Abstract
Fibroblast growth factors (FGF1, FGF2 and FGF4) and fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3 and FGFR4) have been reported to be expressed in preimplantation embryos and be required for their development. However, the functions of these molecules in trophectoderm cells (TEs) that lead to the formation of the blastocyst as well as the underlying mechanism have not been elucidated. The present study has demonstrated for the first time that endogenous FGF2 secreted by TEs can regulate protein expression and distribution in TEs via the FGFR2-mediated activation of PKC and p38, which are important for the development of expanded blastocysts. This finding provides the first explanation for the long-observed phenomenon that only high concentrations of exogenous FGFs have effects on embryonic development, but in vivo the amount of endogenous FGFs are trace. Besides, the present results suggest that FGF2/FGFR2 may act in an autocrine fashion and activate the downstream PKC/p38 pathway in TEs during expanded blastocyst formation.
Keywords: blastocyst formation, fibroblast growth factor 2 (FGF2), fibroblast growth factor receptor 2 (FGFR2), protein kinase C (PKC), p38 MAPK
Introduction
After fertilization, mammalian preimplantation embryos undergo cleavage and differentiation to form a hollow-shaped embryo called the blastocyst, which is crucial for all stages of subsequent embryonic development.1 At the end of the blastocyst stage, the embryo establishes 3 spatially and molecularly distinct cell lineages [the trophoblast, the trophectoderm (TE) and the inner cell mass (ICM)]. The TE forms a polarized epithelial cell layer that encloses a fluid-filled cavity termed the blastocoel. The ICM, an undifferentiated mass of cells, is composed of 2 distinct layers: an inner population of epiblast (EPI) cells and a superficial layer of primitive endoderm (PrE) adjacent to the blastocyst cavity. The EPI will give rise to the embryo proper, the PrE forms some of the extraembryonic membranes, and the TE contributes to the placenta.2,3 Functional tight junctions form a seal between the cells of the TE, which is essential for fluid accumulation and formation of the blastocyst cavity.4,5 The Na-K ATPase is also a critical mediator of blastocyst formation as it establishes a trans-trophectoderm ionic gradient that directs fluid movement across the epithelium of the TE.4,6,7 Aquaporins (AQP3 and AQP9) in the TE membrane facilitate the movement of fluids across the TE from the ‘outside’ to the ‘inside’ of the blastocyst cavity, along the ionic gradient established by the Na-K ATPase localized on the baso-lateral membrane.4,8 After segregation of the early lineages is complete, the blastocyst emerges from the zona pellucida to invade the maternal uterine endometrium, where it implants.9,10,11
Generally, the development of the blastocyst is guided by highly organized interactions of multiple growth factors.12,13,14 Growth factors perform their roles in embryonic development and blastocyst function in an autocrine/paracrine manner.15 Fibroblast growth factors (FGFs) encompass a large family of autocrine, paracrine, endocrine, and intracrine factors. The FGFs affect target cells via the activation of cell-surface tyrosine kinase receptors that are encoded by 4 genes in mammals (designated fgf1/2/3/4).16,17 Evidence has shown that FGFs play important roles in the regulation of pluripotency and lineage segregation in both the early mouse embryo and pluripotent mammalian stem cells.18 In addition to the indispensability of correct lineage segregation, FGFs activated FGF signaling pathways, characterized by the expression of adherens junctions (AJ), tight junctions (TJ), ion channels and AQPs, are essential for fluid accumulation and formation of the blastocyst cavity in the development of a proper blastocyst.4,18,19
We found that some human embryos could develop to expanded blastocysts without ICM in our in vitro fertilization (IVF) laboratory. This phenomenon may infer that blastocyst formation is independent of ICM. Therefore, the FGFs that have effects on blastocyst formation are from TE or outside of embryos. Rappolee's research showed that FGF3 mRNA was not detected in mouse preimplantation embryos.20 Besides, some researchers observed that the expression of FGF4 polypeptide as well as mRNA was limited to the ICM cells in the blastocyst.17,20 One of central interest is FGF2, which is produced by luminal and glandular epithelium and is detectable in the uterine lumen throughout early pregnancy in animals.21,22 But previous studies found FGF2 improved blastocyst formation during bovine embryo culture in vitro unless large amounts of recombinant protein were provided (500–1000 ng/ml.23-25 The previous research found that FGF2 performs its function in an autocrine manner, which is physiologically significant for FGF2 to bind its high-affinity receptor.26,27 So we explored the possibility that FGF2 might bind to FGFRs in TE in an autocrine model to modulate blastocyst formation in early stage embryos.
Results
Endogenous FGF2 from TEs is required for expanded blastocyst formation
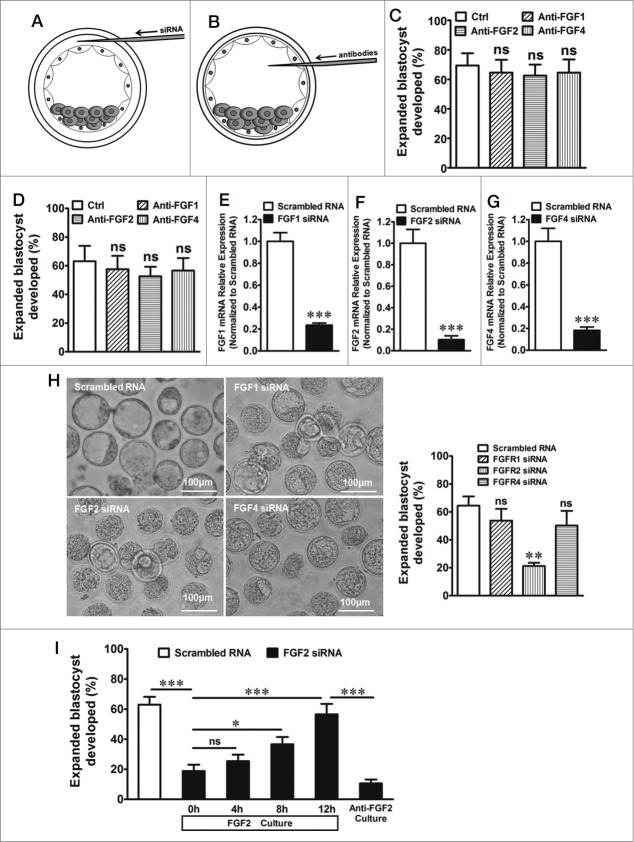
In this study, the micromanipulation system was used to microinject RNA into the cavity gap between the zona pellucida and the trophectoderm to transfect siRNA into trophectoderm (Fig. 1A) and microinject antibodies into blastocoels to eliminate the specific growth factors that may be from ICM (Fig. 1B) (See details in Materials and Methods). As shown in Figure 1C, the formation of murine expanded blastocysts was apparently not affected when exogenous FGF1, FGF2 or FGF4 were eliminated in the medium, respectively, by the corresponding antibodies. In addition, no significant differences were observed between the control and test embryos when endogenous FGF1, FGF2 or FGF4, which may originate from the ICM, was eliminated in the blastocoels by microinjection of the corresponding antibodies (Fig. 1D). The FGF1, FGF2 or FGF4 knockdown (siRNAfgf1, siRNAfgf2 or siRNAfgf4 transfection) was performed to confirm the effect of the 3 FGFs secreted by TEs on expanded blastocyst formation. Quantitative real-time PCR showed that the expression of FGF1, FGF2 and FGF4 sharply decreased 80–90% in the TEs after transfection with siRNA (Fig. 1E–G). We found that the knockdown of FGF1 and FGF4 in the TEs of early blastocysts had no effect on the formation of expanded blastocysts (Fig. 1H). However, the rate of blastocyst expansion significantly decreased after FGF2 knockdown (Fig. 1H). Similarly, FGF2 knockdown also suppressed expanded blastocyst development (Fig. 1I). When exogenous FGF2 (1000 ng/ml) was added to the medium and maintained for 12 h, the inhibition of blastocyst formation was reversed (Fig. 1I). However, this reversal was significantly affected by the addition of 200 ng/ml of FGF2 antibody to the medium (Fig. 1I). These results indicate that endogenous FGF2 from TEs is important for expanded blastocyst formation.
Figure 1 (See previous page).
