Abstract
Interleukine-12 is critical for the differentiation of Th1 cells and can improve the development of Th1 cells with Tfh cell features in mouse model. Human effector CD4+ T cells also exhibit poly-functionality by co-expressing IL-21 and IFN-γ. However, the effects of IL-12 on regulating generation of human IL-21- and IFN-γ-expressing CD4+ T cells are still incompletely understood. Our studies found that IL-12 but not IL-21 could induce the differentiation of human naive CD4+ T cells into multi-cytokine expressing CD4+ T cells in vitro, which co-expressed IL-21 and IFN-γ with or without IL-2 and TNF-α. At early stage of differentiation, addition of excess exogenous IFN-γ could increase the generation of IL-21- and IFN-γ-expressing CD4+ T cells, furthermore, anti-IFN-γ depressed the percentage of poly-functional CD4+ T cells. Phenotypically, IL-21+IFN-γ+CD4+ T cells exhibited more characteristic features about both of Th1 and Tfh cells than IL-21 or IFN-γ single-expressing CD4+ T cells. Mechamistically, IL-12 modulated the differentiation of IL-21+IFN-γ+CD4+ T cells from naive CD4+ T cells via the pathways of STAT-1/4, T-bet and BCL−6. Different from naive CD4+ T cells, IL-12 increasing the generation of IL-21+IFN-γ+CD4+ T cells from memory CD4+ T cells was only involved in STAT-4 pathway but not STAT-1. Poly-functional CD4+ T cells were contributed to generation and progress of varies diseases and our studies provide basic theoretics for the designs of vaccine and therapies of diseases.
Keywords: differentiation, human, IL-12, IL-21, poly-functional CD4+ T cells
Introduction
Naive CD4+ T cells have the remarkable capacities for developing into distinct lineages of T helper (Th) cells that exhibit unique functional properties, including Th1, Th2, Th9, Th17, T follicular helper (Tfh) and even induced regulatory T (iTreg) cells.1,2 Th1 together with Th2 cells were the first distinguished T helper cells in mouse by Mosmann & Coffman.3 Th1 cells mainly secret IFN-γ as their signature cytokine to participate in cell immune response, and also make IL-2 and TNF-α.4 The signature cytokines of Th2 cells are IL-4, IL-5 and IL-13 which contribute to humoral immunity. Th17 linage is the third effector population of CD4+ T cells and these cells were characterized by the production of IL-17A, IL-17F and IL-22 as signature cytokines.5 Follicular T helper (Tfh) cells are the major source of IL-21 and play irreplaceable roles in mediating differentiation of B cells into memory and plasma cells after exposuring to T-dependent antigens in germinal centers (GCs).6 In conclusion, functional helper T cells are distinguished by specifically effector cytokines, however, poly-functional CD4+ T cells which expressed 2 and more different effector cytokines in a single cell level were described in many studies. Mycobacterium tuberculosis (MTB)-specific IFN-γ+IL-2+TNF-α+CD4+ T cells were found in pleural fluid cells (PFCs) from patients with tuberculous pleurisy.7,8 IL-17+IL-21+CD4+ T cells and IFN-γ+IL-21+CD4+ T cell were detected in SIV-infected or uninfected rhesus macaques (RMs) and sooty mangabeys (SMs).9 Mononuclear cells in tissues from nasal polyps (NPs) exhibited poly-functionality by co-expressing IL-21 and IFN-γ, or IL-21 and IL-17.10 Furthermore, we have observed that mononuclear cells form tonsils could co-express 5 cytokines, such as IFN-γ, IL-2, IL-17A, IL-21 and IL-22, or TNF-α, IL-2, IL-17A, IL-21 and IL-22 (data not published). However, the effects of poly-functional CD4+ T cells in particular diseases and relationships between poly-functional CD4+ T cell generation and disease progress are largely unexplored.
It has been proved that all kinds of subpopulation of T helper cells could be derived from naive CD4+ T cells in vitro under suitable polarization.2,11 Special cytokine environment and transcription factor regulation play fate determinations and effector functions on the differentiation of T helper cells. Traditionally, IL-12 and IFN-γ induce the high expression of transcription factor T-bet and STAT-4 in naive CD4+ T cells to improve Th1 cell differentiation, IL-4 induces the high expression of GATA-3 and STAT-6 in naive CD4+ T cells to enhance Th2 cell differentiation. After TCR activation, co-stimulation of TGF-β and IL-6 induces the expression of retinoid-related orphan receptor (ROR) γt to initialize Th17 cell development from human naive CD4+ T cells. The differentiation of Tfh cells is under controversy, naive CD4+ T cells exposure to a signal cytokine IL-6 or IL-21 could differentiate into Tfh cells.12 Traditionally, the differentiation of naive CD4+ T cells into lineages with destine effector has been considered to be an irreversible event,13,14 but nowadays, lots of evidences have proved that part of helper T cells with particular functions exhibit the plasticity.15 Such as iTreg and Th17 cells are more plastic than previously, appreciated multiple studies in vivo and vitro have reported that Foxp3+ Treg cells from intestines have the propensity to differentiate into Th17 or even Tfh cells.16-18 In Peyer's patches, IL-17-producing CD4+ T cells convert into a Tfh cell phenotype and induce germinal center B cells to secrete IgA.19 It has demonstrated that early Th1 cell differentiation induced by IL-12 was marked by a Tfh cell-like transition, generating cells with features of both Tfh and Th1 cells in mouse.20 In human, previous studies declared that dendritic cells could induce the differentiation of IL-21-producing Tfh-like cells through IL-12.21 However, the characteristics of human IL-21- and IFN-γ-producing T cells induced by IL-12 were still unknown.
In current study, we analyzed that recombinational IL-12 but not IL-21 could extremely induce the differentiation of naive CD4+ T cells into multi-cytokine expressing CD4+ T cells, which co-expressed IFN-γ, IL-21, TNF-α and IL-2. Most of IL-21+IFN-γ+CD4+ T cells induced by IL-12 exhibited the features both of Tfh and Th1 cells. Furthermore, the capability of IL-12 on regulating the development of IL-21+IFN-γ+CD4+ T cells could be improved by ectogenic IFN-γ and inhibited by anti-IFN-γ at early differentiation stage. IFN-γ positively induced the phosphorylation of STAT-1 and STAT-4 to improve the generation of IL-21- and IFN-γ-expressing cells. Transcription factors T-bet, BCL−6, STAT-1 and STAT-4 were indispensable for naive CD4+ T cells differentiating into poly-functional CD4+ T cells, nevertheless, only STAT-4 was vitally important for modulating memory CD4+ T cells to co-express IL-21and IFN-γ.
