations were done to confirm the diagnosis and to detect any underlying complications associated with BPH, and also to rule out any contraindications to the study medications.[5] The severity of LUTS was assessed by the IPSS, based on the answers to seven questions regarding urinary symptoms (incomplete emptying, frequency, intermittency, urgency, weak stream, straining, and nocturia). The seven questions were as follows:
Incomplete emptying: How often have you had the sensation of not emptying your bladder?
Frequency: How often have you had to urinate less than every 2 h?
Intermittency: How often have you found you stopped and started again several times when you urinated?
Urgency: How often have you found it difficult to postpone urination?
Weak stream: How often have you had a weak urinary stream?
-
Straining: How often have you had to strain to start urination?
- (scores: 0 - not at all; 1 - <1 in 5 times; 2 - less than half the time; 3 - about half the time; 4 - more than half the time; 5 - almost always)
-
Nocturia: How many times did you typically get up at night to urinate?
- (scores: 0 - none; 1 - 1 time; 2 - 2 times; 3 - 3 times; 4 - 4 times; 5 - 5 times)
- (total IPSS score: 1–7: Mild; 8–19: Moderate: 20–35: Severe)
-
QOL: If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about it?
- (QLSs based on Likert scale: 0 - delighted; 1 - pleased; 2 - mostly satisfied; 3 - mixed; 4 - mostly dissatisfied; 5 - unhappy; 6 - terrible). The QLS is used to assess the impairment in QOL due to urinary symptoms.[6]
The selected patients were randomized into three groups of thirty in each, using a randomization table, to receive alfuzosin SR 10 mg, tamsulosin 0.4 mg, or silodosin 8 mg orally. Alfuzosin sustained release (SR) tablet 10 mg (immediately after meals) and tamsulosin tablet/capsule 0.4 mg (before meals) given once daily at bedtime and silodosin capsule 8 mg (before breakfast) once daily in the morning. Wash out period of 7 days was allowed for those subjects previously receiving any α-blockers. All the three study medications were continued for 12 weeks. The treatment response was monitored during subsequent follow-up visits, after 2 weeks (visit 1), 4 weeks (visit 2), 8 weeks (visit 3), and 12 weeks (visit 4). During each visit, the study subjects were examined for the cardiovascular parameters, pulse rate, and blood pressure (supine and standing). The adverse events (AEs) were recorded and assessed for the causality and severity with the study drugs. The causality was assessed by the World Health Organization-Uppsala Monitoring Center criteria[7] and the severity by adapted Hartwig severity scale.[8] History of concomitant use of drugs increasing the risk of hypotension either through pharmacokinetic or pharmacodynamic interactions such as alcohol, beta-blockers, CYP3A4 inhibitors, phosphodiesterase 5 inhibitors, P-glycoprotein inhibitors, Uridine 5’-diphospho-glucuronosyltransferase 2B7inhibitorsand verapamil, drugs altering the efficacy and tolerability parameters such as 5α-reductase inhibitors, drugs increasing the QTc interval was elicited at each follow-up visit to rule out any undue drug interaction with the study medications. The compliance to the study medication was assessed during each visit. The primary outcome measure was change in total IPSS from baseline; secondary outcome measures were change in Qmax (peak flow rate as assessed by uroflowmetry)[9] (Status Medical Equipment), individual subjective symptom scores of IPSS (voiding and storage scores), and QLS. During the study period, the patients were instructed not to crush, chew, or open the capsule/tablet and also not to drive or operate machinery, and to contact the doctor immediately, in the event of any syncope, priapism, or any other serious AE. History of intake of alcohol was taken during the first/screening visit. Those who consumed alcohol were classified as abstainers, less frequent, frequent, and heavy drinkers (abstainer = [never or <45 ml/year or left for >10 years]; less frequent = [1–3t/month, <225 ml/sitting]; frequent = [1/week, <225 ml/sitting]; heavy = [1/week, >225 ml/sitting])[10] Those who fell under the category of less frequent, frequent, and heavy drinkers were advised against alcohol intake for the next 12 weeks of study period.