The microinjection technique used in the study and the effect of FGFs on expanded blastocyst formation. (A) Working models for RNA microinjection of the embryo. After the blastocysts were dehydrated in drops of 1 M mannitol, each embryo was injected with an RNA solution into the cavity gap between the zona pellucida and trophectoderm using the micromanipulation system. (B) Working models for antibody microinjection of the embryo. Blastocoels of the blastocyst were injected with antibodies against FGFs using the micromanipulation system. Negative control blastocysts were injected with the same volume of normal saline that was substituted for the antibodies. (C) Formation of expanded blastocysts when exogenous FGF1, FGF2 or FGF4 were eliminated in the medium by their respective antibodies. ns indicates P > 0.05 (by one-way ANOVA, n = 5) compared with the control. (D) Formation of expanded blastocysts when endogenous FGF1, FGF2 or FGF4 was eliminated in the blastocoels by the blastocyst microinjection of the respective antibodies. ns indicates P > 0.05 (by one-way ANOVA, n = 5) compared with the control. (E) Expression of FGF1 mRNA in TEs after transfection with FGF1 siRNA. *** indicates P < 0.001 (by unpaired t-test, n = 4) compared with the control (transfection with scrambled RNA). (F) Expression of FGF2 mRNA in TEs after transfection with FGF2 siRNA. *** indicates P < 0.001 (by unpaired t-test, n = 4) compared with the control (transfection with scrambled RNA). (G) Expression of FGF4 mRNA in TEs after transfection with FGF4 siRNA. *** indicates P < 0.001 (by unpaired t-test, n = 4) compared with the control (transfection with scrambled RNA). (H) Left, effect of the knockdown of FGF1, FGF2 or FGF4 in TEs on the formation of expanded blastocysts. Scale bar: 100 μm. Right, summary of the results. ns indicates P > 0.05 and ** indicates P < 0.01 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA). (I) Effect of exogenous FGF2 on expanded blastocyst formation after the knockdown of FGF2 in TEs. ns indicates P > 0.05, * indicates P < 0.05, and *** indicates P < 0.001 (by one-way ANOVA, n = 4) compared with the corresponding controls.
FGFR2 in TEs is required for expanded blastocyst formation
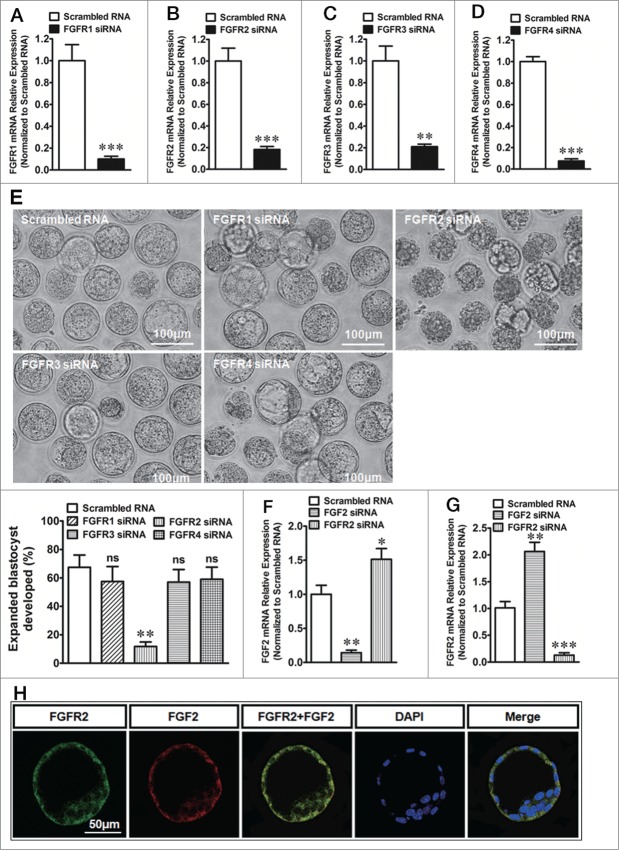
The FGFR1, FGFR2, FGFR3 or FGFR4 knockdown (siRNAfgfr1, siRNAfgfr2, siRNAfgfr3 or siRNAfgfr4 transfection) was performed to examine the function of each of the 4 FGFRs in expanded blastocyst formation. Quantitative real-time PCR showed that FGFR1, FGFR2, FGFR3 and FGFR4 expression also sharply decreased by 80-90% of the control in TEs after the transfection with the corresponding siRNA (Fig. 2A–D). Twenty-four hours after the knockdown of the FGFRs in the TEs of early blastocysts, we found that the knockdown of FGFR1, FGFR3 or FGFR4 had no effect on the development of expanded blastocysts (Fig. 2E). However, the rate of expanded blastocyst formation significantly decreased after FGFR2 knockdown (Fig. 2E). In order to investigate whether FGF2 knockdown affect FGFR2 expression or the vice versa, we examined the mRNA levels of FGFR2 and/or FGF2 when they were knockdown respectively. The results showed that FGF2 knockdown suppressed FGF2 expression and up regulated FGFR2 expression meanwhile (Fig. 2F). On the other side, FGFR2 knockdown suppressed FGFR2 expression and increased FGF2 expression as well. (Fig. 2G). In addition, confocal analysis of double-immunofluorescence staining showed that the co-expression of FGF2 and FGFR2 was detected in the cell membrane and cytoplasm of the TEs (Fig. 2H). The results show that FGF2 acted upon FGFR2 in an autocrine manner in the regulation of expanded blastocyst development.
Figure 2 (See previous page).
The effect of FGFRs in TEs on expanded blastocyst formation. (A) Expression of FGFR1 mRNA in TEs after transfection with FGFR1 siRNA. *** indicates P < 0.001 (by unpaired t-test, n = 4) compared with the control (transfection with scrambled RNA). (B) Expression of FGFR2 mRNA in TEs after transfection with FGFR2 siRNA. *** indicates P < 0.001 (by unpaired t-test, n = 4) compared with the control (transfection with scrambled RNA). (C) Expression of FGFR3 mRNA in TEs after transfection with FGFR3 siRNA. ** indicates P < 0.01 (by unpaired t-test, n = 4) compared with the control (transfection with scrambled RNA). (D) Expression of FGFR4 mRNA in TEs after transfection with FGFR4 siRNA. *** indicates P < 0.001 (by unpaired t-test, n = 4) compared with the control (transfection with scrambled RNA). (E) Up, effect of FGFR1, FGFR2, FGFR3 or FGFR4 knockdown in TEs on the formation of expanded blastocysts. Scale bar: 100 μm. Low-right, summary of the results. ns indicates P > 0.05 and ** indicates P < 0.01 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA). (F) Expression of FGF2 mRNA in TEs after transfection with FGF2 or FGFR2 siRNA. * indicates P < 0.05 and ** indicates P < 0.01 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA). (G) Expression of FGFR2 mRNA in TEs after transfection with FGF2 or FGFR2 siRNA. ** indicates P < 0.01 and *** indicates P < 0.001 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA). (H) Double-immunofluorescence by confocal microscopy that shows the co-expression of FGF2 and FGFR2 in TEs. Total 19 normal blastocysts were used. And all the 19 blastocysts were labeled successfully (19/19 blastocysts). Scale bar: 50 μm.
Expression of E-cadherin, ZO-1, Na-K ATPase, ENaCα, AQP3 and AQP9 mRNAs in TEs after the knockdown of FGF2/FGFR2
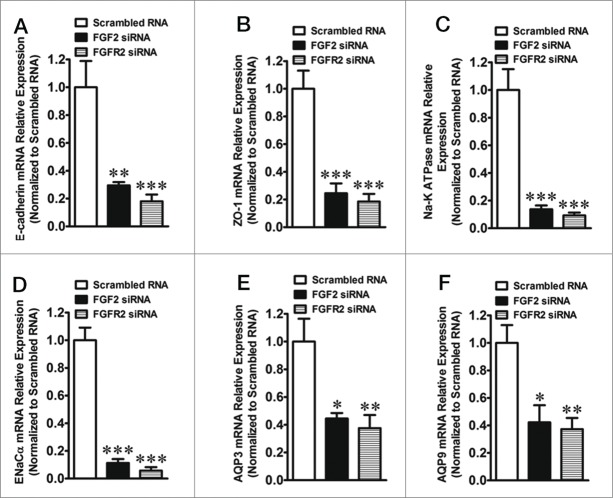
Quantitative real-time PCR showed that FGF2 or FGFR2 knockdown sharply decreased the mRNA expression of E-cadherin, Zonula occluden-1 (ZO-1), Na-K ATPase, Epithelial sodium channel α (ENaCα), AQP3 and AQP9 in the TEs after transfection with their respective siRNAs.(Fig. 3A–F)
Figure 3.
