Abstract
Objectives:
Drugs used for toxoplasmosis have limited efficacy and also severe side effects. A new drug with good efficacy and limited side effects is need of the hour. We studied the effects of artemether on Toxoplasma gondii in vitro conditions.
Materials and Methods:
Artemether (methyl-ether-qinghaosu) was tested for tachyzoites, J774, and Vero cell lines infected by T. gondii. For evaluating the effect of drugs on Vero cells infected with T. gondii, we designed two separate experiments; in the first experiment, the Vero cells were infected with tachyzoites and then treated with artemether; while in the second one, the tachyzoites were exposed to artemether and then Vero cells were infected with treated tachyzoites. For evaluating the apoptotic effect of artemether on tachyzoites and infected J774 macrophages cell line with T. gondii, we used flow cytometry method. Inhibitory concentration (IC50) was evaluated by intracellular replication of tachyzoites in Vero cells.
Results:
IC50 for infected Vero cells with tachyzoites was determined as 49.13 μg/ml. In pretreated tachyzoites with artemether before entering into Vero cells, IC50 was calculated as 13.15 μg/ml. In both experiments, artemether showed a higher inhibitory effect than sulfadiazine (positive control). Artemether even at the highest concentrations only showed low cytotoxicity on Vero and J774 cell lines. Apoptosis in tachyzoites rise with an increasing concentration of artemether.
Conclusions:
Our findings indicate that artemether is effective to control the tachyzoites of T. gondii in vitro and maybe a good alternative drug for toxoplasmosis.
KEY WORDS: Apoptosis, artemether, in vitro, J774, Toxoplasma gondii, Vero
Introduction
Toxoplasma gondii is an obligate intracellular parasite,[1] and it has been estimated that this protozoan has infected about one-third of the world population.[2] Felines in the life cycle of this parasite are definitive hosts that can excrete the oocyst,[3] and all warm-blooded animals including humans and birds plus other mammals as intermediate hosts can be infected. The serological tests for toxoplasmosis in all over the world for domestic cat are stimulated at 30–40%.[4]
The most serious cases of toxoplasmosis are related to congenital and HIV infection. Serological prevalence of toxoplasmosis in the USA is about 40%.[5] Mortality rate of toxoplasmosis in AIDS in the USA is 10% and in Europe it is 30%.[6] In a study about the prevalence of toxoplasmosis in India on pregnant women, Singh et al. showed that 45% were IgG positive, whereas 3.3% were IgM positive.[7]
In a screening study in the Central Parts of Iran, the seroprevalence of Toxoplasma antibodies among pregnant women using indirect fluorescent antibody was 27.6%.[8] The researches on Toxoplasma antibodies demonstrated that about 20–70% of populations among different countries are infected chronically.[9]
Artemisia annua L. is a Chinese herb that has antimalarial activity.[8,9,10] Artemisinin and its derivatives that are made from Artemisia annua L. have end peroxide linkage and heme iron that can play an important role in the mechanism of the action.[9] One of the semi-synthetic derivatives of artemisinin that named artemether is a sesquiterpene lactone endoperoxide. It, moreover, has antimalarial and antileishmanial activity and used extensively for malaria.[11]
The standard therapies which are chosen for toxoplasmosis are sulfadiazine plus pyrimethamine. These drugs are very effective against the tachyzoites, but cannot eliminate bradyzoite stage of T. gondii.[1,12]
Apoptosis is a physiologic programed process which occurs in the maintenance of tissue homeostasis, under pathological conditions and other cases.[13] Flow cytometry is a sensitive method for the detection of apoptosis by using Annexin V. In this study, we applied this method for detection of the apoptosis and necrosis of tachyzoites and J774 (a cell line of macrophages) infected with tachyzoites of T. gondii after treatment.
Artemisinin and derivatives including artemether have been used for the treatment of malaria.[14] T. gondii like Plasmodium falciparum is an apicomplexan, we have predicated the activity of anti-toxoplasma for artemether. Therefore, the purpose of this study was to evaluate the effects of artemether on T. gondii in vitro for prophylaxis and also for therapy with two models.
