Abstract
Maternal obese environment has been reported to induce oxidative stress and meiotic defects in oocytes, however the underlying molecular mechanism remains unclear. Here, using mice fed a high fat diet (HFD) as an obesity model, we first detected enhanced reactive oxygen species (ROS) content and reduced Sirt3 expression in HFD oocytes. We further observed that specific depletion of Sirt3 in control oocytes elevates ROS levels while Sirt3 overexpression attenuates ROS production in HFD oocytes, with significant suppression of spindle disorganization and chromosome misalignment phenotypes that have been reported in the obesity model. Candidate screening revealed that the acetylation status of lysine 68 on superoxide dismutase (SOD2K68) is dependent on Sirt3 deacetylase activity in oocytes, and acetylation-mimetic mutant SOD2K68Q results in almost threefold increase in intracellular ROS. Moreover, we found that acetylation levels of SOD2K68 are increased by ∼80% in HFD oocytes and importantly, that the non-acetylatable-mimetic mutant SOD2K68R is capable of partially rescuing their deficient phenotypes. Together, our data identify Sirt3 as an important player in modulating ROS homeostasis during oocyte development, and indicate that Sirt3-dependent deacetylation of SOD2 plays a protective role against oxidative stress and meiotic defects in oocytes under maternal obese conditions.
Keywords: obese, oocyte, oxidative stress, sirtuin, SOD
Abbreviations
- GV
germinal vesicle
- GVBD
germinal vesicle breakdown
- MI
metaphase I
- MII
metaphase II
- Pb1
first polar body
- PMSG
pregnant mare serum gonadotropin
- hCG
human chorionic gonadotropin
- LH
luteinising hormone
- MO
morpholino
- PI
propidium iodide
- KD
knockdown
- ROS
reactive oxygen species
- HFD
high fat diet
- SOD
superoxide dismutase.
Introduction
As the obesity epidemic continues, the associated medical co-morbidities, including those affecting reproduction, have increased as well. Obese women take longer to conceive, even if cycling regularly, and have a high risk of miscarriage, preeclampsia and congenital defects in the offspring.1-3 A recent large analysis of assisted reproductive technology cycles showed that increasing obesity was associated with a significant rise in failure to achieve a clinical pregnancy with the use of autologous oocytes, but this risk was overcome when donor oocytes from a lean woman were used,4 implying that poor oocyte quality accounts, at least in part, for those reproductive complications in obese women. In support of this idea, emerging evidence derived from animal models revealed that being overweight or obese can have a detrimental effect upon oocyte development. Using mice fed a high fat diet (HFD) as an obesity model, we previously found that maternal obesity results in structural, spatial and metabolic alterations in oocyte mitochondria.5 Approximately 90% of cellular reactive oxygen species (ROS), a natural by-product of cellular respiration, are produced in the mitochondria.6 Deficient management of ROS disturbs the cellular reducing environment and results in oxidative stress. ROS can damage multiple components of the cell, including DNA, RNA, proteins and lipids, and thereby perturb diverse biological processes, such as cell metabolism, apoptosis and aging.7 Interestingly, Igosheva et al. reported that ROS levels were elevated and redox state became more oxidized in oocytes from HFD mice.8 Similarly, feeding a diet enriched in long-chain n-3 polyunsaturated fatty acid (PUFA) induces mitochondrial dysfunction and elevated ROS generation in mouse oocytes, with reduced fertilization capacity and blastocyst formation.9 Importantly, spindle defects and chromosome misalignment are also readily observed in oocytes from HFD mice as well as from obese women.5,10
Sirtuins constitute a family of proteins of NAD+-dependent deacetylases. Seven members (Sirt1-Sirt7) have been identified in mammals, showing differential distribution within the cell and function in numerous physiological processes.11 Among them, Sirt3 has been shown to regulate the production of ROS at the level of the electron transport chain (ETC), as well as the detoxification of ROS through activation of antioxidant enzymes.12-14 Over the last few years, several mitochondrial targets of Sirt3 deacetylation have been identified and functionally characterized.14-19 For example, during caloric restriction, Sirt3 directly deacetylates and activates mitochondrial isocitrate dehydrogenase 2 (Idh2), leading to increased NADPH levels and protecting from oxidative stress-induced cell death.14 Forkhead box O3 (Foxo3a) is also a direct target of Sirt3 and involved in antioxidant defense mechanisms in cultured cells and mice.20,21 Superoxide dismutases (SODs) are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide, which is then converted to oxygen and water by catalase. In mammals, there are 3 forms of SODs (SOD1–3) localized in various compartments.6 Among them, mitochondrial SOD2 is thought to be the major antioxidant enzyme scavenging cellular ROS.11,19 Of note, previous work found that SOD2 activity is strongly regulated by acetylation at several conserved lysine residues.15,16,22 Biochemical studies have demonstrated that SOD2 is a direct substrate of Sirt3, and that deacetylation of SOD2 by Sirt3 leads to SOD2 activation and ROS reduction.15,16,22 In addition, Sirt3 is capable of protecting in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53 mediated developmental arrest.23
Although the phenotypic defects of oocytes from HFD mice are well-recognized, the underlying mechanisms remain to be discovered and management of fertility issues associated with maternal obesity continues to be a challenge. Therefore, the aim of the present study was to investigate whether Sirt3 is a contributing factor in the generation of poor quality oocytes in HFD obese mice, specifically through elevated oxidative stress in oocytes.
Results
Reduced Sirt3 expression in oocytes from HFD mice
To determine whether maternal obesity induces oxidative stress in oocytes, intracellular ROS generation was evaluated in oocytes from mice fed a High Fat-Diet, which are termed HFD oocytes in this study. Consistent with published data,8 we detected a marked increase in the fluorescence intensity emitted by 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) fluorescent dye in HFD MII oocytes relative to control oocytes (Fig. 1A, B), indicating that ROS levels were elevated when exposed to an obese environment.
Figure 1.