Statistical Analysis
There are no studies conducted in India comparing the effectiveness of alfuzosin, tamsulosin, and silodosin in the management of BPH. Therefore, the treatment success rate (>25% improvement in IPSS) was taken as 82% for tamsulosin and 86% for silodosin based on the results of the latest available study done by Yu et al.[11] Since there is no published literature on the success rate of alfuzosin, a pilot study was conducted with 12 patients who showed a 100% treatment success rate. However, the rate of success was taken as 99% for the purpose of calculation of sample size. Based on the noninferiority criteria set at an absolute value of 5%, alpha error at 5%, power of study at 85%, and drop-out rate at 5%, it was estimated that thirty subjects would be required in each study group. Alfuzosin with maximum success rate was kept as standard comparator arm. The success rates for intervention arms were 82% for tamsulosin and 86% for silodosin, each compared against the standard comparator alfuzosin using an online sample size calculator. Tamsulosin intervention versus standard comparator yielded a sample size of 25 in each group whereas silodosin intervention versus standard comparator yielded a sample size of thirty in each group. Thus, instead of taking 25 in tamsulosin, thirty in silodosin, and thirty in alfuzosin, a uniform number of thirty subjects in each group were included, leading to a total sample size of ninety subjects for the present study. Friedman's test was used to study the change in IPSS, individual symptom scores, and QLS during various visits, and repeated measures analysis of variance (ANOVA) was used to study the change in Qmax during various visits within the three study groups. Kruskal–Wallis test was used to compare the changes in IPSS, individual symptom scores, and QLS, and one-way ANOVA was used to compare the changes in Qmax between the three study groups.
Results
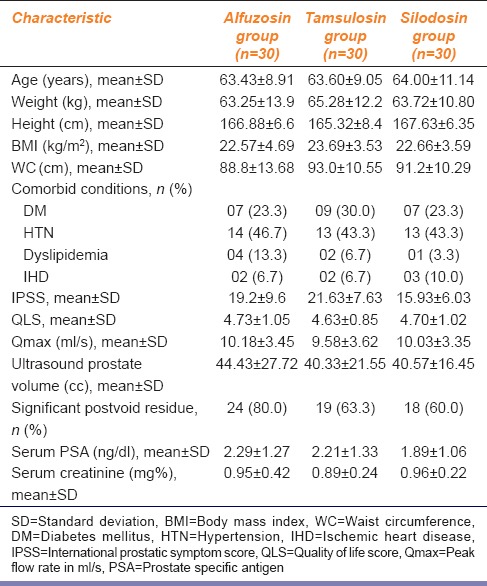
In the present study, there were no drop-outs or protocol violations during the study and all the ninety subjects were included in the analysis [Figure 1]. Table 1 shows the baseline demographic and clinical characteristics of the study subjects. Mean age of patients were 63.43 ± 8.91, 63.60 ± 9.05, and 64.00 ± 11.14 (mean ± standard deviation [SD]) years and mean baseline IPSS were 19.2 ± 9.6, 21.63 ± 7.63, and 15.93 ± 6.03 (mean ± SD) in alfuzosin, tamsulosin, and silodosin groups, respectively.
Figure 1.
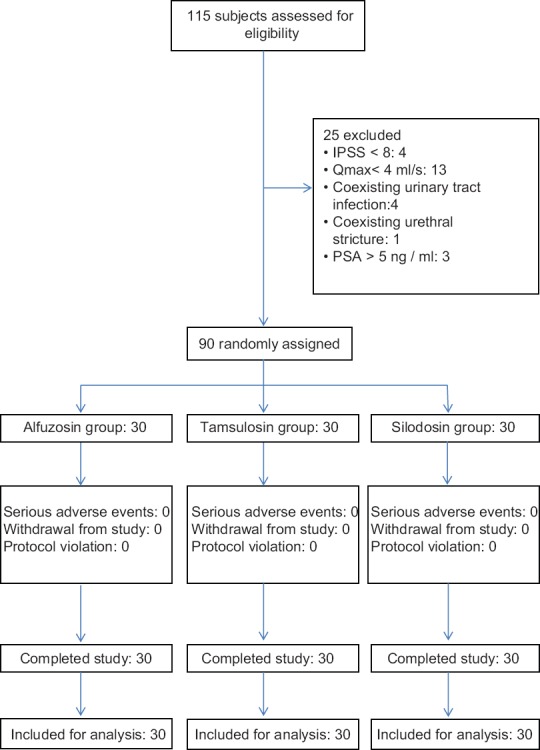
Comparison of alfuzosin, tamsulosin and silodosin in benign prostatic hyperplasia: Flow of study participants
Table 1.