Materials and Methods
In this study, all the tests were repeated 3 times.
Ethics Statement
This experiment was approved by Ethics Committee of Tarbiat Modares University, Faculty of Medical Sciences, permit number: D52/3525 in December 26, 2012.
Vero Cell Culture
Vero cell line (African green monkey kidney cells) cultured in 25 cm2 flasks until confluence in Dulbecco's modified Eagle's medium (DMEM) and high glucose medium (Gibco) containing pyruvate and NaHCO3, supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% heat-inactivated fetal bovine serum (FBS) in an incubator at 37°C and 5% CO2.[15]
J774 Cell Culture
J774 cell line (cell line from mouse BALB/c monocyte macrophage) cultured in 25 cm2 flasks until confluence in RPMI-1640 medium (Gibco), supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% heat-inactivated FBS in a humidified incubator at 37°C and 5% CO2.[16]
Tachyzoites of Toxoplasma Gondii
Tachyzoites of the virulent RH strain of T. gondii were maintained in serial passages in Vero cells in 25 cm2 flasks. Tachyzoites were harvested and washed with phosphate-buffered saline (PBS) by centrifuge (2000 rpm, 10 min, 4°C). Parasites were suspended in RPMI-1640 (Gibco) medium and the number of viable tachyzoites was determined by Trypan blue exclusion in hemocytometric chamber.[17] The tachyzoites were used for in vitro experiment.
Artemether and Sulfadiazine Preparation
Artemether (methyl-ether-qinghaosu) was purchased from Exim Pharm Co. (USA). Artemether was prepared in ethanol–water (v/v) (30–70%) with 1000 µg/ml concentration, then 5, 10, 25, 50, and 100 µg/ml dilutions were made from stock solutions in DMEM.[18] Sulfadiazine was obtained from Sigma (Sigma-Aldrich) and solved in DMSO (dimethyl sulfoxide) as stock solutions with 1000 µg/ml concentration. Following 1.6, 3.12, 6.25, 12.5, 25, 50, 100, and 200 µg/ml dilutions were prepared in DMEM. Provided solutions were stored at 4°C and used in cytotoxicity assays and in vitro experiments.[1]
In Vitro Assay
Cytotoxicity assay
Cytotoxicity of artemether was assessed by determining cellular viability using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT, Sigma-Aldrich) assay. Briefly, Vero cells were cultured in 96-well flat-bottom tissue culture microtiter plates (2 × 104 cells/well/200 µl) in triplicate in complete DMEM and incubated at 37°C in a 5% CO2 incubator. After confluence, the monolayers were washed and test drugs were added with serial dilution of artemether (5–100 µg/ml) or sulfadiazine ranging from 1.56 to 200 µg/ml, and the cultures were incubated as described above for 24 h. Then, cells were washed and 10 µl of thiazolyl blue (MTT, Sigma) at 5 mg/ml in 90 µl of complete DMEM was added and incubated for 4 h. Formazan particles were solubilized in sodium dodecyl sulfate. The optical density was read after 30 min at 570 nm with a plate reader. Results were expressed as percentage of cell viability in comparison to controls.[19,20]
Flow cytometry
We achieved the flow cytometry in three separate experiments. In each experiment, we used artemether in the concentrations of 5, 10, 25, 50, and 100 µg/ml.
In the first experiment, 5 × 105 tachyzoites of T. gondii were cultured in 12-well plates in RPMI-1640 medium with 10% FBS. Artemether was added and placed in the incubator for 3 h at 37°C in a 5% CO2. We used Annexin V-FITC Apoptosis Detection Kit (BioVision, Palo Alto, USA) for the observation of apoptosis. Cell pellets were re-suspended in 500 µl of 1× binding buffer, and then 5 μl of Annexin V-FITC and 5 μl of propidium iodide were added and analyzed by flow cytometry.