Results
IL-12 but not IL-21 induced the differentiation of human Th1 and Tfh co-expression cells
To address the functions of IL-12 on the differentiation of human IL-21- and IFN-γ-producing CD4+ T cells, we first purified naive CD4+ T cells from CBMCs, the cells were cultured for 3–5 d with immobilized monoclonal antibody anti-CD3 and soluble anti-CD28 in the presence of cytokine IL-12, IL-21 or combination of IL-12 plus IL-21. The expression of cytokine IL-21 and IFN-γ was analyzed (Fig. 1). IL-12 efficiently improved the differentiation of IFN-γ-producing CD4+ T cells and IL-21-producing CD4+ T cells, and interestingly produced cells that co-expressed IL-21 and IFN-γ. Although IL-21 could generate cells that expressed IL-21 or IFN-γ compared with neutral condition, IL-21 did not induce the co-expression of IL-21 and IFN-γ (Fig. 1A–1F). We further analyzed the expression of Tfh cell-associated phenotype CXCR5, ICOS, PD-1 and also CXCR3 (Fig. 1G). IL-12 or IL-21 induced high expression of Tfh-related molecular. The proportion of CXCR5+ICOS+ or CXCR5+PD-1+ Tfh-like cells was greater in IL-12 or IL-21 condition than that in neutral condition, but CXCR3 had no significant difference among the variant cultured conditions. In addition, IL-12 enhanced the function of IL-21 on regulating the generation of CXCR5+ICOS+ Tfh-like cells when combination of IL-12 and IL-21 were used as a stimulus. These data indicated that IL-12 but not IL-21 promoted the differentiation of human Th1-Tfh cells and induced the expression of charateristical phenotypes of Tfh cells but not Th1 cells that might be efficient to help B cells.
Figure 1 (See previous page).
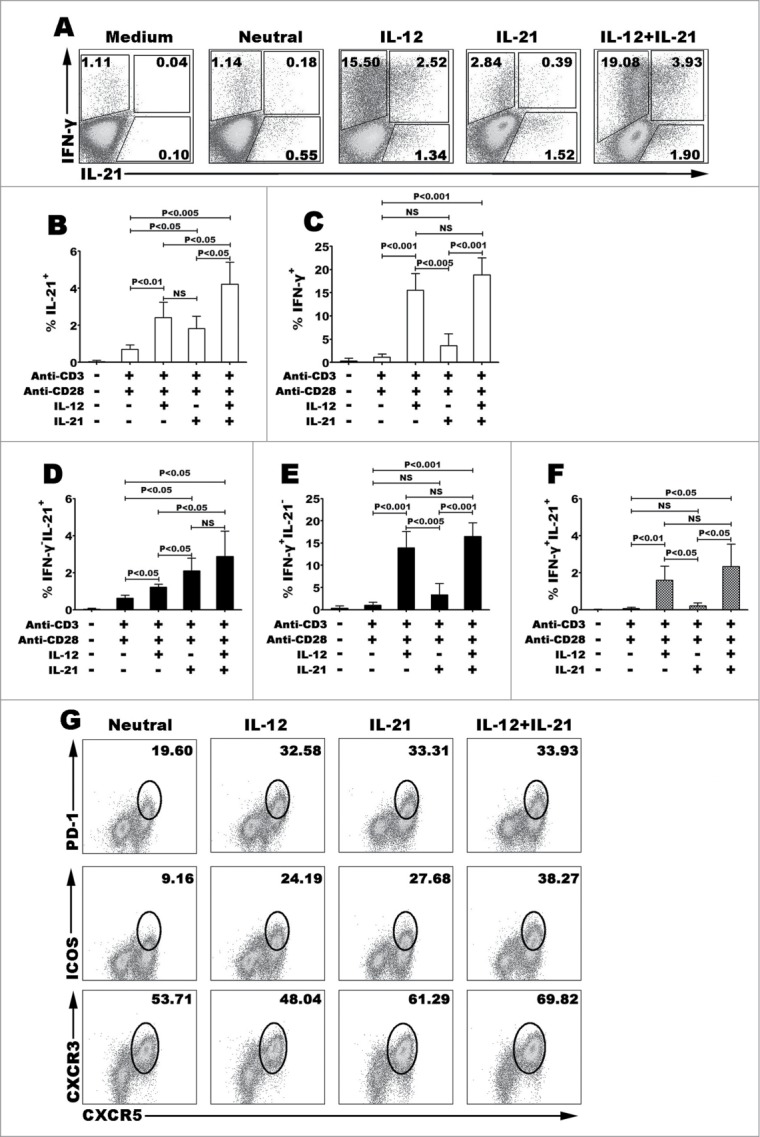
IL-12 but not IL-21 induced the differentiation of human naive CD4+ (T)cells into Th1 and Tfh co-expressing cells. Human naive CD4+ T cells from cord blood were stimulated for 5 d with or without anti-CD3 and anti-CD28 mAbs in the presence or absence of IL-12, IL-21 or IL-12 plus IL-21. The cells were harvested and rested for 2 d with IL-2. The cells were re-stimulated for 6 h with PMA and ionomycin in the presence of BFA. The expression of IL-21 and IFN-γ was detected by FACS. A representative result of different cytokines on the development of Th1 and Tfh cells was shown (A). Statistical data of percentage of IL-21+CD4+ (B), IFN-γ+CD4+ (C), IFN-γ−IL-21+ (D), IFN-γ−IL-21+ (E) and IFN-γ+IL-21+ (F) CD4+ T cells were mean ± SD from 5 independent experiments as described in A. Cell surface expression of CXCR5, PD-1, ICOS and CXCR3 was assessed by surface staining and a representative result was shown (G). P < 0 .05 was considered significant.