Comparison of alfuzosin, tamsulosin and silodosin in benign prostatic hyperplasia: Demographic and baseline characteristics of the study subjects
There was a progressive decrease in the baseline IPSS at different follow-up visits with alfuzosin, the maximum decline (62.14%) occurring at 2 weeks and the overall decrease at the end of the study period being 88.18%, which was highly significant (P < 0.001). The individual symptom scores also showed a corresponding decrease at different visits, the change from the baseline ranging between 80% and 97% (P < 0.001). However, nocturia showed only ~60% decrease from the baseline (P < 0.001). QLS also showed a progressive improvement from baseline, with 90.06% decrease in the score at the end of the study period which was highly significant (P < 0.001). Qmax showed only a modest improvement after 2 weeks, with little further change at subsequent visits, and the overall change from the baseline was 25.34% (P = 0.025).
In patients with tamsulosin, the rate of decrease in the baseline IPSS was similar to alfuzosin group, and the net decrease after 12 weeks was 72.12% (P < 0.001). There was a similar decrease in the individual symptom scores at different visits, with the overall decrease at the end of study period being 66–84% (P < 0.001), but nocturia showed only ~42% decrease from the baseline (P < 0.001). The rate of decrease in QLS was almost similar to alfuzosin group, the overall decrease at the end of study period being 77.75% (P < 0.001). Likewise, only a modest improvement occurred in Qmax, which was maximum after 2 weeks (27.6%), with only a little further change at subsequent visits up to 12 weeks, and the overall change from the baseline was 31.11% (P < 0.001).
Silodosin produced 82.23% decrease in the IPSS after 12 weeks (P < 0.001), with a maximum decrease (61.5%) occurring after 2 weeks. There was a corresponding decrease in the individual symptom scores, which ranged from 80% to 100% (P < 0.001) toward the end of the study period, but nocturia showed only ~ 49% decrease from the baseline (P < 0.001). The overall improvement in QLS was 87.87% (P < 0.001); however, the Qmax showed an improvement of only 14.2% after 2 weeks and 13.46% after 12 weeks (P = 0.153).
The intergroup differences in IPSS, individual symptom scores, QLS, and Qmax are shown in Table 2. Figures 2–4 demonstrate the comparative changes between three study medications in IPSS, QLS, and Qmax, respectively. The improvement in BPH symptoms, IPSS, and peak flow rate were similar between the treatment groups (P > 0.05) [Table 2 and Figures 2–4]. There were no serious AEs in any of the study groups warranting discontinuation of study medication. Upper respiratory tract infection was the most common AE (n = 14, 10, and 14 with alfuzosin, tamsulosin, and silodosin, respectively) followed by dizziness (n = 13, 09, and 10 with alfuzosin, tamsulosin, and silodosin, respectively) in all the three groups. Two patients with alfuzosin and three patients with tamsulosin had a significant QTc prolongation (>45 ms). The incidence of ejaculatory dysfunction was highest with silodosin (n = 9). There were 439 observed AEs with the study medications (inclusive of all three drugs). Five AEs fell under the category of certain, 121 under probable, 242 under possible, and 71 unlikely. The number of certain AEs was 0, 0, and 5; probable were 36, 25, and 60; possible AEs were 91, 51, and 100 and unlikely were 31, 27, and 13 with alfuzosin, tamsulosin, and silodosin, respectively. Compliance, as mentioned in methodology section, was assessed by daily drug reminder chart and pill count method. One hundred percentage compliance means all the prescribed medications taken by the study subjects; >95% compliance means <3 doses missed in a period of 30 days; 80–95% compliance means 3–12 doses missed in a period of 30 days; <80% compliance means >12 doses missed in a period of 30 days. At visit 4, majority of the study subjects (90% with alfuzosin, 93.3% with tamsulosin and 86.7% with silodosin) showed 100% compliance. Only two subjects showed <80% compliance at the end of the study period.
Table 2.