In the second experiment, J774 cells (5 × 105 cells/well/500 µl) were cultured in 24-well plates in complete RPMI-1640 medium for 24 h at 37°C in a 5% CO2. Artemether was added and placed in incubator for 24 h again. The rest of the stages were accomplished as described above.
In the third experiment, after 24 h of incubation of J774 cells in 24-well plates, the tachyzoites (1 × 106 cells/well/500 µl) were added and incubated for 3 h at 37°C in 5% CO2, and then artemether was added and placed in incubator for 24 h, the rests were followed as assay protocol. The results were analyzed using CellQuest software.[21]
Effect of drugs on Vero cells infected with Toxoplasma gondii
In the first set of experiments (treatment of tachyzoites before infecting Vero cells by T. gondii for evaluating prophylaxis role of artemether), tachyzoites were pretreated for 1 h at 37°C and 5% CO2 with different concentrations of artemether (5, 10, 25, 50, and 100 µg/ml) or sulfadiazine (1.56–200 µg/ml), or only with medium as control group and then incubated with Vero cell monolayers with a ratio of 2:1 (parasites: Host cell) (2 × 105 tachyzoites/well/400 µl) for 24 h at 37°C and 5% CO2. Cells were washed and stained with Giemsa. The slides were examined under light microscope with regard to T. gondii infection index (percentage of infected cells per 100 examined cells) and parasite intracellular replication (mean number of parasites per cell in 100 infected cells)[1]
In the second set of experiments (treatment after infection), Vero cell monolayers were washed with medium and infected with T. gondii RH strain tachyzoites at a 2:1 (parasites: Hosts cell) rate of infection (2 × 105 tachyzoites/well/400 µl). After 3 h of infection, cells were washed again to remove nonadherent parasites and then treated with serial dilutions of drugs for 24 h at 37°C and 5% CO2, as described above. As a control group, the infected cells were incubated with only medium. Cells were washed with PBS, stained, and examined as described for the first set of experiments.[1,15,22]
The median inhibitory concentration (IC50) of each drug was calculated by extrapolation of the corresponding dose-response curve on a log-linear plot employing the portions of the curve that transected the 50% response point.
Statistical Analysis
The data were expressed as mean ± standard deviation. IC50 was calculated and one-way analysis of variance was used for the assessment of differences between groups. The log-rank (Mantel–Cox) test was utilized to compare the survival rates and curves of the studied groups. All graphs were plotted using GraphPad prism (version 5.04). Values of P < 0.05 were considered significant.
Results
Cytotoxicity Assay of Artemether
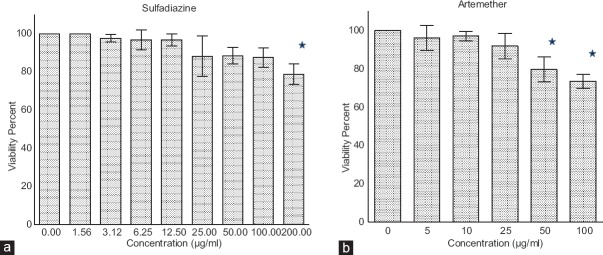
The toxic effects of artemether and sulfadiazine were determined by MTT test. The results showed that viability of Vero cells in the lowest concentrations of artemether and sulfadiazine (5 and 1.56 µg/ml, respectively) were 96.25% and 100%, respectively. In addition, viability rate for the highest concentrations from both treatments of artemether (100 µg/ml) [Figure 1a] and sulfadiazine (200 µg/ml) [Figure 1b] were measured as 73% and 79%, respectively.
Figure 1.