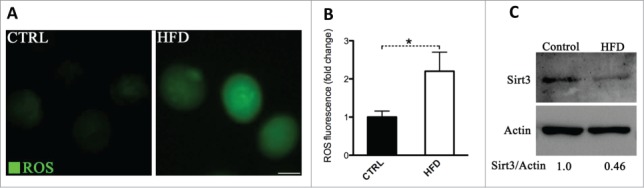
Increased ROS levels and reduced Sirt3 expression in oocytes from HFD mice. Ovulated MII oocytes were collected from mice fed a high fat diet or control diet, and then processed for evaluation of ROS levels via CM-H2DCFDA staining and Sirt3 expression by immunoblotting. (A) Representative images of CM-H2DCFDA fluorescence in oocytes from control and HFD mice. Scale bar: 50 µm. (B) Quantitative analysis of fluorescence intensity shown in panel A. Data were standardized by dividing each value by the average value of the control group in each experiment. Error bars indicate ± SD. (n = 70 oocytes for control and 60 for HFD pooled from 3 replicates). *P < 0.05 vs control. (C) Western blot analysis showed the reduced Sirt3 expression in oocytes from HFD mice compared to controls (100 oocytes were used for each group). Actin served as an internal control. Band intensity was calculated using ImageJ software, and the ratio of Sirt3/Actin expression was normalized and values are indicated. All protein gel blot experiments were repeated at least 3 times, with a representative gel image shown.
Given that Sirt3 functions to trigger mitochondrial reprogramming toward reduced oxidative stress in various cell types,14,16,22 we checked whether Sirt3 expression in oocytes was accordingly changed in response to maternal obesity. Notably, we found that Sirt3 protein levels decrease in HFD oocytes about 2-fold compared to their lean controls (Fig. 1C), suggesting that such a reduction may contribute to the penetrance of observed oxidative stress in HFD oocytes.
Loss of Sirt3 elevates ROS levels in oocytes
To explore the potential involvement of Sirt3 during oocyte maturation, we first examined its subcellular distribution. Sirt3 was initially localized in mitochondria, but recent studies have indicated that it is expressed in the nucleus as well.24,25 Our immunostaining (Fig. 2A) showed that Sirt3 resides throughout the oocyte at the germinal vesicle-stage (GV oocytes), with elevated accumulation in the nucleus (arrowheads). Following germinal vesicle breakdown (GVBD) as the oocytes matured to metaphase II (MII oocytes), Sirt3 levels increase in the cytoplasm, with intense signals surrounding the spindle/chromosome region (arrowheads).
Figure 2.
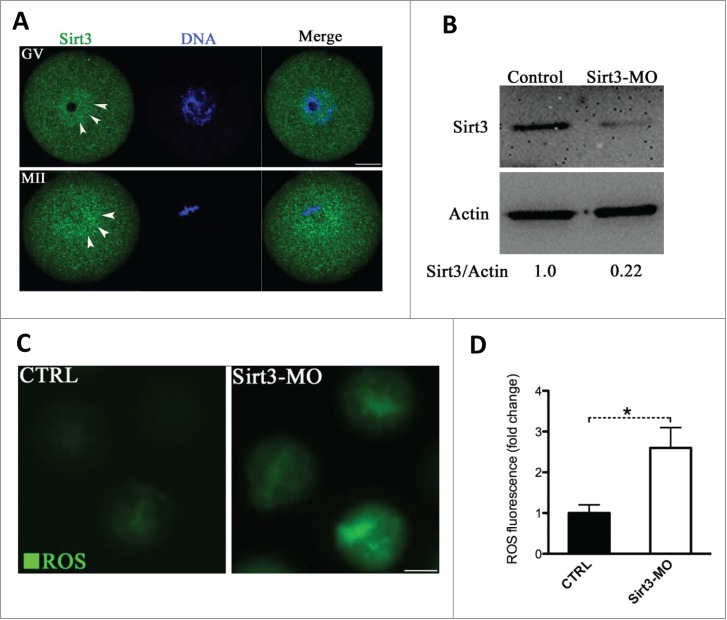
Effects of Sirt3 knockdown on ROS generation in oocytes. (A) Oocytes at GV and metaphase stages were immunolabeled with Sirt3 antibody (green) and counterstained with Hoechst 33342 (blue). Arrowheads point to Sirt3 signals. Scale bar: 20 μm. (B) Extent of knockdown of endogenous Sirt3 protein expression after Sirt3 morpholino (Sirt3-MO) injection was assessed by western blot analysis (100 oocytes were used for each group). Western blot experiments were repeated at least 3 times, with a representative gel image shown. (C) Representative images of CM-H2DCFDA fluorescence in control and Sirt3-MO oocytes. Scale bar: 50 μm. (D) Quantitative analysis of fluorescence intensity shown in C. Error bars indicate ± SD. (In the analysis, n = 120 oocytes for control and 110 for Sirt3 group were included, and pooled from 3 replicates). *P < 0.05 vs control.
Next, to perform functional analysis of Sirt3, we microinjected the Sirt3-targeting morpholino (Sirt3-MO) into fully-grown GV oocytes. This led to an approximately 4-fold knockdown of Sirt3 protein based on immunoblotting analysis (Fig. 2B). We then checked whether loss of Sirt3 influences ROS levels by using CM-H2DCFDA fluorescent dye. Of note, quantitative analysis showed that fluorescence intensity in Sirt3-MO oocytes was significantly higher than that in control oocytes (Fig. 2C, D), indicative of the elevated ROS levels. Nonetheless, Sirt3 depletion appeared to affect neither GVBD nor the polar body extrusion of oocytes (data not shown), indicating that it is not required for normal meiotic progression.