Comparison of alfuzosin, tamsulosin and silodosin in benign prostatic hyperplasia: Summary of the treatment outcome at the end of the study period
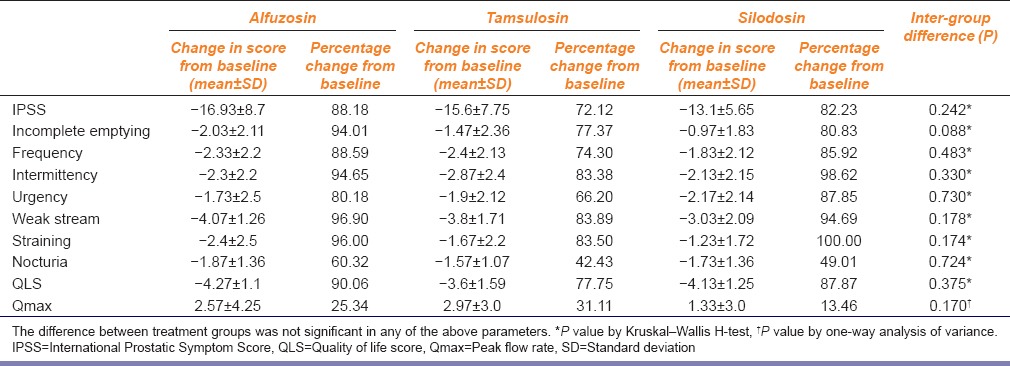
Figure 2.
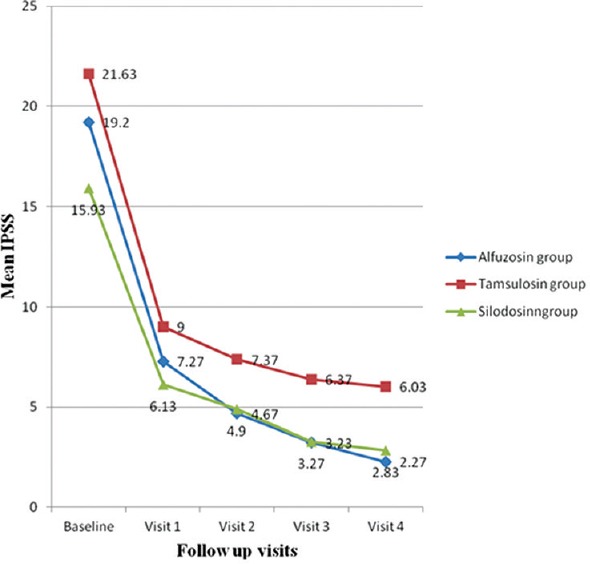
Comparison of alfuzosin, tamsulosin and silodosin in benign prostatic hyperplasia: Change in International Prostatic Symptom Score in the three study groups: The change in mean International Prostate Symptom Score from baseline to 2 weeks (visit 1), 4 weeks (visit 2), 8 weeks (visit 3), and 12 weeks (visit 4) with the three study medications
Figure 4.

Comparison of alfuzosin, tamsulosin and silodosin in benign prostatic hyperplasia: Change in Qmax. The improvement in mean Qmax (peak flow rate) from baseline to 2 weeks (visit 1), 4 weeks (visit 2), 8 weeks (visit 3), and 12 weeks (visit 4) with the three study medications
Figure 3.
