Abstract
Objectives:
To investigate nonsteroidal anti-inflammatory drugs effectiveness in colorectal distension (CRD)-induced visceral pain model.
Materials and Methods:
Male Sprague–Dawley (250–300 g) rats were anesthetized with ketamine (50 mg/kg, intraperitoneally [i.p.]) and chlorpromazine (25 mg/kg, i.p.). Two bipolar Teflon-coated Ni/Cr wire electrodes (80-M diameter) were placed in the abdominal external oblique muscle for the recording of electromyography. Jugular vein catheter was placed for the administration of drugs. CRD method was applied to evaluate of visceral pain. All drugs (paracetamol, meloxicam, metamizole, and dexketoprofen) administered intravenously.
Results:
Paracetamol 200, 400, and 600 mg/kg did not change the visceromotor response (VMR) when compare with the control group. Meloxicam 2 and 4 mg/kg showed no effect but at doses of 6 mg/kg meloxicam significantly ([51.9 ± 6.4%] [P < 0.001]) decreased VMR compared with the control group. Metamizole 200 mg/kg did not change responses but dose of 400 and 600 mg/kg metamizole reduced VMR. Dexketoprofen 2 and 4 mg/kg did not cause a change in VMR but 6 mg/kg dose significantly reduced response compared with the control group ([43.9 ± 3.9%, 36.8 ± 2.8%, 34.8 ± 2.5%, 42.1 ± 4.8%, 40.7 ± 3.5%, 36.4 ± 2.7%, and 26.1 ± 2.2%]; from 10 min to 70 min, respectively, [P < 0.05]).
Conclusion:
Metamizole, dexketoprofen and meloxicam show antinociceptive effect with different duration of action on CRD-induced visceral pain model. This condition can be explained due to different chemical structures and different mechanisms which play a role in modulation of pain.
KEY WORDS: Anti-inflammatory, rat, visceral pain
Introduction
Opioids and nonsteroid anti-inflammotory (NSAIDs) drugs are two of the most commonly used drugs for the treatment of pain.[1] Although opioids are most effective and potent analgesics, they have limited use in chronic pain due to their potentially adverse reactions such as addiction, tolerance development, respiratory depression, and constipation.[1] NSAIDs have been used in acute and chronic pain management such as surgical operations, irritable bowel syndrome, dental procedures, and renal and biliary colic.[2,3] However, NSAIDs have also been associated with a number of side effects including gastrointestinal bleedings, hepatic, and renal impairments.[4,5] Both opioids and NSAIDs may share common indications. Practically, opioids are most likely preferred on the same indications because of their potent effect.[6] Thus, to reduce the side effect of both these two class of analgesics, and to prevent the development of tolerance of opioids, the opioid analgesics have been combined with NSAIDs.[6,7] For this reason, to establish the analgesic efficacy and potency of NSAIDs in comparison to opioid analgesics by performing new experimental and clinical studies are so important.
All of the NSAIDs used in our study are the most commonly preferred analgesics in the pain management. Regarding the pain mechanisms of NSAIDs, most of the knowledge has been obtained from experimental studies performed on somatic pain models rather than visceral pain models. Preclinically, the analgesic effects of NSAIDs have been investigated on visceral chemical pain models such as writhing test[5,6] and on somatic pain models such as hot plate and tail flick.[8] In this regard, there are limited studies available that evaluate the antinociceptive effectiveness of NSAIDs in a comparative manner using colorectal distension (CRD)-induced visceral pain model.[8,9,10,11]
Visceral pain originates from internal organs, and it is one of the most frequent causes for medical attention because of its poor localization and characterization,[12] it differs from the somatic pain of superficial organs such as skin and muscle. In our study, CRD model was used, distension of colon causes a noxious stimuli and a measurable visceromotor response (VMR) seen as a noxious response at the external abdominal muscles.[13] In this study, we aimed to evaluate the putative antinociceptive effect of meloxicam, metamizole sodium, dexketoprofen, and paracetamol by using CRD-induced pain model.
Materials and Methods
Animals
Forty adult male Sprague-Dawley rats weighing 250–300 g were used and all rats were obtained from “Laboratory Animals Application and Research Center” (Ondokuz Mayis University, Samsun). The rats were maintained in an environmentally controlled temperature (22 ± 1°C on a 12-h alternating light–dark cycle). All experiments were approved by the Institutional Animal Care and Use Committee of the Ondokuz Mayis University and adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain.