Quantitative real-time PCR results show the effect of the knockdown of FGF2 or FGFR2 on the expression of protein markers of blastocyst in TEs. (A) Expression of E-cadherin mRNA in TEs after transfection with FGF2 or FGFR2 siRNA. ** indicates P < 0.01 and *** indicates P < 0.001 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA). (B) Expression of ZO-1 mRNA in TEs after transfection with FGF2 or FGFR2 siRNA. *** indicates P < 0.001 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA). (C) Expression of Na-K ATPase mRNA in TEs after transfection with FGF2 or FGFR2 siRNA. *** indicates P < 0.001 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA). (D) Expression of ENaCα mRNA in TEs after transfection with FGF2 or FGFR2 siRNA. *** indicates P < 0.001 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA). (E) Expression of AQP3 mRNA in TEs after transfection with FGF2 or FGFR2 siRNA. * indicates P < 0.05 and ** indicates P < 0.01 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA). (F) Expression of AQP9 mRNA in TEs after transfection with FGF2 or FGFR2 siRNA. * indicates P < 0.05 and ** indicates P < 0.01 (by one-way ANOVA, n = 4) compared with the control (transfection with scrambled RNA).
The alteration of E-cadherin, ZO-1, Na-K ATPase, ENaCα, AQP3 and AQP9 protein expression in TEs after knockdown of FGF2 or FGFR2
The formation and distribution of E-cadherin and ZO-1 in TE was observed by immunofluorescence staining after the knockdown of FGF2 or FGFR2. As shown in Figure 4A, E-cadherin was not expressed in some regions of the TE after FGF2 or FGFR2 knockdown. At the same time, the control embryos demonstrated continuous and well-organized ZO-1 expression only at the cell junction, whereas the FGF2 or FGFR2 knockdown embryos showed disrupted expression of ZO-1 at the cell junction (arrow head) and diffuse localization in the cytoplasm (Fig. 4B). In addition, confocal analysis of immunofluorescence staining showed that the expression of Na-K ATPase on the inner side of the TEs and the expression of ENaCα on the outer side of the TEs were both significantly reduced in the TE after knockdown of FGF2 or FGFR2 (Fig. 4C and D). Moreover, confocal analysis of double-immunofluorescence staining showed that both FGF2 knockdown and FGFR2 knockdown significantly reduced the expression of AQP3 and AQP9 in the TE (Fig. 4E). The down regulation of E-cadherin, ZO-1, Na-K ATPase, ENaCα, AQP3 and AQP9 proteins by FGF2 or FGFR2 knockdown was confirmed by western blots (Fig. 4F). Taken together, these results suggest that FGF2/FGFR2 interaction is involved in mediating the expression and localization of cavitation formation markers in TE in the development of expanded blastocysts.
Figure 4.
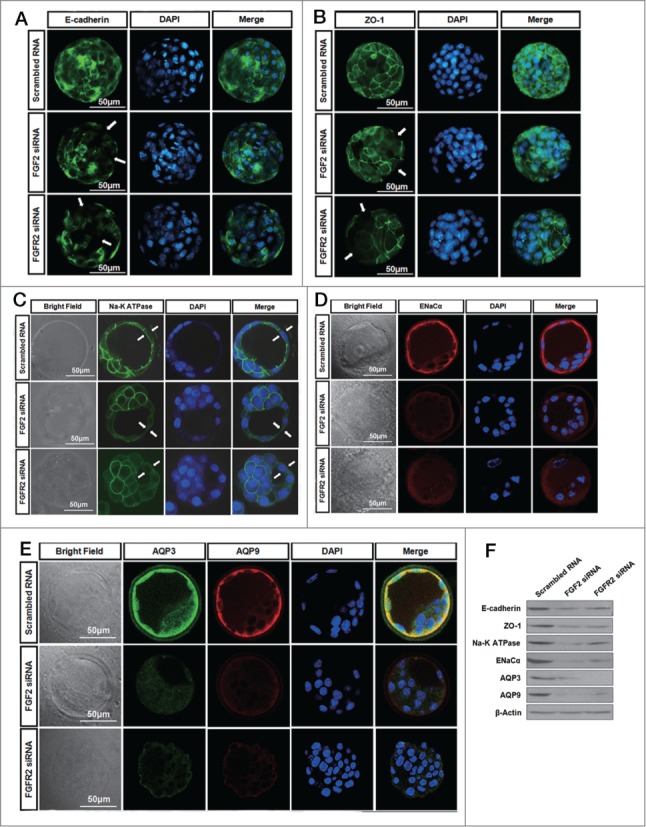
Immunofluorescence microscopy illustrates the effect of the knockdown of FGF2 or FGFR2 on the distribution or expression of protein markers of blastocyst in the TEs. (A) Effect of the knockdown of FGF2 or FGFR2 on the distribution of E-cadherin within the TEs by immunofluorescence. Phenotype as labeled by scrambled RNA (13/15 embryos). Phenotype as labeled by FGF2 siRNA (11/14 embryos). Phenotype as labeled by FGFR2 siRNA (13/17 embryos). Scale bar: 50 μm. (B) Effect of the knockdown of FGF2 or FGFR2 on the distribution of ZO-1 within the TEs by immunofluorescence. Phenotype as labeled by scrambled RNA (12/14 embryos). Phenotype as labeled by FGF2 siRNA (12/16 embryos). Phenotype as labeled by FGFR2 siRNA (17/21 embryos). Scale bar: 50 μm. (C) Effect of the knockdown of FGF2 or FGFR2 on the expression and localization of Na-K ATPase in the TEs by confocal immunofluorescence. Phenotype as labeled by scrambled RNA (21/23 embryos). Phenotype as labeled by FGF2 siRNA (16/19 embryos). Phenotype as labeled by FGFR2 siRNA (16/20 embryos). Scale bar: 50 μm. (D) Effect of the knockdown of FGF2 or FGFR2 on the expression of ENaCα in the TE by confocal immunofluorescence. Phenotype as labeled by scrambled RNA (22/25 embryos). Phenotype as labeled by FGF2 siRNA (15/20 embryos). Phenotype as labeled by FGFR2 siRNA (14/18 embryos). Scale bar: 50 μm. (E) Effect of the knockdown of FGF2 or FGFR2 on the expression of AQP3 and AQP9 in the TE by confocal double-immunofluorescence. Phenotype as labeled by scrambled RNA (26/30 embryos). Phenotype as labeled by FGF2 siRNA (19/25 embryos). Phenotype as labeled by FGFR2 siRNA (23/28 embryos). Scale bar: 50 μm. (F) Western blots showing E-cadherin, ZO-1, Na-K ATPase, ENaCα, AQP3 and AQP9 in TEs after FGF2 or FGFR2 knockdown.
FGF2 binding to FGFR2 in TEs is required for the regulation of the formation of expanded blastocysts by activation of the PKC-p38 cascade
We have established that FGF2 binding to FGFR2 in the TEs is a key event in the regulation of expanded blastocyst formation. Next, we wanted to explore the signaling mechanism involved in this regulation. The expression of certain protein markers in the development of expanded blastocysts has been shown to be mediated by a PKC-dependent pathway.28 The addition of the PKC inhibitor Gö6983 to the culture medium of embryos drastically inhibited the transformation of early blastocysts to expanded blastocysts compared with the DMSO-treated vehicle control (Fig. 5A). The stimulation of PKC-activated p38 mitogen-activated protein kinase (MAPK) or ERK1/2 MAPK, is linked to the induction of blastocyst development.4,29,30 To test this link, we studied the phosphorylated (p-) of p38 or ERK1/2 and their transport between the cytoplasm and nucleus using an immunofluorescence confocal technique. As shown in Figure 5B, p-p38 translocated to the nucleus of TEs in blastocysts that were transfected with scrambled (control) RNA. The p-p38 and p-ERK1/2 levels were examined by protein gel blots in TEs in response to FGF2 or FGFR2 knockdown (Fig. 5C). This translocation and phosphorylation of p38 was inhibited by the knockdown of either FGF2 or FGFR2 or by treatment with the p38 MAPK inhibitor SB202190 (50 µM) (Fig. 5B–E). However, the translocation and phosphorylation of ERK1/2 was not affected obviously by the above treatments (Fig. 5B and C). These results suggest that the translocation or activation of p38 in the TEs of mouse blastocysts depends on both FGF2 and FGFR2, which are known to activate the PKC/p38 pathway. If the activation of PKC or p38 is blocked by the inhibitors Gö6983 or SB202190, respectively, the downregulation of E-cadherin, ZO-1, Na-K ATPase, ENaCα, AQP3 and AQP9 mRNA is observed in the TEs of blastocysts in the presence of FGF2, as demonstrated by quantitative real-time PCR (Fig. 5F). These results indicate that the FGF2/FGFR2-dependent expression of the above proteins, which are important to the development of the expanded blastocyst, is mediated by the PKC-dependent activation of p38 MAPK.