Sirt3 overexpression attenuates ROS production and ameliorates meiotic defects in oocytes from HFD mice
We then asked whether elevating Sirt3 expression is sufficient to attenuate ROS production in HFD oocytes. To address this question, we performed overexpression experiments by injecting exogenous Myc-Sirt3 mRNA into GV oocytes. Following in vitro-maturation, MII oocytes were collected for further analysis (Fig. 3A). Immunoblotting confirmed that exogenous Myc-Sirt3 protein was efficiently overexpressed (Fig. 3B). We found that overexpression of Sirt3 strikingly reduced the ROS levels in HFD MII oocytes (Fig. 3C, D), providing additional support that Sirt3 modulates oxidative stress in oocytes.
Figure 3.
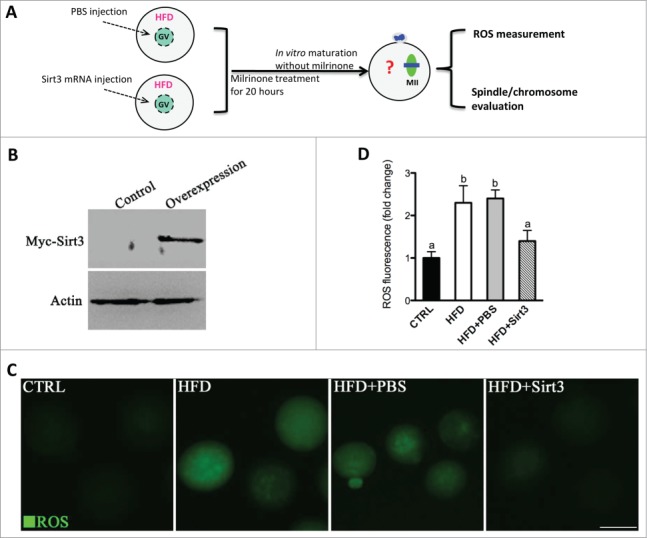
Sirt3 overexpression attenuates the ROS production in oocytes from HFD mice. (A) Cartoon summarizing the experimental protocol to investigate the effects of Sirt3 overexpression on ROS production and meiotic structures in oocytes. PBS or exogenous Myc-Sirt3 mRNA (overexpression group) was microinjected into GV oocytes from HFD mice, which were arrested for 20 hours with milrinone to allow synthesis of new Myc-Sirt3 protein. (B) Western blot analysis showing that exogenous Myc-Sirt3 protein was efficiently overexpressed, probing with anti-Myc Tag antibody. (C) Representative images of CM-H2DCFDA fluorescence in control, HFD, HFD+PBS and HFD+Sirt3 MII oocytes. Scale bar: 100 μm. (D) Quantitative analysis of fluorescence intensity shown in C. 106 oocyte for CTRL group, 118 oocytes for HFD group, 110 oocytes for HFD+PBS group and 115 oocytes for HFD+Sirt3 group were analyzed. Error bars indicate ± SD. Different superscripts indicate significant values (P < 0.05).
Furthermore, we have previously reported a high frequency of spindle defects and chromosomal abnormalities in ovulated oocytes from HFD mice, which may be a consequence of mitochondrial dysfunction.5 Therefore, here we wished to determine whether the beneficial effects of Sirt3 expression on ROS homeostasis in HFD oocytes could ameliorate the meiotic effects simultaneously. For this purpose, Sirt3 was overexpressed in HFD oocytes and then matured eggs were immunolabeled with anti-tubulin antibody to visualize spindle and co-stained with Hoechst for chromosomes. Confocal microscopy (Fig. 4A, B) revealed that most oocytes obtained from control mice displayed a typical barrel-shape spindle and well-aligned chromosomes on the metaphase plate. Only 9.1 ± 3.5% of control oocytes displayed the abnormal spindle and chromosomes. In sharp contrast, 34.6 ± 6.2% of oocytes retrieved from HFD mice showed disorganized spindle (arrows) or misaligned chromosomes (arrows). Remarkably, these defects were only detected in 15.8 ± 5.3% of HFD oocytes overexpressing Sirt3, which is significantly decreased compared to those HFD oocytes injecting PBS (30.7 ± 4.1%). Altogether, these data suggest that forced Sirt3 expression can suppress excessive ROS generation and the meiotic defects observed in oocytes from obese animals.
Figure 4.
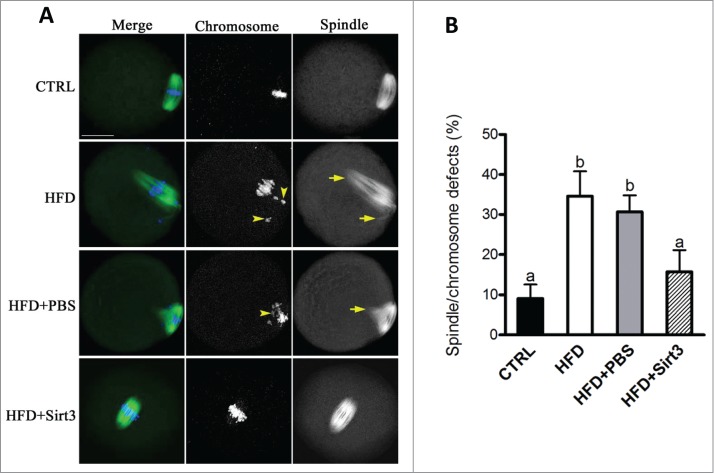
Sirt3 overexpression ameliorates the meiotic defects in oocytes from HFD mice. (A) MII oocytes were stained with α-tubulin antibody to visualize the spindle (green) and counterstained with Hoechst 33342 to visualize chromosomes (blue). Normal metaphase oocytes present a typical barrel-shape spindle and well-aligned chromosomes on the metaphase plate. Spindle defects and chromosome misalignment are indicated by arrows and arrowheads, respectively. Representative confocal sections are shown. (B) Quantification of control (n = 180), HFD (n = 182), HFD+PBS (n = 196) and HFD+Sirt3 (n = 203) MII oocytes with spindle defects or chromosome misalignment. Data are expressed as mean percentage ± SD from 3 independent experiments. Different superscripts indicate significant values (P < 0.05). Scale bar: 30 µm.
