Abstract
Upon wounding, multiple stem cell populations in the hair follicle (HF) and interfollicular epidermis (IFE) converge at the site of injury. Although these cells can contribute permanently to the regenerating epithelium, it remains unclear whether these contributions vary among cells originating from diverse compartments in the skin. By comparing the fates of several keratinocyte lineages, we observed here an initial decrease in both HF- and IFE-derived cells within the transient acanthotic layers of the regenerating epithelium. At the same time, the relative abundance of early-arriving IFE-derived cells specifically in the wound basal layer declined as later-arriving HF-derived cells entered the site of injury. Although laggard bulge-derived cells were typically constrained at the regenerative periphery, these cells persisted in the wound basal layer. Finally, suppressing Notch enabled IFE-derived cells to out-compete HF-derived cells. Taken together, these findings indicate that IFE-, HF- and bulge-derived cells make distinct contributions to regeneration over time. Furthermore, we speculate that extrinsic, non-genetic factors such as spatial constraint, distance from the wound, and basal versus suprabasal position may largely determine whether a cell ultimately persists.
Keywords: hair follicles, skin, stem cells, wound healing
Introduction
External wounds compromise the epithelial barrier that ordinarily protects us from our environment. Upon barrier breach, the most immediate challenges facing the body include sealing the wound, staunching blood loss and preventing infection. This is accomplished by an assortment of cells including platelets, neutrophils, macrophages and other immune cells that are rapidly recruited to sites of injury.1,2
After hemostasis and scab formation, keratinocytes originating from the HF and IFE proliferate and enter the wound to regenerate damaged skin in a process known as re-epithelialization. These cells alter their keratin expression profiles, increase their metabolic activities, and collectively form a thickened epithelial front that traverses a bed of provisional matrix beneath the scab.3,4 Numerous paracrine interactions between keratinocytes, fibroblasts and immune cells promote re-epithelialization, while intercellular signals such as FGF, HGF, TGF-β and EGF family members likely play key modulatory roles.5,6 After the wound has resolved, most of the suprabasal acanthotic layers of the regenerated epithelium are lost as the skin gradually reverts to a more normal, IFE-like phenotype.
Notably, wounding perturbs the compartmentalized organization of different skin stem cell populations.7,8 Consequently, stem cells that do not ordinarily encounter one another during homeostasis, such as those of the HF bulge and the basal layer of the IFE, are suddenly brought into contact when they converge at the wounded area.8 Although multiple reports have documented the overall persistence of different HF stem cell-derived sub-populations following wound healing,9-16 few have quantitated the specific ability of these cells to populate the permanent, basal layer of the regenerating epithelium over time. This layer is likely to be significant, since IFE stem cells are found exclusively in the basal layer, whereas their differentiated progeny stratify into the overlying suprabasal layers of the skin and are eventually sloughed off.17
While it is clear that multiple epithelial stem cell sub-populations can enter the wound, a more general question remains: What is the overall HF and IFE contribution to wound healing over time? Although recent reports have suggested that most HF-derived cells are eventually lost and replaced by IFE-derived cells,18 we initiated this study to better understand the kinetics underlying these population shifts. To do so, we have performed genetic fate mapping studies to document the dynamic rise and fall of HF- and IFE-derived cells in the wound over time. From these data, we speculate that these changes can largely be explained by extrinsic, non-genetic factors such as spatial constraint, distance from the wound, and basal vs. suprabasal position.