Figure 5.
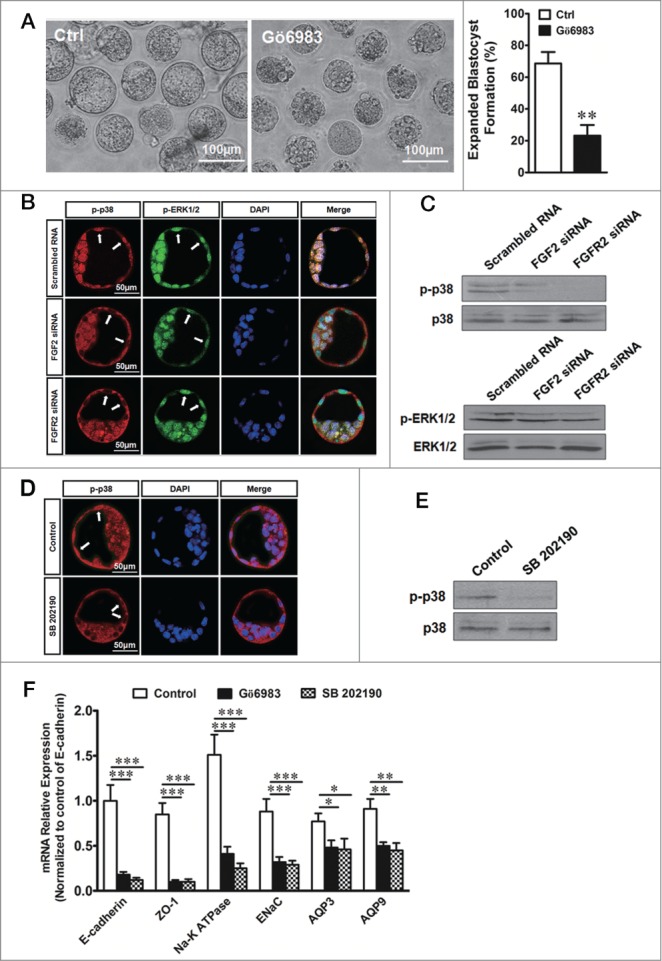
Effect of FGF/FGFR2 on the PKC-p38 pathway during expanded blastocyst formation. (A) Left, effect of the PKC inhibitor Gö6983 (1μM) on expanded blastocyst formation. Scale bar: 100 μm. Right, summary of the results. ** indicates P < 0.01 (by unpaired t-test, n = 5) compared with the control. (B) Effect of FGF2 or FGFR2 knockdown on the nuclear translocation of p-p38 MAPK and p-ERK1/2 MAPK in TEs by confocal double-immunofluorescence. Phenotype as labeled by scrambled RNA (24/30 embryos). Phenotype as labeled by FGF2 siRNA (22/29 embryos). Phenotype as labeled by FGFR2 siRNA (18/24 embryos). Scale bar: 50 μm. (C) Western blots of phosphorylated (p-) p38 (up) or ERK1/2 (down) levels in TEs in response to FGF2 or FGFR2 knockdown with siRNA transfection. (D) Effect of the p38 MAPK inhibitor SB202190 (50 µM) on the nuclear translocation of p-p38 MAPK in TEs by confocal immunofluorescence. The embryos were treated with SB202190 for 10 min. Phenotype as labeled by control (31/35 embryos). Phenotype as labeled by SB202190 treatment (27/32 embryos). Scale bar: 50 μm. (E) Western blots showing p-p38 in response to SB202190 (50 µM). (F) Effect of Gö6983 (1 μM) or SB202190 (50 µM) on the mRNA expression of protein markers of blastocysts in TEs by quantitative real-time PCR. * indicates P < 0.05, ** indicates P < 0.01, and *** indicates P < 0.001 (by one-way ANOVA, n = 4) compared with the corresponding controls.
Discussion
FGFs (FGF1, FGF2 and FGF4) and FGFRs (FGFR1, FGFR2, FGFR3 and FGFR4) have been reported to be expressed in preimplantation embryos and be required for their development.18,20,31-35 However, the functions of these molecules in TEs that lead to the formation of the blastocyst as well as the underlying mechanism have not been elucidated. The present study has demonstrated for the first time that endogenous FGF2 secreted by TEs can regulate protein expression and distribution in TEs via the FGFR2-mediated activation of PKC and p38, which are important for the development of expanded blastocysts (Fig. 6). This finding provides the first explanation for the long-observed phenomenon that only high concentrations of FGFs have effects on embryonic development and suggests that FGF2 acts as an endogenous factor for the regulation of TE function during expanded blastocyst development.23
Figure 6.
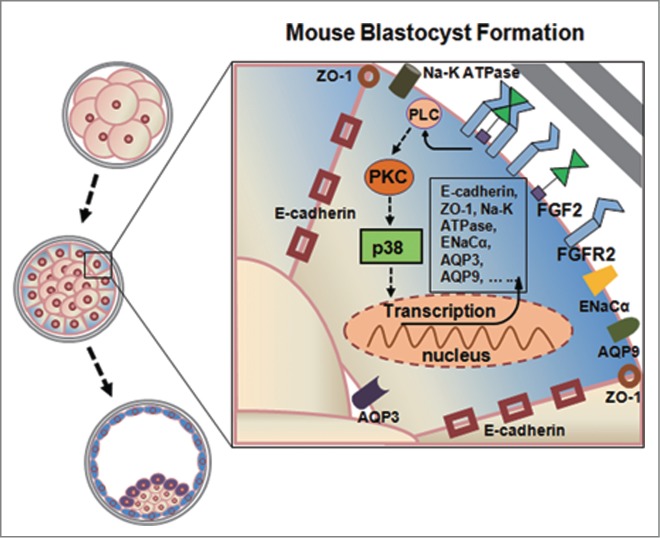
Working model for the regulation of blastocyst formation by the autocrine interaction of FGF2/FGFR2 activated PKC/p38 pathway in mouse TEs. Binding of FGF to cell surface heparin-like molecules in an autocrine fashion, FGF2 activates FGFR2, which stimulates phospholipase C (PLC) to generate diacylglycerol (DAG). In turn, DAG activates protein kinase C (PKC), which stimulates p38 activation, triggering transcription factor to regulate the expression of E-cadherin, ZO-1, Na-K ATPase, ENaCα, AQP3 and AQP9, which is required for blastocyst formation.
The results of the present study show that the development of expanded blastocysts was not affected when the activities of exogenous FGF1, FGF2 or FGF4 in the medium were eliminated by the corresponding antibodies. The same phenomenon was also observed when the activities of FGF1, FGF2 or FGF4 were eliminated by the microinjection of the corresponding antibodies into the blastocoels. This finding suggests that neither exogenous FGFs from the oviduct and endometrium nor endogenous FGFs from the ICM affect the development of expanded blastocysts under normal physiological conditions. These results may provide a useful explanation of why an abnormal ICM was found in some blastocysts that were cultured in vitro while the TE developed normally. FGF1, FGF2 and FGF4 have been reported to be expressed in preimplantation embryos.20,31,32 Here, we found that the formation of expanded blastocysts was arrested only after the knockdown of FGF2 in TEs in vitro, which suggests that FGF2 is required in TEs for the development of expanded blastocysts. The knockdown of FGF2 in TEs affected the growth of expanded blastocysts, which could be reversed when the embryos were cultured with 1000 ng/ml exogenous FGF2 for 12 h. However, this rescue was completely abolished when the activation of FGF2 was eliminated by the application of an FGF2 antibody. This finding suggests that exogenous FGF2 can also perform its function in the formation of expanded blastocysts after the knockdown of FGF2 in TEs.