Surgical Preparation
Rats were anesthetized with 50 mg/kg ketamine and 25 mg/kg chlorpromazine intraperitoneally (i.p.) and enameled nichrome wire electrodes (diameter: 80 µm; Driver-Harris, Cedex, France) were implanted into the external oblique musculature of the rats, just above the inguinal ligament, to record electromyography (EMG) activities. During operation, a jugular vein catheter was applied to administrate of drugs by intravenous (i.v.) route. Then, EMG electrodes and venous catheter were tunneled subcutaneously and externalized at the nape of the neck of rats. After surgery, rats were kept individually with free access to food and water and were adapted in Bollman cages (Bahadır Co., Turkey) to manipulation and to reduce distress during the next 6 days before the experiments to be performed.
Experimental Protocol
CRD was used to induce nociception in rats. A flexible Tygon plastic tubing was inserted into a latex balloon (6–7 cm in length), with the end of the balloon securely tied to the tube. The balloon was lubricated with ultrasound gel (Norm Co., Turkey) and inserted intra-anally into the descending colon so that the balloon was 1 cm put into the rectum. The tubing was taped to the base of the tail to prevent dislocation. Rats were fully awake and inside in Bollman cages during testing. CRD was produced by inflation of the balloon with air via an injector. The catheter was attached to a bridge amplifier (ML221, AD Instruments, Australia) via a pressure transducer (MLT380, AD Instruments). The intracolonic pressure was monitored and recorded by a data acquisition system (ML870/P, PowerLab 8/30, AD Instruments) connected to the bridge amplifier.
The VMR, encompass the contraction of the external oblique musculature (X12), was quantified from EMG activity recorded from the electrodes implanted in the external oblique musculature. The EMG signal was amplified using a Bio Amp (ML132, ADInstruments, Australia) connected to the data acquisition system and integrated offline using the Chart programme (version 5.2, ADInstruments). A stable distension procedure was used for CRD. Briefly, the intracolonic pressure was incremented to the 80 mm Hg directly in 20 s. Then, EMG activity from the first 20 s of this pressure was quantified and taken for data analysis. On the day of testing, five stable distensions, at 5 min intervals, were given to establish the baseline response before drug administration. Stable distension was administered 10, 20, 30, 40, 50, 60, 70, 80, and 90 min after drugs or saline administration. All drugs and saline were given by i.v. route.
Drugs
All drugs were freshly dissolved in saline and administered i.v. in a volume of 1 ml/kg body weight. Paracetamol (a generous gift from Gripin Co., Turkey), meloxicam (Sanovel Co., Turkey), metamizole sodium (Sanofi Aventis, France), dexketoprofen (Ufsa Co., Turkey), and morphine hydrochloride (Galen Co., Turkey) antinociception were evaluated.
Data Analysis
All data are expressed as a mean ± standard error of the mean with six rats per group. The VMR to CRD is represented as a percentage of control (% control), where the mean of the predrug responses to 80 mm Hg is defined as 100%. The overall effect of any treatment was determined by taking the area under the curve (AUC) of the time-response function with an Excel computer program. The AUC was calculated from the time plot of postdrug responses normalized to the baseline response (100%), plotted against time using the trapezoidal rule (AUC = response × 90 min).
Statistical analyses were performed using the GraphPad In Stat (version 3.06) software (GraphPad Software, San Diego, CA, USA). After confirmation of normal distribution of one-way analysis of variance between groups for post hoc test Tukey–Kramer test was used. P < 0.05 was considered statistically significant.
Results
Antinociceptive Effect of Paracetamol
Paracetamol did not cause a statistically significant alteration on VMR in 100 mg/kg and 200 mg/kg doses compared to the control group (saline). Paracetamol caused a statistically antinociceptive effect only at the 20 min at a dose of 400 mg/kg (40.1 ± 3.3%) (P < 0.05) [Figure 1]. The analgesic effect of paracetamol completely finished after the 20 min and not continued after the following minutes.
Figure 1.