Acetylation of SOD2 lysine 68 controlled by Sirt3 functions in ROS generation in oocytes
Previous findings15,16,22 prompted us to hypothesize that SOD2 deacetylation might be a major downstream mediator of Sirt3 in protecting against ROS production in oocytes. It has been shown that substitution of lysine (K) with a glutamine (Q) mimics an acetylated amino acid state, while substitution with an arginine (R) mimics deacetylation.16,26 To test whether SOD2 acetylation, and if so, which lysine residue(s) affects ROS levels in oocytes, we constructed the site-specific mutants (K-to-Q) targeting 3 conserved lysines (K53, 68 and 89) of SOD2, and then the mRNA encoding the SOD2 mutants was singly microinjected into fully-grown oocytes for ROS measurement. As shown in Fig. 5A, B, we found that acetylation mimetic mutant SOD2 (K68Q) exclusively resulted in almost threefold increase in ROS levels compared to WT control, whereas both K52Q and K89Q mutations had little effects on cellular ROS. These results indicate that K68 is likely to be a major acetylation site on SOD2 affecting ROS generation in oocytes.
Figure 5.
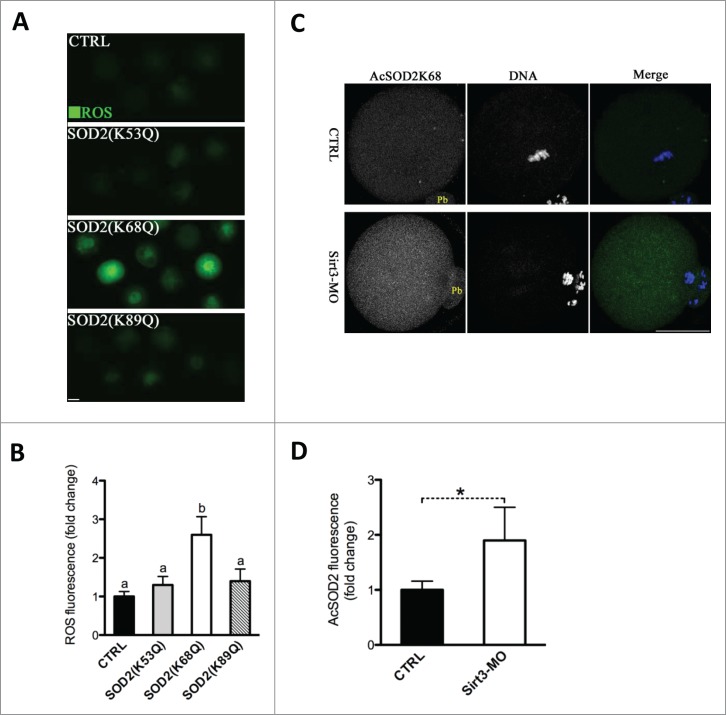
Acetylation of SOD2 lysine 68 controlled by Sirt3 functions in ROS generation in oocytes. (A) Acetylation-mimetic mutant SOD2(K53Q), SOD2(K68Q) or SOD2(K89Q) was microinjected into fully grown oocytes to evaluate the ROS levels via CM-H2DCFDA staining. Representative fluorescent images are shown. (B) Quantitative analysis of fluorescence intensity shown in A. Error bars indicate ± SD. At least 60 oocytes for each group were analyzed, and the experiments were conducted 3 times. (C) Control and Sirt3-MO MII oocytes were labeled with acetyl-SOD2K68 antibody (green) and counterstained with Hoechst 33342 for DNA (blue) to examine the effects of Sirt3 knockdown on acetylation status of SOD2K68 in oocytes. (D) Quantitative analysis of fluorescence intensity shown in C. Error bars indicate ± SD (n = 90 oocytes for control and 75 for Sirt3 group pooled from 3 replicates). Different superscripts indicate significant values (P < 0.05). Scale bars: 50 µm.
To further investigate whether Sirt3 directly acts on acetylation of SOD2K68, Sirt3 was knocked down by MO injection, and then matured oocytes were immunostained with anti-Acetyl-SOD2K68 antibody and counterstained with Hoechst for nuclear status. We observed a twofold increase in AcSOD2K68 fluorescence was detected in Sirt3-MO oocytes compared to control cells (Fig. 5C, D), suggesting that Sirt3 depletion leads to elevated acetylation of SOD2K68 in oocytes. Since Sirt3 expression is reduced in HFD oocytes (Fig. 1C), we postulated that acetylation state of SOD2K68 in HFD oocytes would be altered accordingly. Consistent with the hypothesis, quantitative analysis of fluorescence intensity (Fig. 6A, B) demonstrated that SOD2K68 acetylation was increased by ∼80% in HFD oocytes compared to controls. Importantly, forced expression of exogenous Sirt3 was able to markedly lower the acetylation levels of SOD2K68 in HFD oocytes. In combination with the effects of Sirt3 and SOD2 acetylation on ROS levels, these data collectively indicate Sirt3 reduction in HFD oocytes elevates the acetylation levels of SOD2K68, which contributes to ROS accumulation.
Figure 6.