Some research has suggested that the 4 FGFRs, including FGFR1, FGFR2, FGFR3 and FGFR4, are all expressed in preimplantation embryos.31,32 The development of expanded blastocysts was not affected when the expression of FGFR1, FGFR3 or FGFR4 was suppressed by transfection with specific siRNAs. In addition, using the double-immunofluorescence confocal technique, we found that FGF2 and FGFR2 were co-expressed in the membrane and the cytoplasm of TEs. We also found that the development of expanded blastocysts was inhibited by the addition of FGF2 antibodies to the culture medium, which presumably blocks the extracellular ligand-receptor interaction. This finding implies that FGF2 acts in an autocrine manner when it binds to FGFR2 in TEs to promote the formation of expanded blastocysts. Our results are consistent with the previous report that FGF2 performs its function in an autocrine manner.26 FGF2 was previously demonstrated to bind to the cell membrane via heparin or heparin-like molecules, which is physiologically significant because heparin is required for the binding of FGF2 to its high-affinity cell surface receptor.27,36
The ion channels (including Na-K ATPase and ENaC), tight junctions (including E-cadherin and ZO-1) and AQPs (including AQP3 and AQP9) contribute directly to the mechanism that enables the TEs to regulate cavitation and blastocyst formation.6,37 Functional adheren junctions and tight junctions form a seal between the cells of the TE, which is essential for fluid accumulation and the formation of the blastocyst cavity during the process of expanded blastocyst formation.5,38 In this study, we found that the knockdown of either FGF2 or FGFR2 reduced the expression of E-cadherin and ZO-1 and perturbed their distribution within the TE as determined by quantitative real-time PCR and immunofluorescence staining. The Na-K ATPase plays an important role in the establishment of a trans-trophectoderm ionic gradient that directs fluid movement across the TE epithelium during expanded blastocyst formation.7,39 During the process of Na+ transport, Na+ crosses the apical membrane via ENaC into TEs and is extruded into the blastocoels by Na-K ATPase on the basal side of the membrane.7,39,40 Thus, the activities of ENaCα and Na-K ATPase must be highly coordinated to accommodate variations in Na+ transport during expanded blastocyst formation. In this study, the knockdown of FGF2 or FGFR2 in the TE was accompanied by the down-regulation of the expression of Na-K ATPase and ENaCα in TEs. These outcomes implicate FGF2 and FGFR2 as mediators that coordinate the function of proteins that are involved in the establishment of the trans-TE ionic gradient during expanded blastocyst formation. Additionally, fluid movement across the TE is facilitated by the presence of AQP3 and AQP9 in the TE membrane.8 AQP9 is localized to the apical surface of the TE, whereas AQP 3 is localized to the baso-lateral surface of the TE. Together, they facilitate fluid movement across TEs into the blastocyst cavity along the ionic gradient established by the Na-K ATPase.8 We have demonstrated that the knockdown of FGF2 or FGFR2 in TEs resulted in the downregulation of AQP3 and AQP9 mRNA levels, the reduction of AQP3 and AQP9 localization and the suppression of expanded blastocyst formation.
We have established that the FGF2/FGFR2 interaction plays an important role in the regulation of E-cadherin, ZO-1, Na-K ATPase, ENaCα, AQP3 and AQP9 expression and in the localization in the development of expanded blastocysts. We also explored the signaling mechanism involved in this process. The activation of FGFR2 has been shown to regulate cell differentiation via PKC.41,42 Interestingly, the activation of PKC has been shown to be involved in the regulation of blastocoel formation during mouse preimplantation development,28,43 which is in accordance with our observation that the inhibition of PKC significantly suppressed the formation of expanded blastocysts. More importantly, p38 regulates cavitation and the function of tight junctions, which affects the expanded formation of the blastocyst.4 As PKC is an activator of p38/ERK1/2,19,44,45 the effect of the PKC-mediated pathway on the formation of expanded blastocysts may be mediated by the p38/ERK1/2 pathway. To test this relationship, we studied the inhibition of p38 by examining the shuttling of p-p38 between the cytoplasm and nucleus using double-immunofluorescence confocal techniques. As shown in Figure 5B, the translocation of p-p38 to the nucleus in TEs was inhibited by the knockdown of FGF2 or FGFR2. In contrast, the translocation of p-ERK1/2 remained unchanged. Blocking the activation of p38 by an inhibitor, SB202190, also resulted in the inhibition of its translocation within TEs. These results suggest that the translocation or activation of p38 in mouse TEs depends on FGF2/FGFR2, which are known to activate the PKC/p38 pathway. The inhibition of PKC or the activation of p38 also resulted in the down-regulation of the expression of E-cadherin, ZO-1, Na-K ATPase, ENaCα, AQP3 and AQP9 in the TE. These results indicate that the FGF2/FGFR2-dependent formation of expanded blastocysts is mediated by the PKC/p38-dependent pathway.
The present study has demonstrated a critical role for FGF2/FGFR2 in the mediation of expanded blastocyst formation. The expression and localization of E-cadherin, ZO-1, Na-K ATPase, ENaCα, AQP3 and AQP9 were altered when the expression of FGF2/FGFR2 changed in the TEs of blastocysts. In addition, the autocrine interaction of FGF2/FGFR2 and the activation of the downstream PKC/p38 pathway in TEs suggest that the progression of expanded blastocyst formation is a self-regulatory process under physiological conditions.
Materials and Methods
Animals
The care and use procedures for the ICR mice were performed in accordance with the Institutional Guide for Laboratory Animals established by the Animal Care and Use Committee (ACUC). All animal handling protocols were approved by the ACUC of the School of Medicine of Zhejiang University. The mice were housed under a 12/12-h light/dark cycle at 25 ± 0.5°C and 50 to 60% humidity and were fed ad libitum with a standard diet and water.
Induction of ovulation and collection of mouse embryos
Female ICR mice (7–8 weeks old) were superovulated via intraperitoneal injections of 10 IU of pregnant mare's serum gonadotrophin (PMSG; Hangzhou Animal Pharmaceutical Factory, Hangzhou, Zhejiang, China), followed by 10 IU of human chorionic gonadotrophin (hCG; Hangzhou Animal Pharmaceutical Factory) after 48 h; they were then mated with ICR males (10-11 weeks old). Successful mating was confirmed the following morning by the appearance of a vaginal plug. The early-stage blastocysts were obtained after the sacrifice of the mice at 89 h after the hCG injection. Blastocysts were collected by flushing the uterus with human tubal fluid HEPES (HTF-HEPES, Irvine Scientific, Irvine, CA, USA) medium.
Culture and treatment of embryos
The collected embryos were transferred to human tubal fluid (HTF; Irvine Scientific) medium, cultured in 5% CO2 at 37°C and then prepared for further experiments. The embryos were washed 3 to 4 times in HTF-HEPES medium and transferred to medium containing the following: 20-μl drops of HTF + 0.2% dimethylsulfoxide (DMSO, D2650; Sigma, St. Louis, MO, USA), HTF + 1 μM of the PKC inhibitor Gö6983 (sc-203432; Santa Cruz Biotechnology, Santa Cruz, CA, USA), HTF + 50 µM of the p38 MAP kinase inhibitor SB 202190 (ab120238; Abcam, Cambridge, MA, USA), HTF + 100 ng of FGF1 antibody, HTF + 100 ng of FGF2 antibody, 100 ng of FGF4 antibody or 100 ng of FGF2 (PMG0033; Invitrogen, Gibco, Carlsbad, CA, USA) under light paraffin oil. The information of the antibodies used is given in Table 1. The embryos were maintained in culture in 5% CO2 and atmospheric air at 37°C. After 24 h of treatment, all embryos from each group were imaged at time point equivalent to 113 h post-hCG injection. The diameter of the embryos was used to assess blastocyst expansion. The diameter of each blastocyst was measured in 2 different directions (using Image Pro analysis 6.2 software) and then averaged. After treatment, some embryos were fixed in 4% paraformaldehyde for immunofluorescence staining or frozen at −80°C for quantitative real-time PCR analysis.
Table 1.