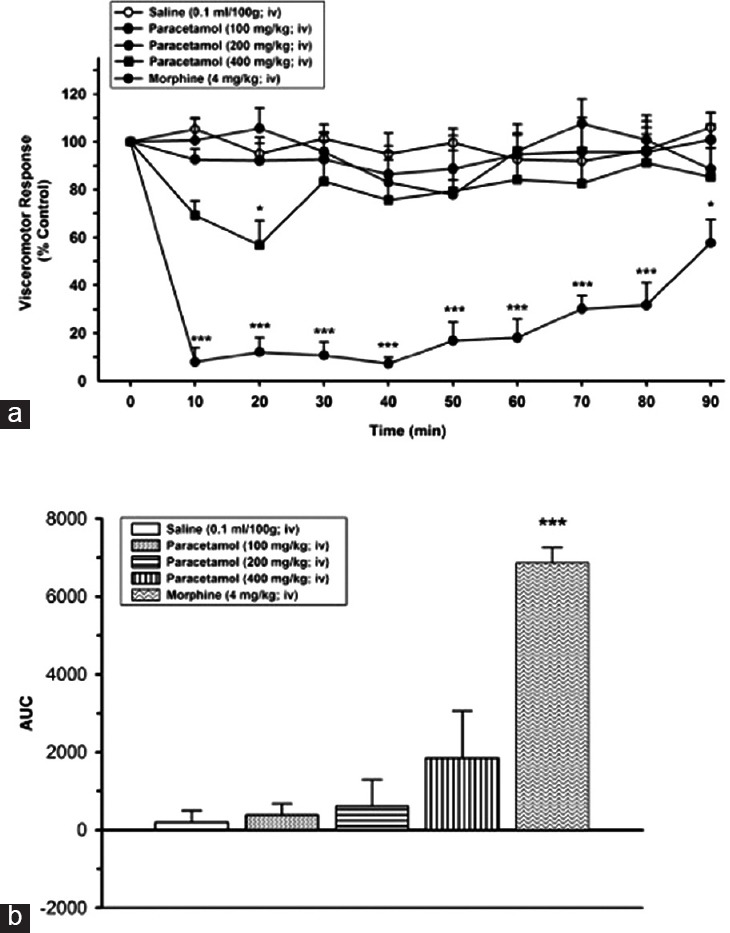
(a) Effects of paracetamol at the doses of 100 and 200 mg/kg, intravenous, P > 0.05, and at the dose of 400 mg/kg, intravenous, *P < 0.05, on visceromotor response when compared to time point of administration of saline group. (b) Area under the curve values of alteration on visceral response
Antinociceptive Effect of Meloxicam
Meloxicam did not cause a statistically significant effect on VMR in 2 mg/kg and 4 mg/kg dosages compared to the control group (saline). Meloxicam reduced the VMR only at the dose of 6 mg/kg (51.9 ± 6.4%) (P < 0.001) and the antinociceptive effect initiated at 30 min and this effect continued to till 60 min; (at 40 [36.9 ± 3.1%], at 50 [45.6 ± 3.7%] and at 60 min [38.4 ± 3.7%] [P < 0.01]). During 60–90 min VMR did not change compared to control group (saline[SF]) (P > 0.05) [Figure 2].
Figure 2.
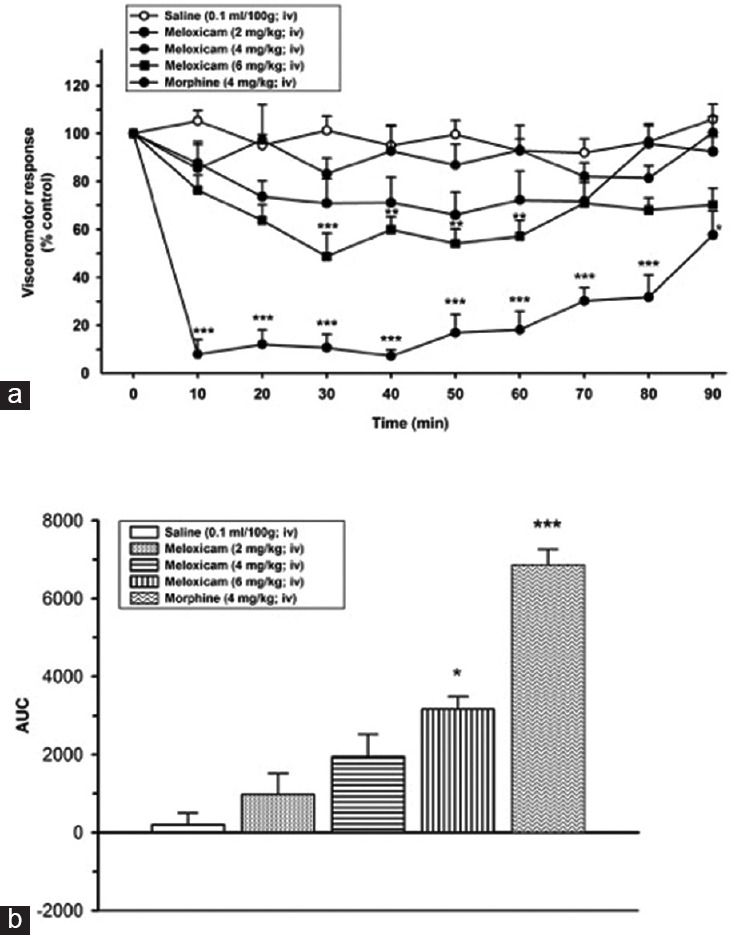
(a) Effects of meloxicam (2, 4, and 6 mg/kg, intravenous) on visceromotor response (*P < 0.05, **P < 0.01, ***P < 0.001); when compared to time point of administration of saline group. (b) Area under the curve values of alteration on visceral response
Antinociceptive Effect of Metamizole Sodium
No statistically significant reduction has been detected at the dose of 200 mg/kg metamizole sodium compared to control group (saline). Metamizole sodium lead to a statistically significant reduction on VMR of at the doses of 400 and 600 mg/kg compared to control group (saline) (P < 0.05). The