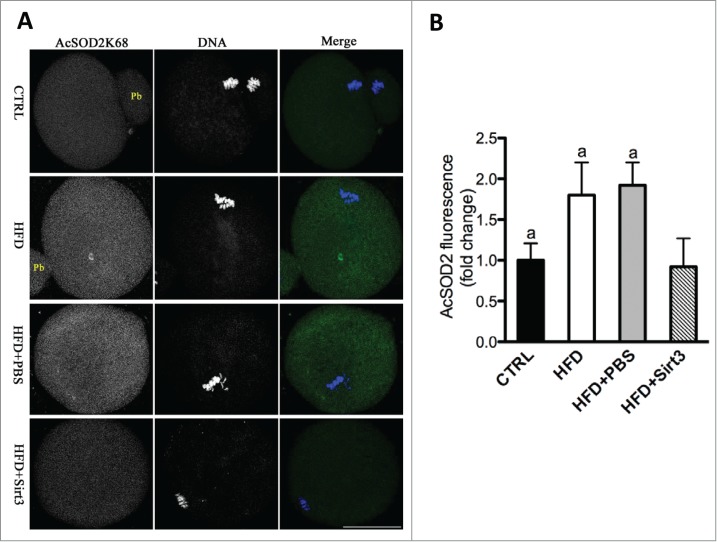
Sirt3 overexpression reduces the acetylation levels of SOD2K68 in oocytes from HFD mice. (A) Control, HFD, HFD+PBS and HFD+Sirt3 MII oocytes were stained with acetyl-SOD2K68 antibody (green) and counterstained with Hoechst 33342 for DNA (blue). Representative confocal sections are shown. (B) Quantitative analysis of fluorescence intensity shown in A. Error bars indicate ± SD (n = 105 oocytes for CTRL, 114 oocytes for HFD, 108 oocyte for HFD+PBS and 120 oocytes for HFD+Sirt3 group pooled from 3 replicates). Different superscripts indicate significant values (P < 0.05). Scale bars: 50 µm.
Deacetylation-mimetic mutant SOD2K68R partially rescues defective phenotypes of oocytes from HFD mice
Given the role of SOD2K68 acetylation in generating ROS, we next examined whether the non-acetylatable-mimetic mutant of SOD2 can rescue at least some of the phenotypic defects in HFD oocytes with reduced expression of Sirt3. To this end, mRNA for WT SOD2 or mutant SOD2 with lysine 68 changed to arginine (K68R) to mimic constitutively non-acetylated form was microinjected into fully grown HFD oocytes (Fig. 7A). Matured oocytes were then stained for evaluation of oxidative stress or the meiotic apparatus. In comparison to WT controls, SOD2K68R significantly lowered ROS levels (Fig. 7B, C) and the proportion of abnormal spindle/chromosomes (Fig. 7D, E) in HFD oocytes. These results suggest that non-acetylated SOD2K68R is capable of protecting oocytes against oxidative stress and associated meiotic defect.
Figure 7.
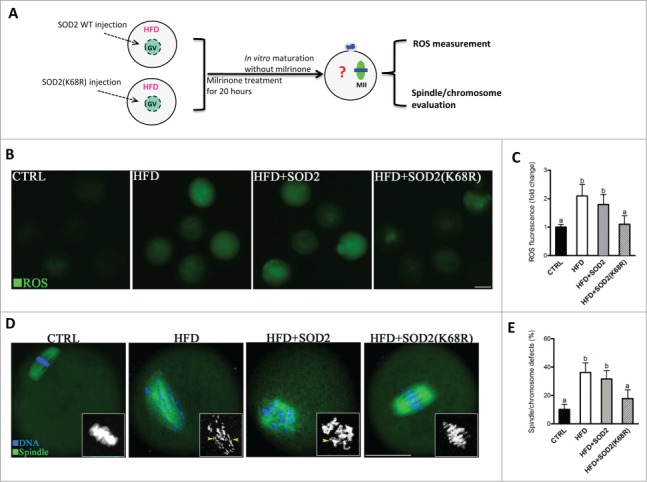
Deacetylation-mimetic mutant SOD2K68R prevents oxidative stress and meiotic defects in oocytes from HFD mice. (A) Cartoon summarizing the experimental protocol to investigate the role of SOD2K68 acetylation in oocyte meiosis and ROS homeostasis. SOD2 WT or SOD2(K68R) mutant mRNA was microinjected into GV oocytes from HFD mice, which were arrested for 20 hours with milrinone to allow synthesis of new proteins and then matured in vitro. (B) Control, HFD, HFD+SOD2 and HFD+SOD2(K68R) MII oocytes were then stained with CM-H2DCFDA to evaluate ROS production. Representative fluorescent images are shown. (C) Quantitative analysis of fluorescence intensity shown in B (n = 110 oocytes for CTRL, 102 oocytes for HFD, 106 oocyte for HFD+PBS and 110 oocytes for HFD+Sirt3 group). (D) Control, HFD, HFD+SOD2 and HFD+SOD2(K68R) MII oocytes were stained with α-tubulin antibody to visualize the spindle (green) and counterstained with Hoechst 33342 to visualize chromosomes (blue). Spindle defects and chromosome misalignment were indicated by arrows and arrowheads, respectively. Representative confocal sections are shown. (E) Quantification of Control (n = 176), HFD (n = 185), HFD+SOD2 (n = 178) and HFD+SOD2(K68R) (n = 192) oocytes with spindle defects or chromosome misalignment. All experiments were conducted 3 times, error bars indicate ± SD. Different superscript letters indicate significant differences (P < 0.05). Scale bars: 50 µm.
Discussion
Mitochondrial function has a dual impact on the intracellular redox state, through anaplerosis which will decrease oxidative stress, and through ROS generation which will increase oxidative stress.7 Formation of ROS is a by-product of oxidative phosphorylation in the mitochondria, and mitochondria are also the main target of ROS-induced damage. Here we showed that ROS levels are markedly increased in oocytes from obese mice (Fig. 1), which is consistent with previous work.8 It is worth noting that prominent mitochondrial dysfunction was observed in HFD oocytes,5 which may be associated with the elevated ROS generation. Accumulating evidence has suggested that ROS plays important roles in female reproduction. Particularly, mammalian oocyte are very sensitive to oxidative stress.27,28 Excessive ROS has been reported to have deleterious effects on oocyte maturation, fertilization and subsequent embryo development.29-31 However, the potential molecules controlling oocyte ROS levels remain largely unknown. In the present study, we noted that Sirt3 expression was reduced in HFD oocytes (Fig. 1). Sirt3 has been shown to regulate multiple intracellular pathways involved in energy production, flow of substrates, ROS production and detoxification.32 This led us to explore the link between Sirt3 expression and oocyte ROS generation. We found the dramatically increased ROS in Sirt3-depleted oocytes, and Sirt3 overexpression could attenuate ROS generation in HFD oocytes (Figs. 2 and 3). These data suggest that Sirt3 plays an important role in ROS clearance during oocyte development.