Primary antibodies
| Poly/monoclonal | Host species | Against | Catalog number | Company | Application |
|---|---|---|---|---|---|
| Polyclonal | Rabbit | FGF1 | sc-7910 | Santa Cruz | EC (100ng in 20μl) |
| Polyclonal | Goat | FGF2 | sc-1360 | Santa Cruz | IF (1:200), EC (100ng in 20μl) |
| Polyclonal | Rabbit | FGF4 | sc-1361 | Santa Cruz | EC (100ng in 20μl) |
| Polyclonal | Rabbit | FGFR2 | Ab10648 | Abcam | IF(1:500) |
| Polyclonal | Rabbit | E-cadherin | sc-7870 | Santa Cruz | IF(1:500),WB(1:1000) |
| Polyclonal | Rabbit | ZO-1 | sc-10804 | Santa Cruz | IF(1:200),WB(1:1000) |
| Polyclonal Polyclonal | Rabbit Goat | Na-K ATPase ENaCα | sc-10804 sc-21012 | Santa Cruz Santa Cruz | IF(1:100), WB(1:1000) IF(1:200), WB(1:1000) |
| Polyclonal | Rabbit | AQP3 | sc-20811 | Santa Cruz | IF(1:100), WB(1:1000) |
| Polyclonal | goat | AQP9 | sc-14988 | Santa Cruz | IF(1:100), WB(1:1000) |
| Polyclonal | rabbit | β-Actin | sc-7210 | Santa Cruz | WB(1:5000) |
| Polyclonal | rabbit | p38 | sc-535 | Santa Cruz | WB(1:1000) |
| Monoclonal | Mouse | p-p38 | Ab45381 | Abcam | IF(1:300), WB(1:1000) |
| Polyclona | rabbit | ERK1/2 | #9102 | Cell Signaling | WB(1:1000) |
| Polyclonal | rabbit | p-ERK1/2 | #9101 | Cell Signaling | IF(1:200), WB(1:1000) |
IF: Immunofluorescence labeling, WB: Western-blots, EC: Embryo culture
Transfection of embryos with siRNA
Five microliters of Lipofectamine 2000 (Invitrogen) and 20 pmole of scrambled RNA (4390843; Ambion, Carlsbad, CA, USA), siRNAfgf1, siRNAfgf2, siRNAfgf4, siRNAfgfr1, siRNAfgfr2, siRNAfgfr3 or siRNAfgfr4 were diluted separately in 250 μl of serum-free Opti-MEM (Invitrogen) and incubated at room temperature for 5 min. The specific siRNA duplexes were synthesized by Sangon (Shanghai, China). The siRNA sequences are listed in Table 3. The diluted scrambled RNA/siRNA pools were then gently mixed with the diluted Lipofectamine 2000 reagent and incubated at room temperature for 20 min. The embryos were washed in HTF-HEPES medium 3 times and transferred to HTF-HEPES drops containing 1 M mannitol (M9647; Sigma) under light paraffin oil. After the blastocysts were dehydrated, each embryo was injected with 200 fmol of RNA solution into the cavity gap between the zona pellucida and the trophectoderm using the micromanipulation system (Olympus Corporation, Tokyo, Japan) (Fig. 1A). After injection, the embryos were transferred to HTF (Irvine Scientific, Santa Ana, CA, USA) medium and allowed to recover in 5% CO2 and at 37°C. Eight hours after injection, the expression of the FGFs and FGFRs was evaluated by quantitative real-time PCR. The embryos were imaged 24 h after RNA injection to assess blastocyst expansion.
Table 3.
Nucleotide sequences of siRNA used for knockdown
| Target RNA(mouse) | Guide (5′–3′) | Passenger (5′–3′) |
|---|---|---|
| FGF1 | AAGUGUUAUAAUGGUUUUCUU | GAAAACCAUUAUAACACUUAC |
| FGF2 | UCGUUCAAAGAAGAAACACUC | GUGUUUCUUCUUUGAACGACU |
| FGF4 | UUUACACUCGUCGGUAAAGAA | CUUUACCGACGAGUGUAAAUU |
| FGFR1 | AAAAACCACUAUGAUACACGG | GUGUAUCAUAGUGGUUUUUUU |
| FGFR2 | UUAUGUCAAGUUAGGAAACAA | GUUUCCUAACUUGACAUAAAA |
| FGFR3 | AUUCUUUGCCAUUCUUCAGCC | CUGAAGAAUGGCAAAGAAUUC |
| FGFR4 | ACAUACAGGAUGAUAUCUGUG | CAGAUAUCAUCCUGUAUGUAU |
Isolation of TEs from transfected embryos
The TEs were isolated from blastocysts (110-113 h after hCG injection; 24 h after siRNA transfection) as previously described with modifications.43,46 After removal of the zona pellucida with Tyrode's solution, the ICMs were shelled out mechanically with a thinly pulled and polished micropipette.43 The freshly isolated TEs were intensively washed in phosphate-buffered saline (PBS), frozen in liquid nitrogen and stored at −80°C for quantitative real-time PCR analysis.
Quantitative real-time PCR
Total RNA extraction and reverse transcription (RT) from 100 pools of TE were performed using the Cells-to-cDNA™ II Kit according to the manufacturer's instructions (Ambion, Austin, TX, USA). Quantitative real-time PCR was performed with the ABI Prism 7900HT detection system (Applied Biosystems, Carlsbad, CA, USA). The specific primers were provided by Sangon. The full list of primer sequences for quantitative real-time PCR (mouse E-cadherin, ZO-1, ENaCα, Na-K ATPase, AQP3, AQP9 and GAPDH) is given in Table 4. Three to 6 replicates were performed.
Table 4.
Nucleotide sequences of primers used for quantitative real-time PCR (SYBR Green)
| Target RNA(mouse) | Forward (5′–3′) | Reverse (5′–3′) | Product size (bp) | Accession No. |
|---|---|---|---|---|
| ENaCα | AACTCCTGGTGACCCTGACAA | GAAGGGACAGGAAATGATGGG | 131 | NM_011324.2 |
| Na-K ATPase | CTCGGCAAGGACAGAACAAAA | AGGCTTTCCCAGAGAAGGTG | 119 | NM_144900.2 |
| ZO-1 | GTTCCGGGGAAGTTACGTGC | AAGTGGGACAAAAGTCCGGG | 187 | NM_001163574.1 |
| E-cadherin | ATGTGGAGGGTCCTGACTCG | CCTCCGGATCCCAACTTTCTT | 101 | NM_009864.2 |
| AQP3 | TAACCCTTGCCTTACTGGGC | AACGGTCTCCCTTCACTCCT | 164 | NM_016689.2 |
| AQP9 | CATTACCGTCCTGGACTTCCC | TGCCCAAGTGCCATGTTCTC | 120 | NM_001271843.1 |
| GAPDH | CCCCAGCAAGGACACTGAGCAAGAG | GCCCCTCCTGTTATTATGGGGGTC | 107 | NM_008084.2 |
Immunofluorescence labeling and confocal microscopy
The embryos were fixed in 4% paraformaldehyde for 1 h and then permeabilized with 0.1% Triton X-100 in PBS for 30 min. After incubation with 5% goat serum and rabbit serum for 1 h to block nonspecific antigens, the embryos were grouped and incubated with the primary antibodies at 4°C overnight. Then, the embryos were washed with 0.1% Triton X-100 in PBS (PBST) and were incubated with the secondary antibodies for 30 min, followed by nuclear counterstaining with 4′, 6-diamidino-2-phenylindole (DAPI, 1:1000; Sigma) for 20 min. Antibodies used in the present study are listed in supplementary material Table 1 (primary antibodies) and Table 2 (secondary antibodies). For the negative controls, we incubated the embryos in PBS without the addition of the primary antibody to determine the levels of non-specific fluorescence. Finally, the fluorescent images were analyzed using a Zeiss LSM 510 META laser scanning confocal microscope (Carl Zeiss, Thornwood, NY, USA).
Table 2.