antinociceptive effect of metamizole sodium at the dose of 400 mg/kg initiated at 40 min and lasted up to 70 min; (at 40 [45 ± 3.2%], at 50 [48.4 ± 3.6%], at 60 min [41.0 ± 4.2%], and at 70 min [37.7 ± 3.2%]); (P < 0.05 during 40 and 70 min).
The antinociceptive effect of metamizole sodium at the dose of 600 mg/kg initiated at 20 min and lasted up to 90 min. The 600 mg/kg dose of metamizole sodium decreased the VMR compared to control group initiated at 20 min and went on until 90 min (65.9 ± 7.3%; 72.3 ± 8.6%, 54.4 ± 5.1%, 56.5 ± 4.8%, 57.3 ± 4.4%, 44.4 ± 3.6%, 45.1 ± 4.7%, and 36.4 ± 2.7%) from 20 to 90 min, respectively, (P < 0.001 from 20 to 60 min, P < 0.01 from 70 to 80 min, P < 0.05 at 90 min) [Figure 3].
Figure 3.
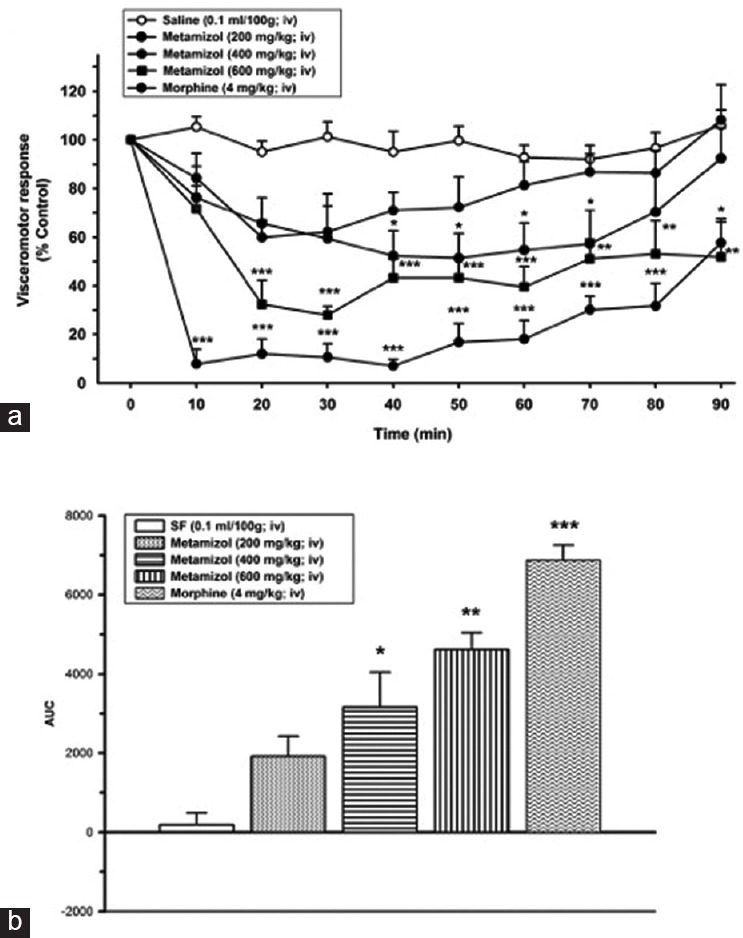
(a) Effects of metamizol sodium (200, 400, and 600 mg/kg, intravenous) on visceromotor response (*P < 0.05, **P < 0.01, ***P < 0.001); when compared to time point of administration of SF group. (b) Area under the curve values of alteration on visceral response
Antinociceptive Effect of Dexketoprofen
Administration of 2 mg/kg and 4 mg/kg i.v. dexketoprofen did not show a statistically significant reduction on VMR compared to control group (SF). Dexketoprofen at the dose of 6 mg/kg led to a statistically significant reduction on VMR at 10 min and it remain until 70 min ([43.9 ± 3.9%, 36.8 ± 2.8%, 34.8 ± 2.5%, 42.1 ± 4.8%, 40.7 ± 3.5%, 36.4 ± 2.7%, and 26.1 ± 2.2% from 10 min to 70 min, respectively, [P < 0.05]) [Figure 4].