In vitro cytotoxicity activity determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test. Vero cells were cultured in 96-well plates in the presence of control, different concentrations of (a) artemether (from 100 to 5 μg/ml) or (b) sulfadiazine (from 200 to 1.56 μg/ml) for 24 h. The results were expressed as viability percent cells in relation with the control. There are statistical differences with control group (P < 0.05)
Flow Cytometry for Apoptotic Effects of Artemether on Toxoplasma Gondii Tachyzoites and J774 Cells
We observed a concentration-dependent apoptosis in tachyzoites after 3 h of treatment with artemether. For tachyzoites treated with artemether in concentrations of 5 µg/ml and 100 µg/ml, the apoptosis was 1.03% and 10.27%, respectively [Figure 2]. For J774 cells, flow cytometry revealed that with increasing concentrations, the apoptosis rate rises. At concentrations of 50 and 100 µg/ml, apoptosis was seen in 20.76% and 21.22%, respectively, after 24 h of treatment, although at a concentration of 25 µg/ml of the artemether, the rate of apoptosis was 15.02%. The results did not show any significant necrosis.
Figure 2.
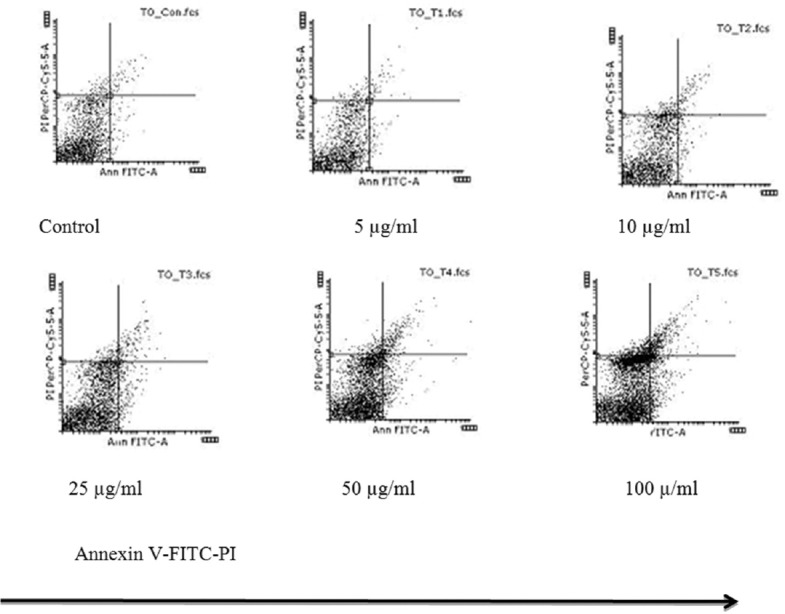
Flow cytometric analysis of the artemether effect on apoptosis of Toxoplasma gondii tachyzoite after 3 h of treatment. Percentage of Toxoplasma gondii tachyzoite apoptosis by artemether at different concentrations given in 5–100 μg/ml. Results are presented as percentage of apoptosis compared with control, that is, normal apoptotic period for Toxoplasma in the absence of artemether
Infected Vero Cells Treated with Artemether
The T. gondii tachyzoites were pretreated with different concentrations of artemether and sulfadiazine for 1 h before entering into Vero cells. At concentrations of 100 and 200 µg/ml, artemether and sulfadiazine resulted above 95% or 77% inhabitation of cell infection, respectively [Figure 3a and b]. IC50 of each drug was calculated as 13.15 µg/ml for artemether and 16.42 µg/ml for sulfadiazine.
Figure 3.