IL-12 induced the expression of poly-functional cytokines in a single CD4+ T cell level
IL-12 is a potent inducer of Th1 cells via accordingly inducing naive CD4+ T cells to secrete IFN-γ, in addition, we found that IL-12 could induce the expression of IL-21, TNF-α and IL-2. After stimulation for 5 d with IL-12 and re-stimulation for 6 h with PMA plus ionomycin, we observed that CD4+ T cells could express 2 kinds of cytokines (Fig. 2A). Besides co-expression of IFN-γ and IL-21, the co-expression of IL-21 and IL-2, IL-21 and TNF-α, IFN-γ and TNF-α, IFN-γ and IL-2, IL-2 and TNF-α were observed in a single cell level. Gated on IL-21+ (or IFN-γ+) and IL-21− (or IFN-γ−) cells, we compared the mean fluorescence intensity (MFI) levels of IFN-γ (or IL-21). IL-21+ cells expressed significantly higher MFI level of IFN-γ than did IL-21− cells, likewise, IL-21 were mainly expressed in IFN-γ+ cells but not IFN-γ− cells (Fig. 2B). We calculated that 40.10 ± 4.59% of IL-21+CD4+ T cells co-expressed IFN-γ, 69.95 ± 3.15% of IL-21+CD4+ T cells co-expressed IL-2, 62.55 ± 7.55% of IL-21+CD4+ T cells co-expressed TNF-α, 49.46 ± 4.29% of IFN-γ+CD4+ T cells co-expressed IL-2, 53.90 ± 10.77% of IFN-γ+CD4+ T cells co-expressed TNF-α, and 46.91 ± 8.74% of IL-2+CD4+ T cells co-expressed TNF-α (Mean ± SD, n=5). Furthermore, we analyzed the poly-functional CD4+ T cells which co-expressed 3 and more kinds of cytokines (Fig. 2C–D). Four major subsets were identified, as following IFN-γ+IL-21+TNF-α+IL-2+, IFN-γ+IL- 21−TNF-α+IL-2+, IFN-γ−IL-21+TNF-α+IL-2+ and IFN-γ−IL-21−TNF-α−IL-2− cells. Statistical results showed that 17.5% of CD4+ T cells co-expressed 4 cytokines IFN-γ, IL-21, TNF-α and IL-2, we also gained this information that only 17.75% of CD4+ T cells did not express any cytokine under the culture condition of IL-12.
Figure 2.
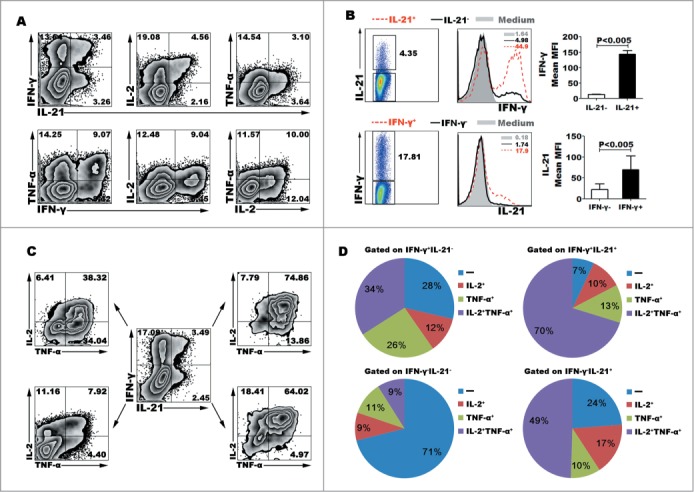
IL-12 induced the differentiation of polyfunctional CD4+ (T)cells. Naive CD4+ T cells were stimulated for 5 d with anti-CD3 and anti-CD28 mAbs in the presence of IL-12. The cells were rested and re-stimulated for 6 h with PMA and ionomycin in the presence of BFA. The expression of IL-21, IFN-γ, TNF-α and IL-2 was detected by FACS. Most of IL-21-expressing CD4+ T cells co-expressed Th1 cytokine IFN-γ, TNF-α or IL-2. The representative dot plots were shown (A). Gated on IL-21− and IL-21+CD4+ T cells or IFN-γ− and IFN-γ+CD4+ T cells, the expression of IFN-γ or IL-21 was analyzed. The representative histogram graphs and statistical data of mean MFI were shown (B). Gated on IL-21−IFN-γ−, IL-21+IFN-γ+, IL-21+IFN-γ− and IL-21−IFN-γ+ CD4+ T cells, the expression of TNF-α and IL-2 in the 4 different subsets were analyzed. The representative dot plots (C) and statistical data of mean percentages (D) of TNF-α−IL-2−, TNF-α−IL-2+, TNF-α+IL-2− and TNF-α+IL-2+ were shown.
Kinetic studies on the expression of cytokine and transcription factor after IL-12 stimulation
To further determine the effect of IL-12 in the production of IL-21 and IFN-γ, purified naive CD4+ T cells were stimulated with anti-CD3 plus anti-CD28 in presence or absence of IL-12, the cells and supernatants were collected at different time points. The levels of IL-21 and IFN-γ in supernatants were assessed by ELISA (Fig. 3A–B) and the expression of IL-21 and IFN-γ by cells were detected by flow cytometry (Fig. 3C). Low percentages and levels of IL-21 and IFN-γ were induced following the stimulation with IL-12 at day 1 and markedly increased at day 3 and day 5. Specially, IL-12 up-regulated the frequency of IL-21+IFN-γ+CD4+ T cells in a time-dependent manner. We evaluated the proportion of T-bet and BCL−6 during Th1 cell differentiation and found that both of T-bet and BCL−6 were upregulated as the term-stimulation extending.
Figure 3.
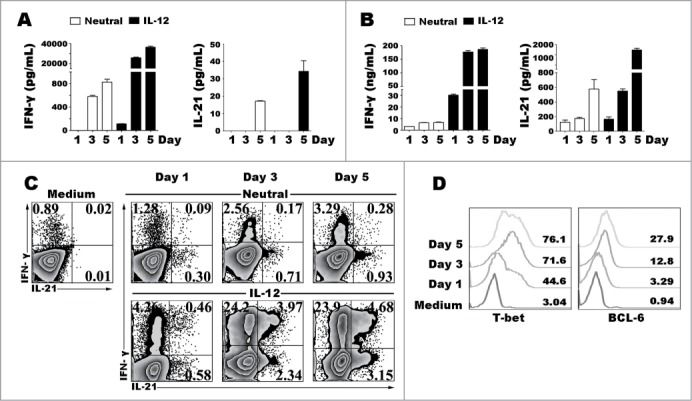
Kinetic studies of the expression of cytokines and transcription factors after IL-12 stimulation. Naive CD4+ T cells were stimulated for 1 to 5 d with anti-CD3 and anti-CD28 mAbs in the presence or absence of IL-12, and the cells and supernatants were harvested at different time-points. The levels of IFN-γ and IL-21 in supernatants were determined by ELISA (A). The cells were re-stimulated for 48 h with PMA and ionomycin, and levels of IFN-γ and IL-21 in supernatants were determined by ELISA (B). The cells were re-stimulated for 6 h with PMA and ionomycin in the presence of BFA, and the expression of IFN-γ, IL-21, T-bet and BCL−6 was detected by FACS. The representative results were shown (C, D).