Figure 4.
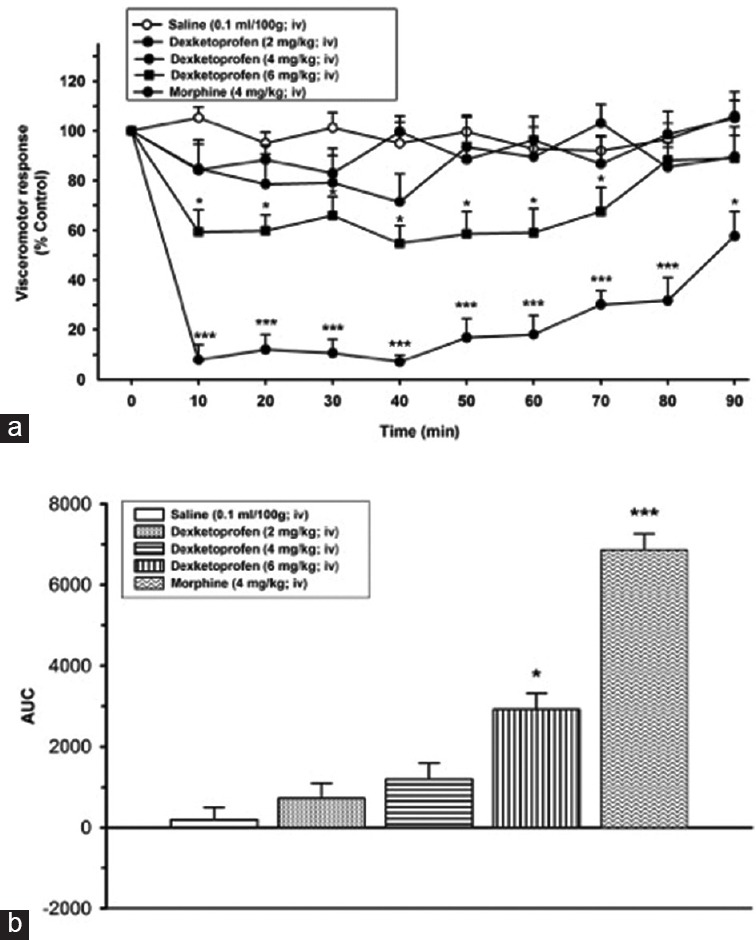
(a) Effects of dexketoprofen (2, 4, and 6 mg/kg, intravenous) on visceromotor response (*P < 0.05, ***P < 0.001); when compared to time point of administration of SF group. (b) Area under the curve values of alteration on visceral response
Discussion
This study was designed to investigate the antinociceptive effects of NSAIDs; paracetamol, dexketoprofen, meloxicam, and metamizole on CRD-induced visceral pain model. Paracetamol did not show any antinociceptive effect at the doses of 100, 200, and 400 mg/kg while meloxicam at a dose of 6 mg/kg, metamizole sodium at the doses of 400 and 600 mg/kg, and dexketoprofen at a dose of 6 mg/kg exerted a statistically significant antinociceptive effect.