A growing body of evidence indicates that mitochondrial activity, specifically redox state, appears essential for proper assembly of meiotic apparatus in oocyte.33 For instance, a treatment of oocytes with only H2O2 causes a decrease in both length and width of metaphase spindle.34 Hydroxyl radical generated by the H2O2-driven Fenton reaction adversely affects spindle formation and chromosome alignment in mouse oocyte.35 Clinical data also showed that the loss of meiotic spindles in oocytes from polycystic ovarian syndrome (PCOS) women is correlated with high levels of oxidative stress.36 Similarly, spindle defects and chromosome misalignment are frequently detected in HFD oocytes with high levels of ROS.8 Notably, in addition to reducing ROS content, we found that Sirt3 overexpression simultaneously reduces the maternal obesity-associated meiotic defects in oocytes (Fig. 4). Accurate control of spindle assembly and chromosome organization is required for orderly oocyte meiosis. Any errors in this process can lead to the generation of aneuploid eggs, which is a major cause of pregnancy loss and, if there is survival to term, will result in developmental disabilities in humans.37 Thus, our data identified Sirt3 as an important factor determining oocyte quality, and we conclude that maternal obese environment disrupts Sirt3-controlled ROS homeostasis in oocytes, thereby contributing to the observed metabolic and meiotic alterations.
The molecular mechanism by which Sirt3 affects oxidative stress in mammalian oocytes has yet to be investigated. Recent studies have shown that Sirt3 can serve as a broad-ranging mitochondrial deacetylase targeting multiple different substrates.38 In particular, acetylation at several conserved lysine residues, i.e. K53, K68, K89 and K122, on SOD2 has been identified to inhibit enzyme activity, and that Sirt3 can deacetylate SOD2, resulting in SOD2 activation and ROS reduction.15,16,22 Through candidate screening, we identified that SOD2K68 is a major target mediating Sirt3 control of ROS generation in oocytes (Fig. 5). Due to the limitation of oocyte number, we have not yet been able to analyze the relationship between SOD2 acetylation and its activity in mouse oocytes, although we assume that acetylation inactivates SOD2, as observed in somatic cells. We further found that expression of the non-acetylatable-mimetic mutant SOD2(K68R) effectively attenuates ROS generation and ameliorates meiotic defects in HFD oocytes (Fig. 7). Together these results suggest that Sirt3-dependent SOD2 deacetylation functions as a protective mechanism in mouse oocytes, although this mechanism is aberrantly blunted in the HFD obese model. This study cannot and does not rule out that other substrates or pathways might be modulated by Sirt3 to influence redox state in oocytes. In addition, Sirt1 signaling has also been indicated to be involved in oocyte maturation by affecting ROS production.39 The potential interaction between Sirt1 and Sirt3 in mammalian oocytes deserves further investigation.
In summary, our data identify a Sirt3 pathway controlling ROS generation in oocytes and uncover a potential protective effect of Sirt3 against oxidative stress-related defects that are induced in oocytes under maternal obese conditions. In light of the importance of mitochondrial ROS in determining oocyte quality, these findings have important implications related to reproductive complications and birth defects.
Materials and Methods
All chemicals and culture media were purchased from Sigma (St. Louis, MO, USA) unless stated otherwise.
Animals and diet
All experiments were approved by the Animal Care and Use Committee of Nanjing Medical University and were performed in accordance with institutional guidelines. Female ICR mice 3 wk of age were housed 5 per cage and given access to water and fed either a high-fat diet (HFD) containing 35.8% fat, 20.7% protein, and 35% carbohydrates (D12492; Research Diets, New Brunswick, NJ, USA), or a matched control diet containing 4.8% fat, 73.9% carbohydrate, and 14.8% protein ad libitum. After 16 weeks of feeding, body weights (26.3 ± 1.8 g, n = 10 control vs. 45.7 ± 2.6 g, n = 10 HFD; P < 0.05) and fasting serum glucose (95 ± 6.3 mg/dL, n = 10 control vs. 132.1 ± 5.8 mg/dL, n = 10 HFD; P < 0.05) were significantly higher in mice fed a HFD compared with controls.
Antibodies
Rabbit polyclonal anti-SIRT3 (Cat#: ab86671) and rabbit monoclonal anti-SOD2(acetyl K68) (Cat#: ab137037) antibodies were purchased from Abcam (Cambridge, MA, USA); Mouse monoclonal anti-α-tubulin-FITC (Cat#:76074)and anti-α-tubulin(Cat#: T6074) antibodies was purchased from Sigma(St. Louis, MO, USA). FITC-conjugated goat anti-rabbit IgG was purchased from Thermo Fisher Scientific (Rockford, IL, USA).
Oocyte collection and culture
Female mice were primed with 5 IU Pregnant Mares Serum Gonadotropin (PMSG), and 48 hours later, cumulus-enclosed oocytes were collected by manual rupturing of antral follicles. Fully-grown GV oocytes were obtained by removing cumulus cells through repeatedly pipetting. For in vitro maturation, GV oocytes were cultured in M2 medium under mineral oil at 37°C in a 5% CO2 incubator. To collect ovulated MII oocytes, mice received an injection of 5 IU human Chorionic Gonadotropin (hCG) 46–48 hours after PMSG priming. Oocytes were retrieved from oviduct ampullae 13.5 h post-hCG, and freed of cumulus cells by exposure to 1 mg/ml hyaluronidase.
Plasmid construction and mRNA synthesis
Total RNA from mouse oocytes was extracted as described previously.40 The primers used to amplify the CDS sequence of Sirt3, SOD2 and mutants are listed in Supplementary Information. PCR products were purified, digested with FseI and AscI (NEB Inc., MA, USA), and then cloned into the pCS2+ vector encoding an N-terminal Myc tag. The pCS2+ vectors encoding the Myc-SOD2 substitution mutants K53Q, K68Q, K89Q and K68R were generated with the use of a QuickChange site-directed mutagenesis kit (Stratagene). For the synthesis of mRNA, the pCS2+ plasmids were linearized by NotI. Capped cRNAs were made using in vitro transcription with SP6 mMESSAGE mMACHINE (Ambion, CA, USA) and purified by RNeasy Micro Kit (Qiagen, Germany) according to the manufacturer's instruction.