IL-12 induced the expression of characteristic phenotypes of Th1 and Tfh cells
Whether IL-21+IFN-γ+CD4+ T cells induced by IL-12 from naive cells were identified as Tfh cells, we next compared the phenotypic differences among different subpopulations that distinguished based on the expression of cytokine IL-21 and IFN-γ. IL-21+IFN-γ+ cells exhibited fundamental features of Tfh cells by expressing higher levels of CXCR5, ICOS, PD-1 and CD40L CXCR3 than did single cytokine-producing cells, and also high expressed CXCR3 (Fig. 4A–B). IL-21+IFN-γ−CD4+ T cells also expressed higher CXCR5 than did IL-21−IFN-γ+CD4+ T cells, however there was no extremely different expression of ICOS or CD40L between the 2 subsets of single cytokine-expressing CD4+ T cells. We then compared the differences of transcription factor T-bet and BCL−6. Both of T-bet and BCL−6 were majorly expressed on cytokine-positive cells and IL-21+IFN-γ+ cells expressed highest levels of T-bet and BCL−6 (Fig. 3C–D). T-bet was correlated with IFN-γ expression, while BCL−6 was not significantly correlated with IL-21 expression. We further analyzed the differences of IL-21 and IFN-γ expression among cells that expressed T-bet and BCL−6 or not. Highest percentages of IL-21 and IFN-γ expression were detected in T-bet- and BCL−6-co-expressing cells, T-bet+BCL−6+ cells exhibited highest co-expression of IL-21 and IFN-γ (Fig. 4E–F). In conclusion, IL-21+IFN-γ+ CD4+ T cells exhibited fundamental features of both Th1 and Tfh cells, and had close correlation with transcription factor T-bet and BCL−6.
Figure 4.
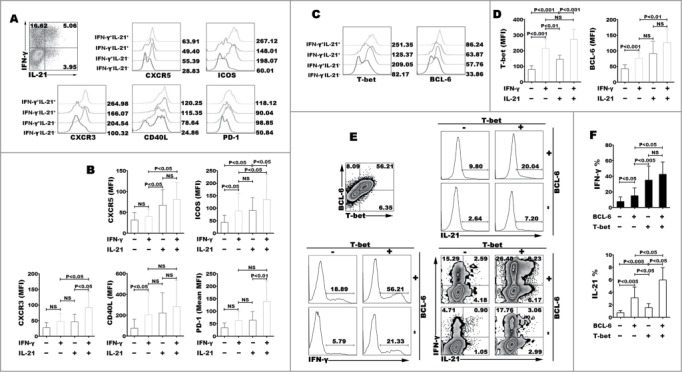
Characteristic phenotypes of Th1 and Tfh cells. Naive CD4+ T cells were stimulated for 5 d with anti-CD3 and anti-CD28 mAbs in the presence of IL-12. The cells were harvested, rested and re-stimulated for 6 h with PMA and ionomycin in the presence of BFA. The expression of cytokines and surface markers were detected by FACS. A representative result of surface expression of CXCR5, CXCR3, PD-1, ICOS and CD40L (A), and expression of transcription factor T-bet and BCL−6 (B) on IFN-γ−IL-21−, IFN-γ+IL-21−, IFN-γ−IL-21+ and IFN-γ+IL-21+ CD4+ T cells were shown in histogram, statistical data of MFI (B) were mean±SD from 5 independent experiments. A representative result of IL-21 and IFN-γ expression gated on T-bet−BCL−6−, T-bet−BCL−6+, T-bet+BCL−6+ and T-bet+BCL−6−CD4+ T cells was shown in histogram graph and dot plots (C). The statistical data were mean ± SD from 5 independent experiments (D).
Effects of IFN-γ on modulating IL-12 to induce IL-21 and IFN-γ expression
In the early stage of Th1 cell differentiation, IFN-γ enhances CD4+ T cells to produce IFN-γ via inducing the phosphorylation of STAT-1.22 To understand the effect of IFN-γ on modulating the generation of IL-21+IFN-γ+ cells, we added exogenous IFN-γ into the IL-12 polarization. Although sole IFN-γ could induce IFN-γ expression, IFN-γ did not influence IL-21 expression. At the early stage, we found that additional IFN-γ into IL-12 condition could enhance the effect of IL-12 on regulating the differentiation of IL-21- and IFN-γ-expressing cells. However, the effect of IFN-γ was decreased when IFN-γ was added at the later program of differentiation (day 3) (Fig. 5A). When naive CD4+ T cells were co-stimulated with IL-12 and IFN-γ for 3 days, we analyzed the expression of pSTAT-1, pSTAT-4, T-bet and BCL−6. The results showed that IFN-γ extremely enhanced the phosphorylation of STAT-1 and anti-IFN-γ blocked the signal pathway. Meanwhile, IFN-γ improved the expression of pSTAT-4, T-bet and BCL−6 (Fig. 5B). Interestingly, IFN-γ did not down- or up-regulate the expression of IL-2 and TNF-α (data not shown). We further found that neutral antibody anti-IFN-γ inhibited the expression and production of IL-21 and IFN-γ, and down-regulated the expression of phenotype CXCR5, ICOS, CXCR3, CD40L and PD-1, and transcription factor T-bet and BCL−6 (Fig. 5C–G). We demonstrated that IFN-γ might improve the differentiation of Th1-Tfh cells via inducing the phosphorylation of STAT-1 and STAT-4 at early time.
Figure 5.
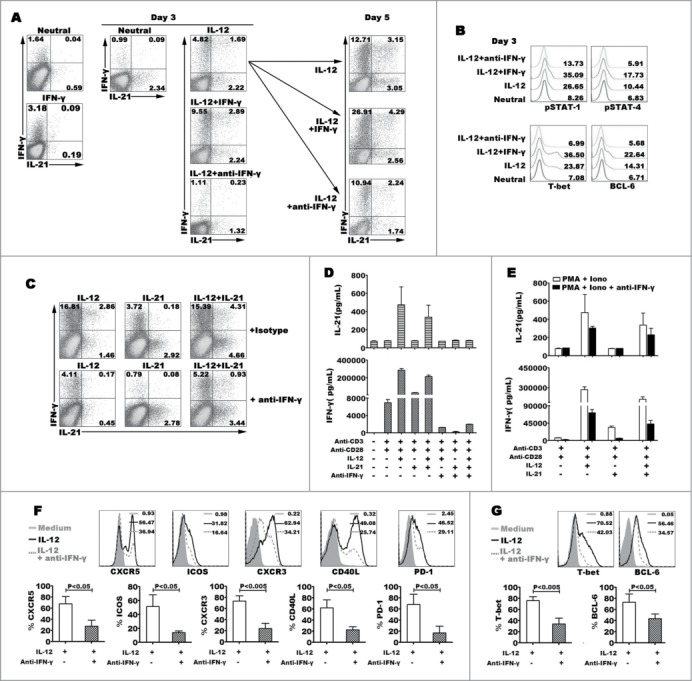
Effect of IFN-γ on modulating the expression and production of IL-21 and IFN-γ. Naive CD4+ T cells were stimulated for 3 d with anti-CD3 and anti-CD28 mAbs plus IL-12, and anti-IFN-γ or IFN-γ was added into the culture in the presence of IL-12 at day 0 and day 3, the expression of IL-21 and IFN-γ production were detected by FACS after re-stimulated with PMA and ionomycin at day 5, a representative dot plots were shown (A). Transcription factors phosphorylated STAT-1 and STAT-4, T-bet and BCL−6 were detected by FACS at day 3, a representative histogram graphs were shown (B). Naive CD4+ T cells were stimulated for 3 d in the presence of IL-12, IL-21 or IL-12 plus IL-21 with or without anti-IFN-γ. The cells were harvested, rested and re-stimulated for 6 h with PMA and ionomycin in the presence of BFA. IFN-γ and IL-21 production were analyzed by FACS (C). The cells were re-stimulated for 48 h with PMA and ionomycin, the levels of IFN-γ and IL-21 in supernatants were determined by ELISA (D). The cells were re-stimulated for 48 h with PMA and ionomycin in the presence of anti-IFN-γ. The levels of IFN-γ and IL-21 in supernatants were determined by ELISA (E). The expression of phenotypic markers (F) and transcription factor T-bet and BCL−6 (G) were analyzed by FACS under the conditions of IL-12 or IL-12 plus anti- IFN-γ. The representative histogram graphs and statistical data from 5 independent experiments were shown.