The major finding of our study was that paracetamol exerted a weak antinociceptive effect, and this effect was only seen at 20 min. Paracetamol had a clear antinociceptive effect in hot plate, tail flick, writhing test, formalin test, and paw withdrawal test.[14,15,16] Furthermore, in clinical practice, paracetamol is a widely used analgesic, especially in postoperative pain management alone and/or in combination with the opioids.[17] Despite its wide use, the mechanism of the clear analgesic action of paracetamol is not fully understood. So far, some possible antinociceptive mechanisms have been proposed; for instance, the inhibition of cyclooxygenase (COX) enzyme,[18] interaction with the cannabinoid receptors,[19] and interaction with the serotonergic system.[20] According to our results, the lack of antinociceptive effect of paracetamol may depend on either pharmacokinetic parameters or different affected nociceptive pathways such as a spinal or supraspinal level. In the previous studies, the antinociceptive effect of paracetamol was observed at doses from 50 mg/kg to 400 mg/kg systemically (i.p. or i.v.), and these doses were shown to be effective to reduce the nociception in pain models.[21,22] Jensen et al.[23] have reported that paracetamol had a clear antinociceptive effect at the dose of 5 µg given intrathecally, but a failure was seen at the dose of 50 mg/kg given by i.v. route in rabbits in CRD model. One of the antinociceptive effect of paracetamol was attributed to its central effect; for instance, regarding the nociception produced by hot plate test, it was suggested that the licking and jumping behavior was controlled by supraspinal centers, for this reason drugs found effective in hot plate test was accepted as they are able to reach effective concentration in brain. In studies conducted on rats and humans, it has been reported that paracetamol was able to cross blood–brain barrier easily and able to decrease prostaglandin (PG) synthesis.[24,25] Hence, the weak antinociceptive effect of paracetamol may result as paracetamol act spinally, rather than supraspinal brain centers, in CRD-induced pain. Thus, 400 mg/kg, i.p. dose of paracetamol did not reach to the spinal areas adequately to produce analgesia.
The second important results in our study were obtained with the other NSAIDs; metamizole sodium, meloxicam, and dexketoprofen. The overall analgesic pattern of these three NSAIDs at their effective doses was very similar to morphine analgesia; the antinociception reached to 80% of morphine analgesia with metamizole. Björkman et al.[8] showed that the analgesic effect of diclofenac was reversed by naloxone. Therefore, the analgesic effect of NSAIDs used in our study may be related to the opioidergic and also other pain inhibitory pathways. Metamizole, a pyrazolone derivative, has potent analgesic and antipyretic effects. It was established that metamizole and its active metabolite 4-methylamino antipyrine acts through the inhibition of central COX enzyme so that the inhibition of the PG synthesis. It has been also suggested that the antinociceptive effect of metamizole sodium was reversed by naloxone.[26] Another study reported that intradermally applied metamizole was shown to be locally effective and delay nociceptive tail-flick responses drastically and this antinociceptive effect was (reversible when) given naloxone.[27] Dexketoprofen is S + enantiomer of ketoprofen which belongs to 2-arilpropionik acid group. It was shown that ketoprofen has antinociceptive effect in acetic acid induced writhing test and formalin test.[28] Dexketoprofen is more potent analgesic than ketoprofen. Following a laminar discectomy surgery, 35% reduction in morphine consumption was reported.[29] Moreover, it was indicated that dexketoprofen decreases the usage of morphine in people who underwent abdominal hysterectomy.[28] In the previous studies, the S-(+)-ketoprofen induced antinociceptive effect has been reported to act through central mechanisms and this effect was naloxone reversible.[29] These results show that opioidergic receptors have a role in the antinociceptive effect of S-(+)-ketoprofen.
Meloxicam is a variant of oxicam, selective COX-2 inhibitor because it exerts a stronger inhibitory effect on COX-2 compared to COX-1. It is believed that nitric oxide-cyclic guanosine monophosphate pathway has an essential role in the antinociceptive effect of meloxicam.[30] Furthermore, it was showed that Ca2+-activated K+ channels take a place in the antinociceptive effect of meloxicam in the formalin test.
Conclusion
NSAIDs used in this study showed a discrepancy in terms of decrease in nociception inhibition and time of action in CRD-induced visceral pain model. This can be explained by different pharmacologic and chemical properties of the drugs used in the study and affecting different mechanisms involved in pain modulation. The next aim of our study is to examine other NSAIDs in this model and investigate their mechanisms. At least, in pain management, NSAIDs used in our study may be combined with opioidergic drugs and may decrease opioid consumption in visceral pain and related syndromes.