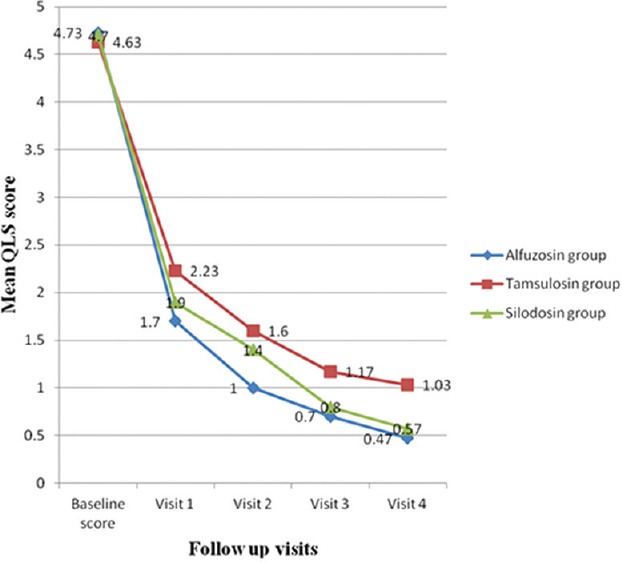
Comparison of alfuzosin, tamsulosin and silodosin in benign prostatic hyperplasia: Change in quality of life score. The improvement in mean quality of life score from baseline to 2 weeks (visit 1), 4 weeks (visit 2), 8 weeks (visit 3), and 12 weeks (visit 4) with the three study medications
Discussion
In all the three study groups, the maximum decrease occurred at visit 1 (2 weeks) indicating a similar onset of action and rate of symptomatic improvement with the three α-blockers. During subsequent visits, there was a gradual further decrease in the IPSS indicating that the improvement was sustained throughout the study period without loss of efficacy. Other studies have shown a quicker onset of action with silodosin (3–4 days) compared to alfuzosin and tamsulosin (1–2 weeks).[1,2,12] However, in the present study, the exact relative onset of action could not be ascertained since the first follow-up visit was at the end of 2 weeks. The rate of improvement in the QLS was similar in all the three study groups, the maximum improvement after 2 weeks with further gradual improvement towards the end of 12 weeks, indicating that the onset of improvement in the QLS corresponds to the decrease in IPSS. Other studies have also observed a similar pattern of parallel improvement in the IPSS and QLS.[1,2,12] Maximum improvement was observed after 2 weeks, and it was sustained throughout the study period with little further improvement. Results of a recent meta-analysis have shown that tamsulosin and silodosin improve Qmax and bladder outlet obstruction index in patients with LUTS/BPH. Lower the Qmax at baseline, higher will be the end of the study improvement.[13] Interestingly, in the present study, a slight decline in the mean Qmax was observed between visit 2 and visit 3, in all the three study groups. Other studies have also reported a similar decline in Qmax after 4 weeks.[1,2,12] The improvement in Qmax was modest with alfuzosin and tamsulosin, minimal with silodosin and did not correlate well with the other outcome measures. Similar observations have been made in other studies with the maximum improvement with tamsulosin, followed by alfuzosin and lesser improvement with silodosin.[1,2,12] This may be probably because of various other factors which may influence voiding such as fullness of bladder, intra-abdominal pressure generated during voiding and voiding conditions. There was a good correlation between the decrease in IPSS and QLS in all the three study groups (P < 0.01), but the improvement in Qmax did not correlate well with the other two treatment outcome measures (data not shown).
The decrease in the IPSS was 88.18%, 72.12%, and 82.23% in alfuzosin, tamsulosin, and silodosin groups, respectively, which showed a good correlation with the decrease in the individual symptom scores. However, there was less improvement in nocturia compared to other symptoms in all the three study groups. Our observations were consistent with other reported studies.[1,2,12] Subjective improvement in nocturia was noted specifically with silodosin in a recent randomized cross-over comparison of half-dose silodosin with tamsulosin.[14] In the present study, the improvement in nocturia was marginally better by 6.78% with silodosin when compared to tamsulosin. The improvement in QLS was 90.06%, 77.75%, and 87.87% in alfuzosin, tamsulosin, and silodosin groups, respectively. Other studies have also reported a similar degree of improvement in the QLS.[1,2,12] Though, a marginal difference in the primary and secondary outcome measures was observed between the three treatment groups in the present study, it was not statistically significant (P > 0.05). A similar observation was made in a recent study on Indian subjects which compared the efficacy and tolerability of silodosin against tamsulosin. The authors concluded that silodosin is comparable to tamsulosin in terms of improvement in IPSS, measures of uroflowmetry and ultrasound prostate volume at the end of 12 weeks of therapy.[15] The network meta-analysis between various pharmacological interventions concluded that the improvement in IPSS was comparable between silodosin, tamsulosin, alfuzosin, naftopidil, dutasteride, vardenafil, sildenafil, and tadalafil.[16]