Signal pathways modulated the co-expression of IL-21 and IFN-γ by naive and memory CD4+ T cells
STAT-1 pathway is important to improve the production of IFN-γ by naive CD4+ T cells under the IL-12 condition with or without IFN-γ, STAT-4 is the key pathway that IL-12 induces the production of IL-21 in mouse.20,22 However, the signal pathways that regulate the generation of IL-21+IFN-γ+CD4+ T cells are not clear. IL-12 did not only induce the differentiation of IL-21+IFN-γ+CD4+ T cells from, we also found that IL-12 could induce memory CD4+ T cells to express more IL-21 and IFN-γ and generate poly-functional Th cells as that in naive CD4+ T cells (Fig. 6A–B). Blockade of STAT-4, T-bet or BCL−6 expression by special inhibitors, the expression of IL-21 and IFN-γ induced by IL-12 in naive cells was significantly reduced, inhibition of STAT-1 did not completely block the expression of IFN-γ but extremely inhibit the expression of IL-21. In memory CD4+ T cells, both of IL-21 and IFN-γ were decreased by blocking STAT-4, while blocking the expression of STAT-1, T-bet and BCL−6 did not completely inhibit the expression of IL-21 and IFN-γ. Phenotypic analysis found that STAT-4 inhibitor AG490 reduced the expression of fundamental features of Tfh cells in memory CD4+ T cells, including CXCR5, ICOS and PD-1 but not CXCR3 and CD40L (Fig. 6C–D). However, transcription factor T-bet and BCL−6 were inhibited in both of naive and memory cells when blocking the pathways of STAT-1 and STAT-4 (Fig. 6E). These results indicated that STAT-4, T-bet and BCL−6 pathway but not STAT-1 participated in the progress of naive CD4+ T cells differentiating into IL-21- and IFN-γ-producing CD4+ T cells under the stimulation of IL-12. However, in memory CD4+ T cells, IL-12 induced IL-21 and IFN-γ production via STAT-4 but not T-bet and BCL−6.
Figure 6 (See previous page).
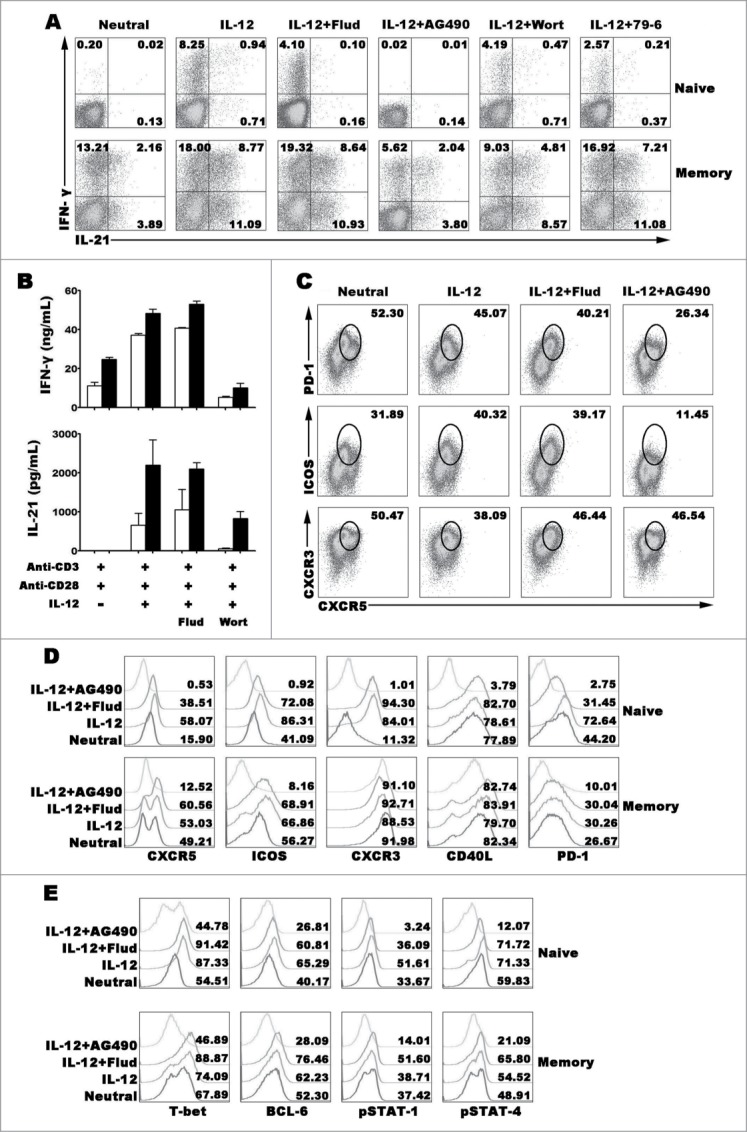
Transcription factors regulating the expression and production of IFN-γ and IL-21. Naive CD4+ T cells (CD4+CD45RA+CD45RO−) and memory CD4+ T cells (CD4+CD45RA−CD45RO+) were stimulated with anti-CD3 and anti-CD28 plus IL-12 in the presence or absence of inhibitors for transcription factor for 5 d. The cells were harvested and re-stimulated with PMA and ionomycin for 6 h in the presence of BFA, and the expression of IFN-γ and IL-21 were detected by FACS (A). Cells were re-stimulated with PMA and ionomycin for 48 h, the levels of IFN-γ and IL-21 in the supernatants were detected by ELISA (B). Memory CD4+ T cells were stimulated with anti-CD3 and anti-CD28 plus IL-12 in the presence or absence of inhibitors for transcription factor for 5 d. The cells were harvested and re-stimulated with PMA and ionomycin for 6 h in the presence of BFA, and the expression of IFN-γ, IL-21, CXCR5, CXCR3, ICOS and PD-1 were detected by FACS (C). Expression of Tfh and Th1 cell phenotypes (D) and transcription factors (E) was detected by FACS.