Financial Support and Sponsorship
Ondokuz Mayis University Research Fund, PYO.TIP. 1904.11.024.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
The authors thank Selami Turel for his technical support.
References
- 1.Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3:263–75. doi: 10.3978/j.issn.2224-5820.2014.10.02. [DOI] [PubMed] [Google Scholar]
- 2.Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig. 2012;32(Suppl 1):53–63. doi: 10.2165/11630080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Habib I, Mazulis A, Roginsky G, Ehrenpreis ED. Nonsteroidal anti-inflammatory drugs and inflammatory bowel disease: Pathophysiology and clinical associations. Inflamm Bowel Dis. 2014;20:2493–502. doi: 10.1097/MIB.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 4.Süleyman H, Demircan B, Karagöz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. 2007;59:247–58. [PubMed] [Google Scholar]
- 5.Nash MS, McIntyre P, Groarke A, Lilley E, Culshaw A, Hallett A, et al. 7-tert-Butyl-6-(4-chloro-phenyl)-2-thioxo-2,3-dihydro-1H-pyrido[2,3-d] pyrimidin-4-one, a classic polymodal inhibitor of transient receptor potential vanilloid type 1 with a reduced liability for hyperthermia, is analgesic and ameliorates visceral hypersensitivity. J Pharmacol Exp Ther. 2012;342:389–98. doi: 10.1124/jpet.112.191932. [DOI] [PubMed] [Google Scholar]
- 6.Miranda HF, Pinardi G. Lack of effect of naltrexone on the spinal synergism between morphine and nonsteroidal anti-inflammatory drugs. Pharmacol Rep. 2009;61:268–74. doi: 10.1016/s1734-1140(09)70031-3. [DOI] [PubMed] [Google Scholar]
- 7.Rivkin A, Rivkin MA. Perioperative nonopioid agents for pain control in spinal surgery. Am J Health Syst Pharm. 2014;71:1845–57. doi: 10.2146/ajhp130688. [DOI] [PubMed] [Google Scholar]
- 8.Björkman R. Central antinociceptive effects of non-steroidal anti-inflammatory drugs and paracetamol. Experimental studies in the rat. Acta Anaesthesiol Scand Suppl. 1995;103:1–44. [PubMed] [Google Scholar]
- 9.Ohmori H, Iwasaki H, Omote K, Kobayashi I, Namiki A. Differential effects of morphine and non-steroidal anti-inflammatory drugs on somatic and visceral pain in rats. Masui. 1994;43:1310–3. [PubMed] [Google Scholar]
- 10.Hultin L, Nissen TD, Kakol-Palm D, Lindström E. Colorectal distension-evoked potentials in awake rats: A novel method for studies of visceral sensitivity. Neurogastroenterol Motil. 2012;24:964–e466. doi: 10.1111/nmo.12005. doi: 101111/nmo12005. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez C, Zegpi C, Noriega V, Prieto JC, Miranda HF. Synergism between dexketoprofen and meloxicam in an orofacial formalin test was not modified by opioid antagonists. Pharmacol Rep. 2011;63:433–40. doi: 10.1016/s1734-1140(11)70509-6. [DOI] [PubMed] [Google Scholar]
- 12.Tomic MA, Vuckovic SM, Stepanovic-Petrovic RM, Ugresic ND, Prostran MS, Boskovic B. Synergistic interactions between paracetamol and oxcarbazepine in somatic and visceral pain models in rodents. Anesth Analg. 2010;110:1198–205. doi: 10.1213/ANE.0b013e3181cbd8da. [DOI] [PubMed] [Google Scholar]
- 13.Jones RC, 3rd, Gebhart GF. Models of visceral pain: Colorectal distension (CRD) Curr Protoc Pharmacol 2004 Sep 1. doi: 10.1002/0471141755.ph0536s25. Chapter 5:Unit 5.36. doi: 10.1002/0471141755. ph0536s25. [DOI] [PubMed] [Google Scholar]
- 14.Pinardi G, Sierralta F, Miranda HF. Adrenergic mechanisms in antinociceptive effects of nonsteroidal anti-inflammatory drugs in acute thermal nociception in mice. Inflamm Res. 2002;51:219–22. doi: 10.1007/pl00000296. [DOI] [PubMed] [Google Scholar]