Knockdown and overexpression analysis
Microinjections of morpholino or mRNA, with a Narishige microinjector, were used to knock down or overexpress specific proteins in mouse oocytes, respectively. 10 pl mRNA solution (10 ng/µl) was injected into oocyte cytoplasm for overexpression analysis. The same amount of RNase-free PBS was injected as control.
For knockdown analysis, morpholino (MO) of Sirt3 (Gene Tools, Philomath, OR, USA) targeting initiation of translation was diluted with water to give a stock concentration of 1 mM, and then 2.5 picoliter MO solution was injected into oocytes. Sirt3-MO: 5′-CCACCATGACCACCACCCTACTGCA-3′; a MO standard control was injected as control.
After injections, oocytes were arrested at the GV stage in M2 medium containing 2.5 µM milrinone for 20 hours to facilitate either knockdown of mRNA translation or permit overexpression. Following three washes, oocytes were cultured in milrinone-free medium for different time periods to evaluate the cellular events during maturation.
Western blotting
A pool of 100 oocytes was lysed in Laemmli sample buffer, and the proteins were separated by SDS–PAGE and then electrophoretically transferred to polyvinylidene fluoride membranes. After transfer, membranes were blocked in TBS containing 0.1% Tween 20 and 5% low fat dry milk for 1 hour, and then incubated with rabbit anti-Sirt3 antibody (1:500), rabbit anti-Myc antibody (1:1000) overnight at 4°C. After three washes, 10 min each in TBS containing 0.1% Tween 20 and incubation with HRP-conjugated secondary antibodies, the protein bands were visualized using an ECL Plus Western Blotting Detection System. Blots were striped and re-probed with anti-actin antibody (1:5000). All western blot experiments were repeated at least 3 times.
Measurement of intracellular ROS
Intracellular reactive oxygen species (ROS) production was measured using CM-H2DCFDA (Life Technologies, Invitrogen TM, Cat#: C6827), which is highly reactive with hydrogen peroxide and has been widely used in evaluating ROS generation in mammalian cells.41 To detect ROS levels in living oocytes, CM-H2DCFDA from Invitrogen was prepared in DMSO prior to loading. Oocytes were incubated with 5 μM CM-H2DCFDA for 30 minutes at 37°C in a 5% CO2 incubator. After three washes, 5 ∼ 10 oocytes were loaded on a slide with a microdrop of medium, and fluorescence images were recorded using a Zeiss laser scanning confocal microscope, as previously described by others,23,42 with minor modifications. The mean fluorescence intensity of cells detected at 488 nm excitation and 585 nm emission was determined.
Immunofluorescence
Oocyte staining and confocal microscopy were conducted as described previously.40 In brief, fixed oocytes were incubated overnight at 4°C with primary antibodies [anti-SIRT3 antibody (1:100) or anti-SOD2K68ac antibody (1:300)], and then with goat anti-rabbit FITC-conjugated secondary antibody for 1 hour. For spindle examination, oocytes were incubated with FITC-conjugated anti-tubulin antibody (1:200). Hoechst 33342 staining for 10 minutes was used to evaluate chromosomes. After three washes, oocyte samples were mounted on anti-fade medium (Vectashield, Burlingame, CA, USA), and examined under a Laser Scanning Confocal Microscope (LSM 710, Zeiss, Germany) equipped with the 40x or 63x oil objectives. Fluorescence intensity was quantified using Image J software (NIH) as described previously.43
Statistical analysis
Data are presented as mean ± SD, unless otherwise indicated. Differences between 2 groups were analyzed by Student's t test. Multiple comparisons between more than 2 groups were analyzed by 1-way ANOVA test using Prism 5.0. P values less than 0.05 were considered to be significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Yi Zhu (Washington University in St. Louis) for technical assistance in site-directed mutagenesis.
Funding
This work was supported by National Key Scientific Research Projects (2014CB943200), National Natural Science Foundation (31301181) of China, Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 13KJA310001), Jiangsu Entrepreneurship and Innovation Award and GM100756 to Tim Schedl.