Discussion
Tfh cells were primordially described in tonsils, the distinguishing features of Tfh cells are the expression of CXCR5, PD-1, IL-21, ICOS and master regulator transcription factor BCL−6.23-25 In human peripheral blood, part of memory CD4+ T cells (CD45RO+) were positively expressed Tfh-like phenotype CXCR5 and IL-21, especially in some infection diseases.25,26 The most important roles of Tfh cells are to modulate the formation, maintenance and function of germinal centers (GCs) and regulate germinal center B cell differentiation into plasma cells and memory B cells. At the beginning of GC formation stage, Tfh cells induce the activation, differentiation and proliferation of GC B cells initially by improving BCL−6 expression and later via the CD40L or/and IL-21 pathway.27,28 Besides, IL-21 as the major effector cytokine of Tfh cells is the critical factor for improving plasma cell differentiation and positively induces purified CD4+ T cells to produce IL-21.29 It has been reported that IL-21 improves the differentiation of Tfh cells by inducing Bcl6 and Cxcr5 mRNA in vitro.30-32 Recently, Th1-like Tfh cells were proposed in many studies, which could express Th1 effector cytokine IFN-γ and Tfh effector cytokine IL-21 in the same time.20,21
In our previous studies, we found that the percentage of IL-21+IFN-γ+CD4+ cells was significantly higher in nasal polyp (NP) tissues compared with control tissues and matched PBMCs.10 Furthermore, higher percentage of IL-12+CD14+ cells was observed in the lymphocytes of NP tissue than PBMCs of NP patients, and IL-12p40 level in NP tissue was higher than that in serum of NP patients and we provoked that exogenous IL-12 exhibited stronger effect on improving CD4+ T cells to produce IL-21 and IFN-γ compared with IL-21 (data not published). We first observed that exogenous IL-12 but not IL-21 could improve the generation of IL-21- and IFN-γ-co-expressing CD4+ T cells from naive CD4+ T cells. In addition, IL-12 induced human naive CD4+ T cells to express Tfh-like phenotypes, including high expression of CXCR5, PD-1 and ICOS. The percentages of CXCR5+ICOS+ T cells induced by IL-21 were increased by additional IL-12 into the culture. As we understood, effector Th1 cells also produce cytokine IL-2 and TNF-α. Under the polarization of IL-12, up-regulation of IL-2 and TNF- were observed in IL-12 condition. Effector memory CD4+ T cells are often thought to be the terminal stage of cell development, which produce lots of cytokines to response to the same pathogens in adaptive immune response. The plasticity of CD4+ T cell lineage differentiation broke the concept that the differentiation of CD4+ T cells was an irreversible event. Polyclonal stimulation could induce memory CD4+ T cells to express 2 and more cytokines in a single cell level, IL-21+IFN-γ+ CD4+ T cells have been observed in PBMCs from health donors. We found that IL-12 could induce naive CD4+ T cells differentiate into poly-functional CD4+ T cells which were identified by co-expressing cytokine IL-21, IFN-γ, IL-2 and TNF-α. We further evaluated that high concentration of IL-12 also induced the development of IL-21+IFN-γ+, IL-21+IFN-γ− and IL-21−IFN-γ+ CD4+ T cells from naive CD4+ T cells in a time dependent manner. Furthermore, IL-12 generated more percentages of IL-21+IFN-γ+CD4+ T cells from purified memory CD4+ T cells (CD45RA−CD45RO+) than neutral condition.
In mouse model, Tfh-cell like features were thought to be not sustained during later stage of Th1 cell differentiation because of high expression of T-bet and IFN-γ.20 In contrast, we found that Tfh-related transcription factor BCL−6 and Th1-related transcription factor T-bet were increased under the IL-12 stimulation as time extending. In vitro, some studies demonstrate that T-bet expression induced by high concentration of IL-2 has the ability to combine with BCL−6 to inhibit the DNA binding domain of BCL−6 but left the DNA-binding domain of T-bet available.33-35 We interestingly observed the blockade of T-bet or BCL−6 signaling pathway by inhibitors in naive CD4+ T cells decreased IL-21 and IFN-γ expression. Nevertheless, the expression of IL-21 or IFN-γ was not affected by inhibiting T-bet and BCL−6 in memory CD4+ T cells. These results indicated that T-bet and BCL−6 were indispensable and irreplaceable for the differentiation of IL-21- and IFN-γ-co-expressing cells from naive CD4+ T cells but not memory cells under IL-12 polarization. Interestingly, we found that although IL-12 enhanced naive CD4+ T cells to express Tfh cell phenotypes, such as CXCR5, PD-1 and ICOS, IL-12 did not significantly increase the expression of Tfh like cell phenotypes in memory CD4+ T cells.
Under the Th1 polarization condition, STAT-1 activation by IFN-γ is important for T-bet expression in vitro.36 There is a positive feedback loop in which IFN-γ induces more IFN-γ by acting through T-bet. We found that exogenous IFN-γ alone had not prominent effect on inducing IL-21 expression while it could promote slight IFN-γ expression. At the early phase of Th1 cell differentiation under IL-12 polarization, additional IFN-γ could enhance the development of IL-21+IFN-γ+ cells and improved the phosphorylation of STAT-1 and STAT-4 which were correlated with up-regulated expression of T-bet and BCL−6. However, the positive feedback loop through IFN-γ/STAT-1 was no observed at the later phase of Th1 cell differentiation. Furthermore, neutralization of IFN-γ by anti-IFN-γ diminished IL-21 expression and decreased the expression of Tfh-like phenotypes at early time but not later time. The co-expression of IL-21 and IFN-γ induced by IL-12 was especially decreased by anti-IFN-γ. Similarly, transcription factor T-bet and BCL−6 were predominantly decreased by anti-IFN-γ. It's worth noted that the differentiation of single IL-21-expressing CD4+ T cells induced by IL-21 was not influenced by IFN-γ and anti-IFN-γ. Blockade of STAT-4 only diminished the generations of IL-21 and IFN-γ from naive CD4+ T cells but not memory cells, CXCR5, ICOS and PD-1 but not CXCR3 and CD40L expression were reduced by inhibiting transcription factor STAT-4. These results demonstrated that IFN-γ improved function of IL-12 but not IL-21 on modulating the development of poly-functional Th1/Tfh cells from naive CD4+ T cells at early course via STAT-1 signaling pathway.