- 15.Gong YH, Yu XR, Liu HL, Yang N, Zuo PP, Huang YG. Antinociceptive effects of combination of tramadol and acetaminophen on painful diabetic neuropathy in streptozotocin-induced diabetic rats. Acta Anaesthesiol Taiwan. 2011;49:16–20. doi: 10.1016/j.aat.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Reid AR, Sawynok J. Antinociception by systemically-administered acetaminophen (paracetamol) involves spinal serotonin 5-HT7 and adenosine A1 receptors, as well as peripheral adenosine A1 receptors. Neurosci Lett. 2013;536:64–8. doi: 10.1016/j.neulet.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 17.Beloeil H. Postoperative non-opioid analgesics management. Presse Med. 2015;44(6 Pt 1):601–9. doi: 10.1016/j.lpm.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Hinz B, Brune K. Paracetamol and cyclooxygenase inhibition: Is there a cause for concern? Ann Rheum Dis. 2012;71:20–5. doi: 10.1136/ard.2011.200087. [DOI] [PubMed] [Google Scholar]
- 19.Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006;531:280–1. doi: 10.1016/j.ejphar.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Pickering G, Estève V, Loriot MA, Eschalier A, Dubray C. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008;84:47–51. doi: 10.1038/sj.clpt.6100403. [DOI] [PubMed] [Google Scholar]
- 21.Sandrini M, Romualdi P, Capobianco A, Vitale G, Morelli G, Pini LA, et al. The effect of paracetamol on nociception and dynorphin A levels in the rat brain. Neuropeptides. 2001;35:110–6. doi: 10.1054/npep.2001.0852. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey L, Yan L, Clarke GD, Ledent C, Kitchen I, Hourani SM. Modulation of paracetamol antinociception by caffeine and by selective adenosine A2 receptor antagonists in mice. Eur J Pharmacol. 2006;531:80–6. doi: 10.1016/j.ejphar.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Jensen FM, Dahl JB, Frigast C. Direct spinal effect of intrathecal acetaminophen on visceral noxious stimulation in rabbits. Acta Anaesthesiol Scand. 1992;36:837–41. doi: 10.1111/j.1399-6576.1992.tb03574.x. [DOI] [PubMed] [Google Scholar]
- 24.Courade JP, Chassaing C, Bardin L, Alloui A, Eschalier A. 5-HT receptor subtypes involved in the spinal antinociceptive effect of acetaminophen in rats. Eur J Pharmacol. 2001;432:1–7. doi: 10.1016/s0014-2999(01)01464-9. [DOI] [PubMed] [Google Scholar]
- 25.Slosky LM, Thompson BJ, Sanchez-Covarrubias L, Zhang Y, Laracuente ML, Vanderah TW, et al. Acetaminophen modulates P-glycoprotein functional expression at the blood-brain barrier by a constitutive androstane receptor-dependent mechanism. Mol Pharmacol. 2013;84:774–86. doi: 10.1124/mol.113.086298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tortorici V, Vásquez E, Vanegas H. Naloxone partial reversal of the antinociception produced by dipyrone microinjected into the periaqueductal gray of rats. Possible involvement of medullary off- and on-cells. Brain Res. 1996;725:106–10. doi: 10.1016/0006-8993(96)00196-5. [DOI] [PubMed] [Google Scholar]
- 27.Dogrul A, Gülmez SE, Deveci MS, Gul H, Ossipov MH, Porreca F, et al. The local antinociceptive actions of nonsteroidal antiinflammatory drugs in the mouse radiant heat tail-flick test. Anesth Analg. 2007;104:927–35. doi: 10.1213/01.ane.0000258773.46897.34. [DOI] [PubMed] [Google Scholar]
- 28.Tuncer S, Reisli R, Keçecioglu M, Erol A. The effects of intravenous dexketoprofen on postoperative analgesia and morphine consumption in patients undergoing abdominal hysterectomy. Agri. 2010;22:98–102. [PubMed] [Google Scholar]
- 29.Kesimci E, Gümüs T, Izdes S, Sen P, Kanbak O. Comparison of efficacy of dexketoprofen versus paracetamol on postoperative pain and morphine consumption in laminectomy patients. Agri. 2011;23:153–9. doi: 10.5505/agri.2011.86548. [DOI] [PubMed] [Google Scholar]
- 30.Lizarraga I, Chambers JP. Involvement of opioidergic and alpha2-adrenergic mechanisms in the central analgesic effects of non-steroidal anti-inflammatory drugs in sheep. Res Vet Sci. 2006;80:194–200. doi: 10.1016/j.rvsc.2005.06.001. [DOI] [PubMed] [Google Scholar]