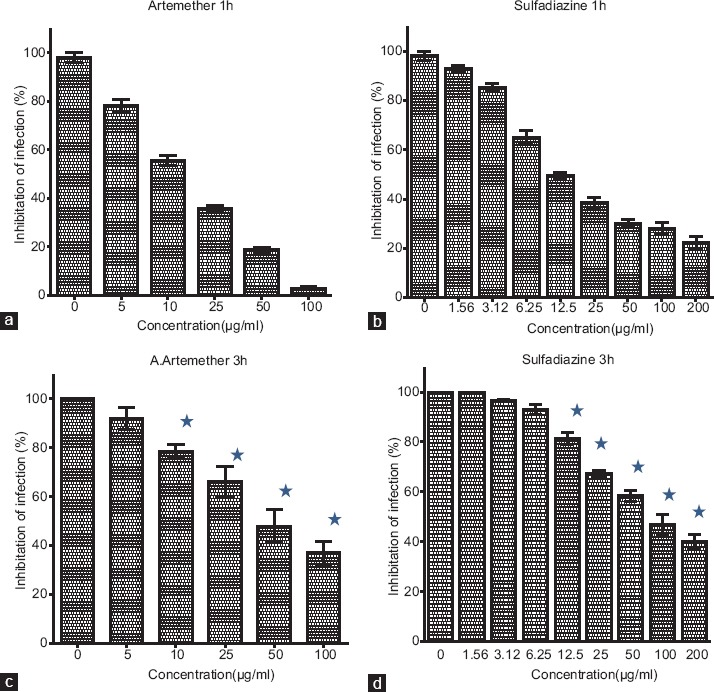
Effect of artemether or sulfadiazine on Toxoplasma gondii tachyzoites infection index in Vero cells. Treatments were performed on tachyzoites before Vero cells infection (a and b) or on Vero cells after parasite infection (c and d). Outcomes are definitive as percentage of inhibition of infection related to controls. The vertical line represents the standard deviation. There are statistical differences with control group (P < 0.05)
In another study, Vero cells were infected with tachyzoites for 3h, then the infected cells were treated with different concentrations of artemether and sulfadiazine. IC50 of the infected cells was determined as 49.13 µg/ml for artemether and 80.27 µg/ml for sulfadiazine. Artemether showed higher inhibitory than sulfadiazine [Figure 3c and d].
Figure 4.
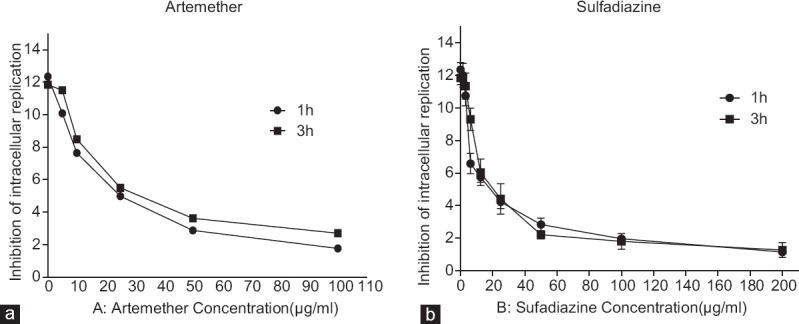
Effect of treatment with artemether (a) or sulfadiazine (b) on Toxoplasma gondii intracellular proliferation index in Vero cells. Treatments were done on Toxoplasma gondii tachyzoites before Vero cells infection or on Vero cells after Toxoplasma gondii infection. Results are expressed as mean number of parasite per cell in 100 infected cells
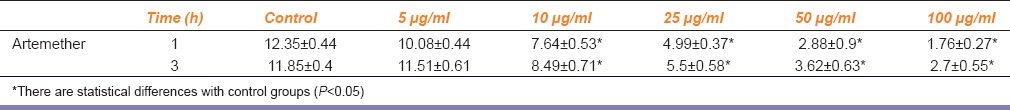
The percentage of intracellular replication of parasites was investigated in both tests [Figure 4]. In the first experiment, the results showed that in the highest concentrations of sulfadiazine and artemether, the average amount of intracellular replication of tachyzoites in the infected Vero cells decreased by 7–10.5 times in comparison to untreated cells [Table 1]. For the second test, intracellular proliferation of tachyzoites decreased by 4–9 times for artemether and sulfadiazine in comparison with untreated control [Table 1]. In our assay, both compounds were effective against tachyzoites and either sulfadiazine or artemether treatments were able to control T. gondii in Vero cells infected by RH strain.