The ejaculatory dysfunction is usually not related to BPH, but may occur due to various other factors such as aging, comorbid conditions such as diabetes, autonomic dysfunction, and also it is a known side effect of α-blockers due to impaired contraction of vas deferens.[1,2] In the present study, the total number of subjects with ejaculatory dysfunction at baseline was 13 (data not shown), which was not worsened by alfuzosin and tamsulosin. The overall incidence of the ejaculatory dysfunction was more common in silodosin group (30%). This is consistent with the observations in other studies which have reported up to 28% with silodosin.[1,2] In fact, worsening of sexual function scores was observed with silodosin alone in one of the studies but, not with tamsulosin at the end of 12 weeks treatment.[15] Abnormal ejaculation is the most commonly reported AE with silodosin and has been reported as the main reason behind its discontinuation.[17] The occurrence of orthostatic hypotension was similar in the three study groups, but symptomatic only in two subjects in silodosin group, probably because of the patients receiving antihypertensive therapy. Other studies have also reported a similar incidence of orthostatic hypotension.[1,2,12] Hence, it is reasonable to presume that symptomatic orthostatic hypotension may not be a major problem while treating BPH with these three uroselective α-blockers unless the person is receiving antihypertensive medications. A population-based cohort study on men aged ≥66 years showed that α-blockers significantly increase the risk of falling, sustaining a fracture, hypotension, and trauma when compared with the unexposed cohort.[18] Syncope which occurred in only one subject in each group in the present study was an isolated episode, which did not recur with the continuation of the medications, and hence did not warrant discontinuation of the medications. Syncope has been reported in the previous studies in <1% of the subjects treated with alfuzosin, but there are no reports of the occurrence with tamsulosin and silodosin.[1,2,12] Persistent diarrhea is a cause of concern because of hypokalemia which may increase the risk of arrhythmia in patients with QTc prolongation. However, diarrhea was self-limiting. Significant QTc prolongation (>45 ms) at the end of the study period was present in two subjects with alfuzosin and three subjects with tamsulosin, but not seen in any of the subjects who received silodosin. QTc prolongation has been reported only with alfuzosin, but not with tamsulosin and silodosin.[19] Hence, the observation of significant QTc prolongation in three subjects with tamsulosin in the present study can be considered as an unexpected AE. Though the causality assessment was found to be possible in all these subjects, because of the risk of torsades de pointes, a serious type of cardiac arrhythmia, alfuzosin and tamsulosin need to be used very cautiously in patients with preexisting QTc prolongation. Based on the observation in the present study and the reports from the previous studies, it may be considered that silodosin appears to be relatively safer in such patients. Causality assessment of the AEs found that most of the AEs were possible and probable, and a very few were certain and unlikely the probable AEs were most common from silodosin group. All the AEs with the study medications were of Level 1 severity,[8] not warranting discontinuation or substitution indicating a good tolerability in the dose employed. There were no clinically significant drug interactions with the study drugs and the concomitant medications. The patient compliance for the study medications was good. The missing of the doses was because of good symptomatic improvement, cost, and nonavailability, but not due to poor tolerability. The good tolerability and high level of compliance to the study medications are indicators for good patient acceptability. The lack of QTc prolongation with silodosin is of a particular advantage while using in elderly patients with preexisting cardiac disease and risk of arrhythmia, but the higher cost of silodosin would be a limiting factor for long-term usage. Some of the limitations of the study were small sample size, short duration of follow-up, exclusion of subjects likely for acute urinary retention, and the study involving a single center. Further, elaborate multi-centric studies involving a larger number of subjects from different geographical regions, with different comorbid conditions and risk factors, concomitant medications, and a longer duration of therapy, may be undertaken to generate more data regarding the relative efficacy and tolerability of these medications and also to detect very rare or unusual adverse effects.