We demonstrated that IL-12 might play critically different functions on modulating the generation of Th1/Tfh like cells from naive and memory CD4+ T cells. These results also indicated that the mechanisms of Th1/Tfh cell development in early and later courses were extremely different. Although poly-functional T helper cells were observed in lots of animal model and human diseases, the physiological and pathological functions of poly-cytokine expressing CD4+ T cells are still under controversial.
Materials and Methods
Subjects
Umbilical cord blood from healthy full-term newborn infants was collected from the Guangzhou Women and Children's Medical Center, China. The parents/guardian of the newborns gave written consent and the study was approved by the Medical School Review Board at Zhongshan School of Medicine, Sun Yat-sen University, China. Individuals that had been diagnosed with HIV, hepatitis B virus (HBV), or hepatitis C virus (HCV) infection or with a history of autoimmune diseases were excluded from the study.
Antibodies and reagents
The following monoclonal antibodies were used for phenotypic, intracellular cytokine and transcription factor analyses: phycoerythrin (PE) labeled-anti-pSTAT-1, PE-anti-pSTAT-4, PE-anti-IL-21, PE-anti-IL-12, PE-anti-PD-1, PE-anti-ICOS, PE-anti-isotype IgG2b,κ, PE-anti-isotype IgG2a, PE-anti-isotype IgG1κ, fluourescein isothiocyanate (FITC)-anti-IFN-γ, PE-Cy7-labeled anti-TNF-α, PE-Cy7-anti-CD14, allophycocyanin (APC)-anti-IFN-γ, APC-anti-CXCR3, APC-anti-CD40L, Alexa Fluor®647-labeled anti-IL-21, Alexa Fluor®647-anti-BCL−6, Alexa Fluor®488-anti-CXCR5, PE-CF594-labeled anti-T-bet, PE-CF594-labeled anti-BCL−6 and peridin-chlorophyll protein-Cy5.5 (PerCP-Cy5.5)-labeled anti-IL-2 were purchased from BD Biosciences (San Jose, CA); PE-anti-T-bet was purchased from eBiosciences (SanDiego, CA). Human ELSIA kits for cytokine IFN-γ was purchased from BD Biosciences, IL-21 was purchased from eBioscience. Purified anti-CD3, anti-CD28, IFN-γ and anti-IFN-γ monoclonal antibodies (mAbs) were purchased from BD Bioscience. Recombination IL-12 and IL-21 were purchased from Peprotech (Rocky Hill, NJ, USA). PMA, ionomycinand Brefeldin A were purchased from Sigam-Aldrich (St. Louis, USA).
Preparation of CBMCs, PBMCs and isolation of CD4+ T cell
For preparation of cord blood mononuclear cells (CBMCs), heparinized cord blood was mixed sufficiently with Dextran 500 solution (GE Healthcare Bio-Sciences, Uppsala, Sweden) and incubated at 37°C in a 5% CO2 incubator for 30 min to remove erythrocytes. CBMCs were obtained by Ficoll-Hypaque density gradient centrifugation. Naive CD4+ T cells were isolated from CBMCs by positive selection with anti-CD4 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. PBMCs from heparinized blood were isolated by Ficoll–Hypaque (Hao Yang Biological Manufacture, Tianjin, China) gradient centrifugation within 24 h of blood drawing and washed twice in Hanks' balanced salt solution. CD4+ T cells were negatively isolated from PBMCs using human CD4+ T cell isolation kits II, positive separations of naive and memory CD4+ T cells were based on CD45RO expression using anti-CD45RO-conjugated magnetic MACS microbeads. The purity of naive and memory cells was >97 %, as assessed by flow cytometry analysis. Cells were suspended at a concentration of 2 × 106 /mL in complete RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% FCS (Sijiqing, China), 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μM 2-mercaptoethanol and 2 mM L-glutamine (all from Gibco BRL).
Cell culture conditions
Purified naive CD4+ T cells were stimulated with immobilized anti-CD3 (1 μg/mL) plus anti-CD28 (1 μg/mL) mAbs. IL-12 (10 ng/mL), IL-21 (50 ng/mL), IFN-γ (200 ng/mL), IL-12 plus IL-21, IL-12 plus IFN-γ, IL-12 plus anti-IFN-γ (10 μg/mL) or IL-12 plus inhibitors were added to the cultures. The concentrations of inhibitor for transcription factor STAT-1 (Fludarabin), STATs (AG490), BCL−6 (79–6) and PI3K (Wortmannin) were 250 μM/mL, 50 μM/mL, 250 μM/mL and 200nM/mL. The cells were harvested and re-stimulated with PMA (20ng/mL) plus ionomycin (1 μg/mL) in the presence or absence of Brefeldin A (10 μg/mL) and used for flow cytometry analysis. Culture supernatants for 48 h were used for cytokine measurement by ELISA.
Cell surface and intracellular cytokine staining
Purified naive CD4+ T cells from CBMCs were stimulated as described above, and re-stimulated with PMA plus ionomycin for 5 h in the presence of Brefeldin A, and the cells were washed twice with PBS buffer containing 0.1% BSA and 0.05% sodium azide. For surface staining, cells were incubated with respective monoclonal antibodies at 4°C in the dark for 30 min, washed twice and fixed in 0.5% paraformaldehyde before acquisition. For the detection of intracellular cytokines and transcription factors, the cells were fixed with BD cytofix buffer (BD Biosciences) at 37°C for 10 min, permeabilized with ice-cold 90% methanol at 4°C for 30 min, and stained with specific transcription factor or isotype antibodies at 4°C for 50 min in the dark. Flow cytometry was performed using a BD FACS Calibur or Aria II cytometer. Lymphocytes were gated on forward and side scatter profiles and analyzed using FlowJo software (Treestar, San Carlos, CA).