Reference
- 1.Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod 2013; 19:486-94; PMID:23612738; http://dx.doi.org/ 10.1093/molehr/gat026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod 2007; 22:414-20; PMID:17095518; http://dx.doi.org/ 10.1093/humrep/del400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jungheim ES, Moley KH. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol 2010; 203:525-30; PMID:20739012; http://dx.doi.org/ 10.1016/j.ajog.2010.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod 2011; 26:245-52; PMID:21071489; http://dx.doi.org/ 10.1093/humrep/deq306 [DOI] [PubMed] [Google Scholar]
- 5.Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PloS One 2012; 7:e49217; PMID:23152876; http://dx.doi.org/ 10.1371/journal.pone.0049217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005; 120:483-95; PMID:15734681; http://dx.doi.org/ 10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 7.Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol 2009; 20:346-53; PMID:19530278; http://dx.doi.org/ 10.1016/j.semcdb.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 8.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PloS One 2010; 5:e10074; PMID:20404917; http://dx.doi.org/ 10.1371/journal.pone.0010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Pphysiol Endocrinol Metabol 2008; 294:E425-34; PMID:18073322; http://dx.doi.org/ 10.1152/ajpendo.00409.2007 [DOI] [PubMed] [Google Scholar]
- 10.Machtinger R, Combelles CM, Missmer SA, Correia KF, Fox JH, Racowsky C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reprod 2012; 27:3198-207; PMID:22968161; http://dx.doi.org/ 10.1093/humrep/des308 [DOI] [PubMed] [Google Scholar]
- 11.Sack MN, Finkel T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Rerspect Biol 2012; 4:1-10; PMID:23209156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bause AS, Haigis MC. SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol 2013; 48:634-9; PMID:22964489; http://dx.doi.org/ 10.1016/j.exger.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 13.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Scie U S A 2008; 105:14447-52; PMID:18794531; http://dx.doi.org/ 10.1073/pnas.0803790105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010; 143:802-12; PMID:21094524; http://dx.doi.org/ 10.1016/j.cell.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep 2011; 12:534-41; PMID:21566644; http://dx.doi.org/ 10.1038/embor.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 2010; 40:893-904; PMID:21172655; http://dx.doi.org/ 10.1016/j.molcel.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama T, Kume S, Koya D, Araki S, Isshiki K, Chin-Kanasaki M, Sugimoto T, Haneda M, Sugaya T, Kashiwagi A, et al. SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radic Biol Med 2011; 51:1258-67; PMID:21664458; http://dx.doi.org/ 10.1016/j.freeradbiomed.2011.05.028 [DOI] [PubMed] [Google Scholar]
- 18.Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D. SIRT3 reverses aging-associated degeneration. Cell Rep 2013; 3:319-27; PMID:23375372; http://dx.doi.org/ 10.1016/j.celrep.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong Y, Guan KL. Mechanistic insights into the regulation of metabolic enzymes by acetylation. J Cell Biol 2012; 198:155-64; PMID:22826120; http://dx.doi.org/ 10.1083/jcb.201202056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med 2013; 63:222-34; PMID:23665396; http://dx.doi.org/ 10.1016/j.freeradbiomed.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 2009; 119:2758-71; PMID:19652361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metabol 2010; 12:662-7; PMID:21109198; http://dx.doi.org/ 10.1016/j.cmet.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 23.Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J Clin Invest 2010; 120:2817-28; PMID:20644252; http://dx.doi.org/ 10.1172/JCI42020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev 2007; 21:920-8; PMID:17437997; http://dx.doi.org/ 10.1101/gad.1527307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol 2002; 158:647-57; PMID:12186850; http://dx.doi.org/ 10.1083/jcb.200205057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci U S A 2006; 103:10224-9; PMID:16788062; http://dx.doi.org/ 10.1073/pnas.0603968103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update 2009; 15:553-72; PMID:19414527; http://dx.doi.org/ 10.1093/humupd/dmp016 [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol Reprod 2000; 62:1745-53; PMID:10819779; http://dx.doi.org/ 10.1095/biolreprod62.6.1745 [DOI] [PubMed] [Google Scholar]
- 29.Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction 2002; 123:479-86; PMID:11914110; http://dx.doi.org/ 10.1530/rep.0.1230479 [DOI] [PubMed] [Google Scholar]
- 30.Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod 1998; 13:998-1002; PMID:9619561; http://dx.doi.org/ 10.1093/humrep/13.4.998 [DOI] [PubMed] [Google Scholar]
- 31.Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, Thornton J, Agarwal A. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril 2004; 82:593-600; PMID:15374701; http://dx.doi.org/ 10.1016/j.fertnstert.2004.02.121 [DOI] [PubMed] [Google Scholar]
- 32.Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J 2012; 444:1-10; PMID:22533670; http://dx.doi.org/ 10.1042/BJ20120030 [DOI] [PubMed] [Google Scholar]
- 33.Eichenlaub-Ritter U, Wieczorek M, Luke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion 2011; 11:783-96; PMID:20817047; http://dx.doi.org/ 10.1016/j.mito.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res 2006; 16:841-50; PMID:16983401; http://dx.doi.org/ 10.1038/sj.cr.7310095 [DOI] [PubMed] [Google Scholar]
- 35.Shaeib F, Banerjee J, Maitra D, Diamond MP, Abu-Soud HM. Impact of hydrogen peroxide-driven Fenton reaction on mouse oocyte quality. Free Rad Biol Med 2013; 58:154-9; PMID:23261938; http://dx.doi.org/ 10.1016/j.freeradbiomed.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chattopadhayay R, Ganesh A, Samanta J, Jana SK, Chakravarty BN, Chaudhury K. Effect of follicular fluid oxidative stress on meiotic spindle formation in infertile women with polycystic ovarian syndrome. Gynecol Obstet Invest 2010; 69:197-202; PMID:20051691; http://dx.doi.org/ 10.1159/000270900 [DOI] [PubMed] [Google Scholar]
- 37.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2001; 2:280-91; PMID:11283700; http://dx.doi.org/ 10.1038/35066065 [DOI] [PubMed] [Google Scholar]
- 38.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biology 2007; 27:8807-14; PMID:17923681; http://dx.doi.org/ 10.1128/MCB.01636-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Emidio G, Falone S, Vitti M, D'Alessandro AM, Vento M, Di Pietro C, Amicarelli F, Tatone C. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod 2014; PMID:24963165 [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Hou X, Ma R, Moley K, Schedl T, Wang Q. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J 2014; 28:1435-45; PMID:24334550; http://dx.doi.org/ 10.1096/fj.13-244111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng HT, Richani D, Sutton-McDowall ML, Ren Z, Smitz JE, Stokes Y, Gilchrist RB, Thompson JG. Prematuration with Cyclic Adenosine Monophosphate Modulators Alters Cumulus Cell and Oocyte Metabolism and Enhances Developmental Competence of In Vitro-Matured Mouse Oocytes. Biol Reprod 2014; 91:47; PMID:24966394; http://dx.doi.org/ 10.1095/biolreprod.114.118471 [DOI] [PubMed] [Google Scholar]
- 42.Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod 2013; 88:67; PMID:23365415; http://dx.doi.org/ 10.1095/biolreprod.112.106450 [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Chi MM, Moley KH. Live imaging reveals the link between decreased glucose uptake in ovarian cumulus cells and impaired oocyte quality in female diabetic mice. Endocrinology 2012; 153:1984-9; PMID:22294751; http://dx.doi.org/ 10.1210/en.2011-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