Conclusion
Alfuzosin, tamsulosin, and silodosin produced effective subjective and symptomatic improvement in subjects with BPH and LUTS, with a comparable efficacy in improving primary and secondary outcome measures. The response was maximum within first 2 weeks with sustained progressive improvement throughout the study period. All the three study drugs showed good tolerability and acceptability with excellent patient compliance.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Rossi M, Roumeguère T. Silodosin in the treatment of benign prostatic hyperplasia. [Last cited on 2013 Mar 01];Drug Des Devel Ther. 2010 4:291–7. doi: 10.2147/DDDT.S10428. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2990389/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida M, Kudoh J, Homma Y, Kawabe K. Safety and efficacy of silodosin for the treatment of benign prostatic hyperplasia. Clin Interv Aging. 2011;6:161–72. doi: 10.2147/CIA.S13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007;9:181–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Nickel JC, Sander S, Moon TD. A meta-analysis of the vascular-related safety profile and efficacy of alpha-adrenergic blockers for symptoms related to benign prostatic hyperplasia. Int J Clin Pract. 2008;62:1547–59. doi: 10.1111/j.1742-1241.2008.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNicholas TA, Kirby RS, Lepor H. Evaluation and nonsurgical management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Novick AC, Peters CA, editors. Campbell-walsh Urology. 10th ed. Philadelphia, PA: Saunders; 2012. pp. 2611–54. [Google Scholar]
- 6.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 7.Uppsala, Sweden: Pharmacovigilance; [Last updated on 2013 Jan 30; Last cited on 2013 Mar 01]. The Uppsala Monitoring Centre. Available from: http://www.who-umc.org/Graphics/26649.pdf . [Google Scholar]
- 8.Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: A prospective analysis of 3695 patient-episodes. PLoS One. 2009;4:e4439. doi: 10.1371/journal.pone.0004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roehrborn CG. Benign prostatic hyperplasia: An overview. Rev Urol. 2005;7(Suppl 9):S3–S14. [PMC free article] [PubMed] [Google Scholar]
- 10.Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism (NIAAA); 2013. [Last updated on 2014 Jul 01; Last cited on 2014 Jul 01]. National Institute on Alcohol Abuse and Alcoholism (NIAAA) Available from: http://www.pubs.niaaa.nih.gov/publications/Social/Module1Epidemiology/Module1.html . [Google Scholar]
- 11.Yu HJ, Lin AT, Yang SS, Tsui KH, Wu HC, Cheng CL, et al. Non-inferiority of silodosin to tamsulosin in treating patients with lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) BJU Int. 2011;108:1843–8. doi: 10.1111/j.1464-410X.2011.10233.x. [DOI] [PubMed] [Google Scholar]
- 12.Roehrborn CG, Rosen RC. Medical therapy options for aging men with benign prostatic hyperplasia: Focus on alfuzosin 10 mg once daily. Clin Interv Aging. 2008;3:511–24. doi: 10.2147/cia.s3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusco F, Palmieri A, Ficarra V, Giannarini G, Novara G, Longo N, et al. α1-blockers improve benign prostatic obstruction in men with lower urinary tract symptoms: A systematic review and meta-analysis of urodynamic studies. Eur Urol. 2016 doi: 10.1016/j.eururo.2015.12.034. pii: S0302-283801254-3. [DOI] [PubMed] [Google Scholar]
- 14.Takeshita H, Moriyama S, Arai Y, Washino S, Saito K, Chiba K, et al. Randomized crossover comparison of the short-term efficacy and safety of single half-dose silodosin and tamsulosin hydrochoride in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. Low Urin Tract Symptoms. 2016;8:38–43. doi: 10.1111/luts.12106. [DOI] [PubMed] [Google Scholar]
- 15.Pande S, Hazra A, Kundu AK. Evaluation of silodosin in comparison to tamsulosin in benign prostatic hyperplasia: A randomized controlled trial. Indian J Pharmacol. 2014;46:601–7. doi: 10.4103/0253-7613.144912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan JQ, Mao C, Wong SY, Yang ZY, Fu XH, Dai XY, et al. Comparative effectiveness and safety of monodrug therapies for lower urinary tract symptoms associated with benign prostatic hyperplasia: A network meta-analysis. Medicine (Baltimore) 2015;94:e974. doi: 10.1097/MD.0000000000000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keating GM. Silodosin: A review of its use in the treatment of the signs and symptoms of benign prostatic hyperplasia. Drugs. 2015;75:207–17. doi: 10.1007/s40265-014-0344-z. [DOI] [PubMed] [Google Scholar]
- 18.Welk B, McArthur E, Fraser LA, Hayward J, Dixon S, Hwang YJ, et al. The risk of fall and fracture with the initiation of a prostate-selective α antagonist: A population based cohort study. BMJ. 2015;351:h5398. doi: 10.1136/bmj.h5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepor H, Lepor NE, Hill LA, Trohman RG. The QT interval and selection of alpha-blockers for benign prostatic hyperplasia. Rev Urol. 2008;10:85–91. [PMC free article] [PubMed] [Google Scholar]