Secondary antibodies
| Host species | Against | Conjugated to | Catalog | Company | Application |
|---|---|---|---|---|---|
| Goat | rabbit IgG | Alexa Fluor 488 | A31627 | Invitrogen (Carlsbad, CA, USA) | IF (1:500) |
| Rabbit | goat IgG | Alexa Fluor 594 | A11080 | Invitrogen (Carlsbad, CA, USA) | IF (1:500) |
| Rabbit | mouse IgG | Alexa Fluor 594 | A11005 | Invitrogen (Carlsbad, CA, USA) | IF (1:500) |
| Goat | Mouse IgG | Alexa Fluor®790 | A11375 | Invitrogen (Carlsbad, CA, USA) | WB (1:2000) |
| Goat | Rabbit IgG | Alexa Fluor®790 | A11367 | Invitrogen (Carlsbad, CA, USA) | WB (1:2000) |
| Donkey | Goat IgG | Alexa Fluor®790 | A11370 | Invitrogen (Carlsbad, CA, USA) | WB (1:2000) |
IF: Immunofluorescence labeling, WB: Western-blots
Western blot analysis
After isolation of TEs from blastocysts (200 embryos/group) were pooled, lysed by loading buffer. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane. After blocking with 4% milk, the blots were probed overnight at 4 °C with the primary antibodies (Table 1). The signal was detected with HRP-conjugated secondary antibody (Table 2) and visualized using ECL Western Blot Detection Reagent (GE Healthcare).
Microinjection of FGF antibodies
The blastocoel of the blastocyst was injected with 100 ng of FGF1 polyclonal antibody, FGF2 polyclonal antibody or FGF4 polyclonal antibody using the micromanipulation system (Fig 1B). The information of the antibodies used is given in Table 1. The negative control was injected with the same volume of saline, which substituted for the antibodies. After the injection, the embryos were cultured in HTF (Irvine Scientific) medium in 5% CO2 at 37°C. The expansion rate of the blastocyst was assessed 24 h after the injection.
Statistical analysis
Each experiment was repeated at least 3 times. The data are presented as the means ± standard error (SEM). Student's unpaired t-test was used for comparisons between 2 groups. One-way analysis of variance (ANOVA) was used for comparisons among 3 or more groups. A probability of P < 0.05 was considered statistically significant.
Funding
This work was supported by the National Natural Science Foundation of China 81370684 (to Y.C.L.), 81500648 (to J.Y.) and 81471421 (to D.Z.) and by National Basic Research Program of China 2012CB944900 (to H.F.H.)
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lu YC, Chen H, Fok KL, Tsang LL, Yu MK, Zhang XH, Chen J, Jiang X, Chung YW, Ma AC, et al.. CFTR mediates bicarbonate-dependent activation of miR-125b in preimplantation embryo development. Cell Res 2012; 22:1453-66; PMID:22664907; http://dx.doi.org/ 10.1038/cr.2012.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest 2010; 120:995-1003; PMID:20364097; http://dx.doi.org/ 10.1172/JCI41229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 2009; 136:701-13; PMID:19201946; http://dx.doi.org/ 10.1242/dev.017178 [DOI] [PubMed] [Google Scholar]
- 4.Bell CE, Watson AJ. p38 MAPK regulates cavitation and tight junction function in the mouse blastocyst. PLoS One 2013; 8:59528; http://dx.doi.org/ 10.1371/journal.pone.0059528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niessen CM. Tight junctions/adherens junctions: Basic structure and function. J Invest Dermatol 2007; 127:2525-32; PMID:17934504; http://dx.doi.org/ 10.1038/sj.jid.5700865 [DOI] [PubMed] [Google Scholar]
- 6.Giannatselis H, Calder M, Watson AJ. Ouabain stimulates a Na+/K+-ATPase-mediated SFK-activated signalling pathway that regulates tight junction function in the mouse blastocyst. PLoS One 2011; 6:23704; http://dx.doi.org/ 10.1371/journal.pone.0023704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madan P, Rose K, Watson AJ. Na/K-ATPase beta1 subunit expression is required for blastocyst formation and normal assembly of trophectoderm tight junction-associated proteins. J Biol Chem 2007; 282:12127-34; PMID:17317668; http://dx.doi.org/ 10.1074/jbc.M700696200 [DOI] [PubMed] [Google Scholar]
- 8.Barcroft LC, Offenberg H, Thomsen P, Watson AJ. Aquaporin proteins in murine trophectoderm mediate transepithelial water movements during cavitation. Dev Biol 2003; 256:342-54; PMID:12679107; http://dx.doi.org/ 10.1016/S0012-1606(02)00127-6 [DOI] [PubMed] [Google Scholar]
- 9.Lu YC, Yang J, Ding GL, Shi S, Zhang D, Jin L, Pan JX, Lin XH, Zhu YM, Sheng JZ, et al.. Small conductance calcium-activated potassium channel 3 (SK3) is a modulator of endometrial remodeling during endometrial growth. J Clin Endocrinol Metab 2014; 99:3800-10; PMID:24978672; http://dx.doi.org/ 10.1210/jc.2013-3389 [DOI] [PubMed] [Google Scholar]
- 10.Lu YC, Ding GL, Yang J, Zhang YL, Shi S, Zhang RJ, Zhang D, Pan JX, Sheng JZ, Huang HF. Small-conductance calcium-activated K(+) channels 3 (SK3) regulate blastocyst hatching by control of intracellular calcium concentration. Hum Reprod 2012; 27:1421-30; PMID:22416006; http://dx.doi.org/ 10.1093/humrep/des060 [DOI] [PubMed] [Google Scholar]
- 11.Seshagiri PB, Roy SS, Sireesha G, Rao RP. Cellular and molecular regulation of mammalian blastocyst hatching. J Reprod Immunol 2009; 83:79-84; PMID:19879652; http://dx.doi.org/ 10.1016/j.jri.2009.06.264 [DOI] [PubMed] [Google Scholar]
- 12.Kawamura K, Chen Y, Shu Y, Cheng Y, Qiao J, Behr B, Pera RA, Hsueh AJ. Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors. PLoS One 2012; 7:49328; http://dx.doi.org/ 10.1371/journal.pone.0049328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Klein C, Liang C, Rauch R, Kawamura K, Hsueh AJ. Autocrine regulation of early embryonic development by the artemin-GFRA3 (GDNF family receptor-α 3) signaling system in mice. FEBS Lett 2009; 583:2479-85; PMID:19580811; http://dx.doi.org/ 10.1016/j.febslet.2009.06.050 [DOI] [PubMed] [Google Scholar]
- 14.Richter KS. The importance of growth factors for preimplantation embryo development and in-vitro culture. Curr Opin Obstet Gynecol 2008; 20:292-304; PMID:18460945; http://dx.doi.org/ 10.1097/GCO.0b013e3282fe743b [DOI] [PubMed] [Google Scholar]
- 15.Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Nat Aca Sci USA 1990; 87:4756-60; http://dx.doi.org/ 10.1073/pnas.87.12.4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev 2005; 26:63-77; PMID:15689573; http://dx.doi.org/ 10.1210/er.2003-0040 [DOI] [PubMed] [Google Scholar]
- 17.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocrine-Related Cancer 2000; 7:165-97; PMID:11021964; http://dx.doi.org/ 10.1677/erc.0.0070165 [DOI] [PubMed] [Google Scholar]
- 18.Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development 2010; 137:3351-60; PMID:20876656; http://dx.doi.org/ 10.1242/dev.050146 [DOI] [PubMed] [Google Scholar]
- 19.Lee SE, Jeong SI, Yang H, Park CS, Jin YH, Park YS. Fisetin induces Nrf2-mediated HO-1 expression through PKC-δ and p38 in human umbilical vein endothelial cells. J Cell Biochem 2011; 112:2352-60; PMID:21520244; http://dx.doi.org/ 10.1002/jcb.23158 [DOI] [PubMed] [Google Scholar]
- 20.Rappolee DA, Basilico C, Patel Y, Werb Z. Expression and function of FGF-4 in peri-implantation development in mouse embryos. Development 1994; 120:2259-69; PMID:7925026 [DOI] [PubMed] [Google Scholar]
- 21.Ocon-Grove OM, Cooke FN, Alvarez IM, Johnson SE, Ott TL, Ealy AD. Ovine endometrial expression of fibroblast growth factor (FGF) 2 and conceptus expression of FGF receptors during early pregnancy. Domest Anim Endocrinol 2008; 34:135-45; PMID:17223006; http://dx.doi.org/ 10.1016/j.domaniend.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 22.Michael DD, Alvarez IM, Ocon OM, Powell AM, Talbot NC, Johnson SE, Ealy AD. Fibroblast growth factor-2 is expressed by the bovine uterus and stimulates interferon-tau production in bovine trophectoderm. Endocrinology 2006; 147:3571-79; PMID:16574787; http://dx.doi.org/ 10.1210/en.2006-0234 [DOI] [PubMed] [Google Scholar]