Table 1.
Mean±standard deviation of intracellular replication of parasites in infected Vero cells after treating with artemether in different concentrations (5-100 μg/ml) for 24 h
In treatment before infection, the highest concentrations of artemether (100 µg/ml) and sulfadiazine (200 µg/ml) had the lowest IC50 values of all the concentrations tested for preventing the intracellular proliferation. Before infection in Vero cells experiment, artemether demonstrated an IC50 of 0.66 µg/ml for the intracellular replication index, whereas the treatment with sulfadiazine showed an IC50 of 0.27 µg/ml [Figure 3].
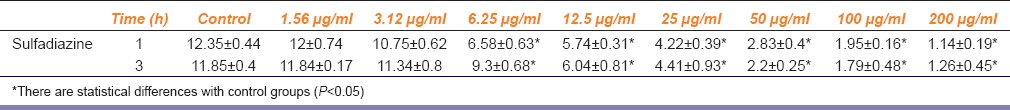
The similar results were calculated for intracellular replication of parasites in infected Vero cells after treating with sulfadiazine in different concentrations (1.56–200 µg/ml) for 24 h [Table 2]. The artemether indicated an IC50 of 0.76 µg/ml and sulfadiazine represented an IC50 of 0.29 µg/ml.
Table 2.
Mean±standard deviation of intracellular replication of parasites in infected Vero cells after treating with sulfadiazine in different concentrations (1.56-200 μg/ml) for 24 h
Discussion
Our results showed that artemether demonstrated low toxicity against Vero cells. Furthermore, several studies investigated the toxicity of artemisinin and its derivatives such as artemether on different cells, and the results showed a low cytotoxicity.[1,23] T. gondii isa parasite that can both stimulate and inhibit apoptosis in macrophages. In this study, we evaluated the effect of artemether on T. gondii, macrophages, and macrophages infected with T. gondii. Artemether enhanced the programed cell death in parasite or infected macrophages.
In vitro condition, our experiment demonstrated that before the infection of Vero cells with T. gondii, pretreatment of tachyzoites with artemether was significantly effective as compared to the pretreatment with sulfadiazine. However, when treatment of Vero cells with artemether was done after T. gondii tachyzoites infection, both drugs had similar efficacy results. A comparison of two methods displayed that treatment of tachyzoites before the infection of Vero cells is more effective than treatment of cells after parasite infection. According to the severe side effects of chemical drugs and not tolerated by many patients, more studies have been done on the effects of different plant compounds against T. gondii. The results have been shown satisfactory and inhibitory activity against a variety of cells infected with T. gondii tachyzoites.[17] The other studies performed on artemisinin derivatives as anti-toxoplasma agents confirm the inhibitory activity of all derivatives from artemisinin.[24] Furthermore, the IC50 values obtained for testing the treatment of T. gondii tachyzoites before infection on Vero cells and treatment of tachyzoites after infection of Vero cells were very low. In the same concentration (data not shown), our results showed similar IC50 values for each drug. These findings indicated the artemether as herb compound that can inhibit intracellular replication of parasites such as sulfadiazine (the choice drug for toxoplasmosis). Inhibition of parasite intracellular replication before and after infection on Vero cells was significantly high for both drugs of artemether and sulfadiazine. Very low IC50 values were determined in both drugs. In another study, the results showed high inhibitory efficacy on the tachyzoites of parasite before infection on human foreskin fibroblast cells (HFF) for both the treatment of artemisinin and sulfadiazine.[1] A previous study investigated the efficacy of artesunate (artemisinin derivatives) on the T. gondii and indicated best effect with low IC50 value compared to other examined drugs such as pentamidine and pyrimethamine.[25] These findings showed that artemisinin and its derivatives are not only a potent new compound of antimalarial,[26] but also they are able to control various strains of T. gondii in vitro and in vivo.[24]
D’Angelo et al. found that five out of seven new derivatives of artemisinin could inhibit the growth of T. gondii tachyzoites in HFF cells and after 5 days, IC50 was 1.0–4.4 µM.[24] In our study, the 24 h IC50 for infected Vero cells with tachyzoites was determined as 49.13 µg/ml.