ELISA
Purified naive CD4+ T cells from CBMCs were stimulated as described above, supernatants were harveasted and cells were re-stimulated with PMA plus ionomycin for 48 h in a round-bottom 96-well plate at a concentration of 2×106 cells/mL in triplicate at 37°C with 5%CO2. Concentrations of cytokines IFN-γ and IL-21 in supernatants were detected by ELISA. The detection limits of IFN-γ and IL-21 assay kits were 4.7 and 62.5 pg/mL, respectively.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by grants from National Science Foundation of China (31470888) and Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010; 28:445-89; PMID:20192806; http://dx.doi.org/ 10.1146/annurev-immunol-030409-101212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev 2010; 238:247-62; PMID:20969597; http://dx.doi.org/ 10.1111/j.1600-065X.2010.00951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986; 136:2348-57; PMID:2419430 [PubMed] [Google Scholar]
- 4.Han Q, Bagheri N, Bradshaw EM, Hafler DA, Lauffenburger DA, Love JC. Polyfunctional responses by human T cells result from sequential release of cytokines. Proc Natl Acad Sci U S A 2012; 109:1607-12; PMID:22160692; http://dx.doi.org/ 10.1073/pnas.1117194109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 2009; 27:485-517; PMID:19132915; http://dx.doi.org/ 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- 6.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011; 29:621-63; PMID:21314428; http://dx.doi.org/ 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- 7.Li L, Qiao D, Fu X, Lao S, Zhang X, Wu C. Identification of M. tuberculosis-specific Th1 cells expressing CD69 generated in vivo in pleural fluid cells from patients with tuberculous pleurisy. Plos One 2011; 6:e23700; PMID:21887301; http://dx.doi.org/ 10.1371/journal.pone.0023700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Qiao D, Li Q, Zhang X, Lao S, Wu C. Distinct polyfunctional CD4+ T cell responses to BCG, ESAT-6 and CFP-10 in tuberculous pleurisy. Tuberculosis (Edinb) 2012; 92:63-71; PMID:22154006; http://dx.doi.org/ 10.1016/j.tube.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 9.Micci L, Cervasi B, Ende ZS, Iriele RI, Reyes-Aviles E, Vinton C, Else J, Silvestri G, Ansari AA, Villinger F, et al.. Paucity of IL-21-producing CD4(+) T cells is associated with Th17 cell depletion in SIV infection of rhesus macaques. Blood 2012; 120:3925-35; PMID:22990011; http://dx.doi.org/ 10.1182/blood-2012-04-420240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao L, Wei Y, Zhang YN, Luo X, Yang BY, Yu SF, Wu XM, Wu CY, Li HB. Increased IL-21 expression in chronic rhinosinusitis with nasalpolyps. Clin Exp Allergy 2015; 45:404-13; PMID:25495679; http://dx.doi.org/ 10.1111/cea.12475 [DOI] [PubMed] [Google Scholar]
- 11.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev 2013; 252:12-23; PMID:23405892; http://dx.doi.org/ 10.1111/imr.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linterman MA, Liston A, Vinuesa CG. T-follicular helper cell differentiation and the co-option of this pathway by non-helper cells. Immunol Rev 2012; 247:143-59; PMID:22500838; http://dx.doi.org/ 10.1111/j.1600-065X.2012.01121.x [DOI] [PubMed] [Google Scholar]
- 13.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity 1998; 9:229-37; PMID:9729043; http://dx.doi.org/ 10.1016/S1074-7613(00)80605-6 [DOI] [PubMed] [Google Scholar]
- 14.Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity 2001; 14:205-15; PMID:11290331; http://dx.doi.org/ 10.1016/S1074-7613(01)00103-0 [DOI] [PubMed] [Google Scholar]
- 15.Baxter AG, Jordan MA. Plasticity is the differentiated state of CD4 T cells. Cell Mol Immunol 2013; 10:375-8; PMID:23921435; http://dx.doi.org/ 10.1038/cmi.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 2008; 112:2340-52; PMID:18617638; http://dx.doi.org/ 10.1182/blood-2008-01-133967 [DOI] [PubMed] [Google Scholar]
- 17.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235-8; PMID:16648838; http://dx.doi.org/ 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 18.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 2009; 323:1488-92; PMID:19286559; http://dx.doi.org/ 10.1126/science.1169152 [DOI] [PubMed] [Google Scholar]
- 19.Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol 2013; 14:372-9; PMID:23475182; http://dx.doi.org/ 10.1038/ni.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, Sun HW, Vahedi G, Hakim O, Handon R, et al.. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 2011; 35:919-31; PMID:22195747; http://dx.doi.org/ 10.1016/j.immuni.2011.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 2009; 31:158-69; PMID:19592276; http://dx.doi.org/ 10.1016/j.immuni.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, et al.. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A 2001; 98:15137-42; PMID:11752460; http://dx.doi.org/ 10.1073/pnas.261570598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med 2001; 193:1373-81; PMID:11413192; http://dx.doi.org/ 10.1084/jem.193.12.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandes M, Legler DF, Spoerri B, Schaerli P, Moser B. Activation-dependent modulation of B lymphocyte migration to chemokines. Int Immunol 2000; 12:1285-92; PMID:10967023; http://dx.doi.org/ 10.1093/intimm/12.9.1285 [DOI] [PubMed] [Google Scholar]
- 25.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 2000; 192:1553-62; PMID:11104798; http://dx.doi.org/ 10.1084/jem.192.11.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shekhar S, Yang X. The darker side of follicular helper T cells: from autoimmunity to immunodeficiency. Cell Mol Immunol 2012; 9:380-5; PMID:22885524; http://dx.doi.org/ 10.1038/cmi.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol 2010; 105:193-210; PMID:20510734; http://dx.doi.org/ 10.1016/S0065-2776(10)05007-8 [DOI] [PubMed] [Google Scholar]
- 28.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol 2006; 177:5236-47; PMID:17015709; http://dx.doi.org/ 10.4049/jimmunol.177.8.5236 [DOI] [PubMed] [Google Scholar]
- 29.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol 2005; 5:688-98; PMID:16138102; http://dx.doi.org/ 10.1038/nri1688 [DOI] [PubMed] [Google Scholar]
- 30.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, et al.. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008; 29:138-49; PMID:18599325; http://dx.doi.org/ 10.1016/j.immuni.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spolski R, Leonard WJ. IL-21 and T follicular helper cells. Int Immunol 2010; 22:7-12; PMID:19933709; http://dx.doi.org/ 10.1093/intimm/dxp112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl−6 expression and germinal center responses. J Exp Med 2010; 207:353-63; PMID:20142429; http://dx.doi.org/ 10.1084/jem.20091738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl−6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol 2012; 13:405-11; PMID:22406686; http://dx.doi.org/ 10.1038/ni.2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oestreich KJ, Weinmann AS. T-bet employs diverse regulatory mechanisms to repress transcription. Trends Immunol 2012; 33:78-83; PMID:22133865; http://dx.doi.org/ 10.1016/j.it.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oestreich KJ, Weinmann AS. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. NAT Rev Immunol 2012; 12:799-804; PMID:23059426; http://dx.doi.org/ 10.1038/nri3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol 2002; 3:549-57; PMID:12006974; http://dx.doi.org/ 10.1038/ni794 [DOI] [PubMed] [Google Scholar]