- 23.Fields SD, Hansen PJ, Ealy AD. Fibroblast growth factor requirements for in vitro development of bovine embryos. Theriogenology 2011; 75:1466-75; PMID:21295834; http://dx.doi.org/ 10.1016/j.theriogenology.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 24.Neira JA, Tainturier D, Pena MA, Martal J. Effect of the association of IGF-I, IGF-II, bFGF, TGF-β1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology 2010; 73:595-604; PMID:20035987; http://dx.doi.org/ 10.1016/j.theriogenology.2009.10.015 [DOI] [PubMed] [Google Scholar]
- 25.Larson RC, Ignotz GG, Currie WB. Transforming growth factor β and basic fibroblast growth factor synergistically promote early bovine embryo development during the fourth cell cycle. Mol Reprod Dev 1992; 33:432-35; PMID:1472373; http://dx.doi.org/ 10.1002/mrd.1080330409 [DOI] [PubMed] [Google Scholar]
- 26.Mignatti P, Morimoto T, Rifkin DB. Basic fibroblast growth factor released by single, isolated cells stimulates their migration in an autocrine manner. Proc Natl Acad Sci USA 1991; 88:11007-11; PMID:1763016; http://dx.doi.org/ 10.1073/pnas.88.24.11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 1991; 64:841-48; PMID:1847668; http://dx.doi.org/ 10.1016/0092-8674(91)90512-W [DOI] [PubMed] [Google Scholar]
- 28.Eckert JJ, McCallum A, Mears A, Rumsby MG, Cameron IT, Fleming TP. Specific PKC isoforms regulate blastocoel formation during mouse preimplantation development. Dev Biol 2004; 274:384-401; PMID:15385166; http://dx.doi.org/ 10.1016/j.ydbio.2004.07.027 [DOI] [PubMed] [Google Scholar]
- 29.Heo JS, Han HJ. PKC and MAPKs pathways mediate EGF-induced stimulation of 2-deoxyglucose uptake in mouse embryonic stem cells. Cell Physiol Biochem 2006; 17:145-58; PMID:16543731; http://dx.doi.org/ 10.1159/000092076 [DOI] [PubMed] [Google Scholar]
- 30.Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Protein kinase C α requirement in the activation of p38 mitogen-activated protein kinase, which is linked to the induction of tumor necrosis factor α in lipopolysaccharide-stimulated microglia. Neurochem Int 2004; 44:205-14; PMID:14602083; http://dx.doi.org/ 10.1016/S0197-0186(03)00163-3 [DOI] [PubMed] [Google Scholar]
- 31.Ozawa M, Yang QE, Ealy AD. The expression of fibroblast growth factor receptors during early bovine conceptus development and pharmacological analysis of their actions on trophoblast growth in vitro. Reproduction 2013; 145:191-201; PMID:23241344; http://dx.doi.org/ 10.1530/REP-12-0220 [DOI] [PubMed] [Google Scholar]
- 32.Rappolee DA, Patel Y, Jacobson K. Expression of fibroblast growth factor receptors in peri-implantation mouse embryos. Mol Reprod Dev 1998; 51:254-64; PMID:9771645; http://dx.doi.org/ 10.1002/(SICI)1098-2795(199811)51:3%3c254::AID-MRD4%3e3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- 33.Morris SA, Graham SJ, Jedrusik A Zernicka-Goetz M. The differential response to Fgf signalling in cells internalized at different times influences lineage segregation in preimplantation mouse embryos. Open Biol 2013; 3:130104; PMID:24258274; http://dx.doi.org/ 10.1098/rsob.130104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang QE, Fields SD, Zhang K, Ozawa M, Johnson SE, Ealy AD. Fibroblast growth factor 2 promotes primitive endoderm development in bovine blastocyst outgrowths. Biol Reprod 2011; 85:946-53; PMID:21778141; http://dx.doi.org/ 10.1095/biolreprod.111.093203 [DOI] [PubMed] [Google Scholar]
- 35.Chai N, Patel Y, Jacobson K, McMahon J, McMahon A, Rappolee DA. FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Dev Biol 1998; 198:105-15; PMID:9640334; http://dx.doi.org/ 10.1006/dbio.1997.8858 [DOI] [PubMed] [Google Scholar]
- 36.Jeanny JC, Fayein N, Moenner M, Chevallier B, Barritault D, Courtois Y. Specific fixation of bovine brain and retinal acidic and basic fibroblast growth factors to mouse embryonic eye basement membranes. Exp Cell Res 1987; 171:63-75; PMID:2442016; http://dx.doi.org/ 10.1016/0014-4827(87)90251-5 [DOI] [PubMed] [Google Scholar]
- 37.Bell CE, Lariviere NM, Watson PH, Watson AJ. Mitogen-activated protein kinase (MAPK) pathways mediate embryonic responses to culture medium osmolarity by regulating aquaporin 3 and 9 expression and localization, as well as embryonic apoptosis. Hum Reprod 2009; 24:1373-86; PMID:19258345; http://dx.doi.org/ 10.1093/humrep/dep010 [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Gye MC, Kim MK. Role of occludin, a tight junction protein, in blastocoel formation, and in the paracellular permeability and differentiation of trophectoderm in preimplantation mouse embryos. Mol Cells 2004; 17:248-54; PMID:15179038 [PubMed] [Google Scholar]
- 39.Baltz JM, Tartia AP. Cell volume regulation in oocytes and early embryos: Connecting physiology to successful culture media. Hum Reprod Update 2010; 16:166; PMID:19825850; http://dx.doi.org/ 10.1093/humupd/dmp045 [DOI] [PubMed] [Google Scholar]
- 40.Wang YB, Leroy V, Maunsbach AB, Doucet A, Hasler U, Dizin E, Ernandez T, de Seigneux S, Martin PY, Féraille E. Sodium transport is modulated by p38 kinase-dependent cross-talk between ENaC and Na,K-ATPase in collecting duct principal cells. J Am Soc Nephrol 2014; 25:250-9; PMID:24179170; http://dx.doi.org/ 10.1681/ASN.2013040429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lonic A, Powell JA, Kong Y, Thomas D, Holien JK, Truong N, Parker MW, Guthridge MA. Phosphorylation of serine 779 in fibroblast growth factor receptor 1 and 2 by protein kinase C(epsilon) regulates Ras/mitogen-activated protein kinase signaling and neuronal differentiation. J Biol Chem 2013; 288:14874-85; PMID:23564461; http://dx.doi.org/ 10.1074/jbc.M112.421669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miraoui H, Oudina K, Petite H, Tanimoto Y, Moriyama K, Marie PJ. Fibroblast growth factor receptor 2 promotes osteogenic differentiation in mesenchymal cells via ERK1/2 and protein kinase C signaling. J Biol Chem 2009; 284:4897-904; PMID:19117954; http://dx.doi.org/ 10.1074/jbc.M805432200 [DOI] [PubMed] [Google Scholar]
- 43.Eckert JJ, McCallum A, Mears A, Rumsby MG, Cameron IT, Fleming TP. PKC signalling regulates tight junction membrane assembly in the pre-implantation mouse embryo. Reproduction 2004; 127:653-67; PMID:15175502; http://dx.doi.org/ 10.1530/rep.1.00150 [DOI] [PubMed] [Google Scholar]
- 44.Chakraborti S, Roy S, Chowdhury A, Mandal A, Chakraborti T. Role of PKCα-p38 MAPK-Giα axis in peroxynitrite-mediated inhibition of β-adrenergic response in pulmonary artery smooth muscle cells. Cell Signal 2013; 25:512-26; PMID:23159577; http://dx.doi.org/ 10.1016/j.cellsig.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 45.Wrann CD, Winter SW, Barkhausen T, Hildebrand F, Krettek C, Riedemann NC. Distinct involvement of p38-, ERK1/2 and PKC signaling pathways in C5a-mediated priming of oxidative burst in phagocytic cells. Cell Immunol 2007; 245:63-9; PMID:17507002; http://dx.doi.org/ 10.1016/j.cellimm.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 46.Sheth B, Fesenko I, Collins JE, Moran B, Wild AE, Anderson JM, Fleming TP. Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1α+. Development 1997; 124:2027-37; PMID:9169849 [DOI] [PubMed] [Google Scholar]