Brun-Pascaud et al. evaluated the activity of artemether for prophylaxis of toxoplasmosis in vivo. They found that artemether did not prevent toxoplasmosis. They used the artemether for 2 and 5 days per week.[27] The half-life of artemether in the plasma is only 2–3 h and eliminated quickly from the sera.[28] The short half-life of this drug is the reason for not being suitable for prophylaxis of toxoplasmosis in vivo; However, our results showed that in vitro artemether has an acceptable ability for prophylaxis. For solving this problem, we need to use some new drug delivery systems such as polyvinyl alcohol that let the drug to release enough amounts during 24 h.[29] The drug delivery system let us to get the drug every 24 h instead of 6 h.
Conclusion
Our findings in this study indicate that artemether showed good results in control of infected cells by tachyzoites of T. gondii. Other finding is the prophylaxis role of artemether in toxoplasmosis. Artemether is effective to control the tachyzoites of T. gondii invitro and maybe a good alternative drug for toxoplasmosis.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
The sponsor of this study is Tarbiat Modares University, Tehran, Iran. The authors wish to thank all colleagues in the Parasitology Departments of Tarbiat Modares University.
References
- 1.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: From animals to humans. Int J Parasitol. 2000;30:1217–58. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montoya JM, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 3.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38:1257–78. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26:190–6. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Hill DE, Chirukandoth S, Dubey JP. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- 6.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–22. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Pandit AJ. Incidence and prevalence of toxoplasmosis in Indian pregnant women: A prospective study. Am J Reprod Immunol. 2004;52:276–83. doi: 10.1111/j.1600-0897.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- 8.Covello PS. Making artemisinin. Phytochemistry. 2008;69:2881–5. doi: 10.1016/j.phytochem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Tonmunphean S, Parasak V, Kokpol S. Theoretical investigations on reaction mechanisms of artemisinin compounds: Effect of structure on kinetic energy profile and antimalarial activity. J Mol Struct. 2005;724:99–105. [Google Scholar]
- 10.Ke OY, Krug EC, Marr JJ, Berens RL. Inhibition of growth of Toxoplasma gondii by qinghaosu and derivatives. Antimicrob Agents Chemother. 1990;34:1961–5. doi: 10.1128/aac.34.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehkordi NM, Ghaffarifar F, Hassan ZM, Esavand Heydari F. In vitro and in vivo studies of anti leishmanial effect of artemether on Leishmania infantum. Jundishapur J Microbiol. 2013;6:1–6. [Google Scholar]
- 12.Lai BS, Witola WH, El Bissati K, Zhou Y, Mui E, Fomovska A, et al. Molecular target validation, antimicrobial delivery, and potential treatment of Toxoplasma gondii infections. Proc Natl Acad Sci U S A. 2012;109:14182–7. doi: 10.1073/pnas.1208775109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarciron ME, Saccharin C, Petavy AF, Peyron F. Effects of artesunate, dihydroartemisinin, and an artesunate-dihydroartemisinin combination against Toxoplasma gondii. Am J Trop Med Hyg. 2000;62:73–6. doi: 10.4269/ajtmh.2000.62.73. [DOI] [PubMed] [Google Scholar]
- 15.Lee WC, Mahmud R, Noordin R, Piaru SP, Perumal S. Alkaloids content, cytotoxicity and anti-Toxoplasma gondii activity of Psidium guajava L. and Tinospora crispa. Banglad J Pharmacol. 2012;7:272–6. [Google Scholar]
- 16.Yang X, Huang B, Chen J, Huang S, Zheng H, Lun ZR, et al. In vitro effects of aqueous extracts of Astragalus membranaceus and Scutellaria baicalensis GEORGI on Toxoplasma gondii. Parasitol Res. 2012;110:2221–7. doi: 10.1007/s00436-011-2752-2. [DOI] [PubMed] [Google Scholar]
- 17.Furtado GC, Slowik M, Kleinman HK, Joiner KA. Laminin enhances binding of Toxoplasma gondii tachyzoites to J774 murine macrophage cells. Infect Immun. 1992;60:2337–42. doi: 10.1128/iai.60.6.2337-2342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum W, Pfaar U, Kuhnol J. Rapid characterization of artemether and its in vitro metabolites on incubation with bovine hemoglobin, rat blood and dog blood by capillary gas chromatography-chemical ionization mass spectrometry. J Chromatogr. 1998;710:101–13. doi: 10.1016/s0378-4347(98)00113-3. [DOI] [PubMed] [Google Scholar]
- 19.Kavitha N, Noordin R, Chan KL, Sasidhara S. Cytotoxicity activity of root extract/fractions of Eurycoma longifolia jack root against vero and Hs27 cells. J Med Plants Res. 2010;4:2383–7. [Google Scholar]
- 20.Pillai S, Mahmud R, Lee WC, Perumal S. Anti-parasitic activity of Myristica fragrans Houtt. Essential oil against Toxoplasma gondii parasite. APCBEE Procedia. 2012;2:92–6. [Google Scholar]
- 21.Xu X, Liu T, Zhang A, Huo X, Luo Q, Chen Z, et al. Reactive oxygen species-triggered trophoblast apoptosis is initiated by endoplasmic reticulum stress via activation of caspase-12, CHOP, and the JNK pathway in Toxoplasma gondii infection in mice. Infect Immun. 2012;80:2121–32. doi: 10.1128/IAI.06295-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Luis FM, Alves LM, Rodrigues VM, Silva DA, Mineo JR. Bothrops pirajai snake venom L-amino acid oxidase: in vitro effects on infection of Toxoplasma gondii in human foreskin fibroblasts. Rev Bras Farmacognosia. 2011;21:477–85. [Google Scholar]
- 23.Jones-Brando L, D’Angelo J, Posner GH, Yolken R. In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob Agents Chemother. 2006;50:4206–8. doi: 10.1128/AAC.00793-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Angelo JG, Bordón C, Posner GH, Yolken R, Jones-Brando L. Artemisinin derivatives inhibit Toxoplasma gondii in vitro at multiple steps in the lytic cycle. J Antimicrob Chemother. 2009;63:146–50. doi: 10.1093/jac/dkn451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes T, de Andrade Junior H, Zevallos S, Amatoneto V. In vitro action of antiparasitic drugs, especially artesunate, against Toxoplasma gondii. Rev Soc Bras Med Trop. 2012;45:485–90. doi: 10.1590/s0037-86822012000400014. [DOI] [PubMed] [Google Scholar]
- 26.Balint GA. Artemisinin and its derivatives: An important new class of antimalarial agents. Pharmacol Ther. 2001;90:261–5. doi: 10.1016/s0163-7258(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 27.Brun-Pascaud M, Chau F, Derouin F, Girard PM. Lack of activity of artemether for prophylaxis and treatment of Toxoplasma gondii and Pneumocystis carinii infections in rat. Parasite. 1996;3:187–9. doi: 10.1051/parasite/1996032187. [DOI] [PubMed] [Google Scholar]
- 28.Novartis Drug Regulatory Affairs Coartem®/Riamet® Basic Prescribing Information. 2007 [Google Scholar]
- 29.Ebrahimisadr P, Ghaffarifar F, Hassan ZM, Sirousazar M, Mohammadnejad F. Effect of polyvinyl alcohol (PVA) containing artemether in treatment of cutaneous leishmaniasis caused by Leishmania major in BALB/c mice. Jundishapur J Microbiol. 2014;7:e9696. doi: 10.5812/jjm.9696. [DOI] [PMC free article] [PubMed] [Google Scholar]