Results
Both HF bulge- and IFE-derived cells are lost from the acanthotic wound epithelium
To examine the contributions of HF bulge- and IFE-derived keratinocytes to wound healing, we re-analyzed different mouse strains that have previously been reported to express Cre recombinase in discrete compartments in the skin.19-21 These animals were coupled with a Cre-inducible ROSA26R promoter-driven YFP reporter allele, enabling us to genetically label different cell lineages prior to injury. After creating a 0.25 cm2 full thickness dorsal wound in 7.5 week old telogen skin, we quantitated the number of YFP+ cells in the wound up to 50 days post-injury. This regenerated epithelium was devoid of neogenic HFs, which arise only in younger mice inflicted with larger injuries.22
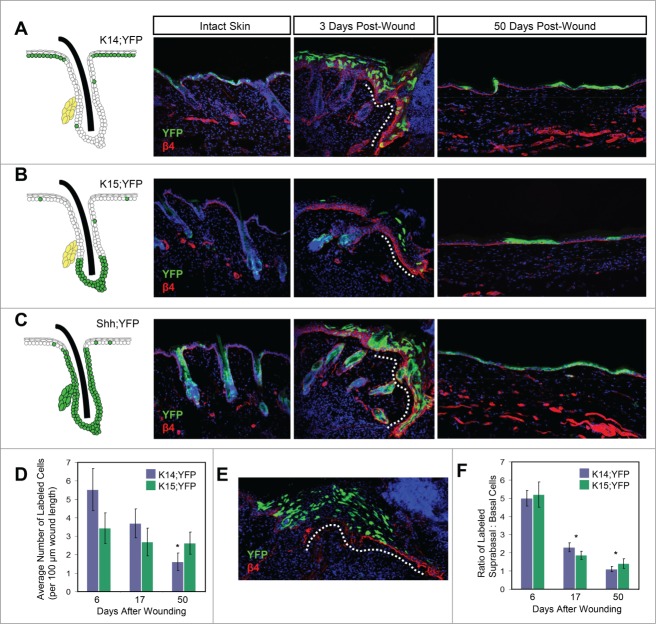
To assess the contribution of IFE-derived keratinocytes, we performed lineage tracing studies using mice expressing an inducible Keratin 14 (K14) promoter-driven CreERT allele coupled with the YFP reporter (K14;YFP mice).21 We and others have previously shown that upon tamoxifen treatment, these mice display recombination primarily in the IFE23,24 (Fig. 1A). We also utilized mice harboring an inducible Keratin 15 (K15) promoter-driven CrePR1 allele along with the YFP reporter (K15;YFP mice), which enables labeling of HF bulge stem cells20 (Fig. 1B). Previous studies have indicated that K15+ bulge-derived cells contribute initially to the wound epithelium, but do not persist long-term.9
Figure 1.
Wound healing contributions by different cell lineages. (A–C) Left, schematic of recombination patterns in intact skin of K14;YFP (A), K15;YFP (B), and Shh;YFP (C) mice. Photos depict YFP-labeled cells (green) in intact skin, in early wound margins 3 days after injury, and in regenerated skin 50 days after injury, as labeled. Basal layer cells express β4 integrin (β4, red). Dotted lines, early wound margin. The wound edge is to the right side in the 3 day images. (D) Graph showing average number of labeled cells (both basal and suprabasal) per 100 µm wound length for K14;YFP and K15;YFP mice. The overall decline in K15;YFP animals is modest due to a concomitant increase in labeled basal layer cells over time. *, p < 0.05 for 50 day wound versus either 6 or 17 day wounds in K14;YFP mice. (E) Representative image showing bulge-derived cells in the thickened wound epithelium (dotted line), 6 days after injury, in a K15;YFP mouse. Note the abundance of suprabasal cells. (F) Ratio of labeled suprabasal:basal cells at different times after wounding in K14;YFP and K15;YFP mice. *, p < 0.01 for 17 or 50 day wounds vs. 6 day wounds.
Upon quantitating the total number of YFP+ cells in the wound, we observed in K15;YFP mice a modest decline in labeled cells between 6-50 days after injury (Fig. 1D). We also observed a decrease in total labeled cells in the wounds of K14;YFP mice over time (Fig. 1D). This decline was likely due to the fact that the early regenerating epithelium appears acanthotic and possesses multiple layers of suprabasal cells that are subsequently lost as the wound heals. Indeed, we noticed that soon after injury, the majority of labeled cells from both K15;YFP and K14;YFP animals were found in the suprabasal layers of the thickened regenerative epithelium, and that the ratio of labeled suprabasal:basal cells in the wound decreased significantly between 6-17 days after wounding (Fig. 1E–F). These findings indicate that both HF- and IFE-derived cells are removed from the wound over time, and that this occurs independently of lineage, likely due to the general loss of suprabasal cells from the thickened wound epithelium.
The relative abundance of HF-derived cells increases, while IFE-derived cells decreases, in the wound basal layer
Since the indiscriminate loss of suprabasal cells from the early wound margin may obscure the actual regenerative capabilities of different keratinocyte lineages, we focused all our subsequent analyses specifically on cells located in the wound basal layer. For these studies, we again utilized K14;YFP and K15;YFP mice, in addition to animals expressing a Sonic hedgehog (Shh) promoter-driven Cre recombinase along with the YFP reporter allele (Shh;YFP mice).19 Since epidermal progenitors expressing Shh at embryonic day 14.5 give rise to HFs, but not IFE, these mice can be used to assess the contribution of labeled HF-derived cells to the wound (Fig. 1C).
Previous studies have noted that the proportion of HF-derived cells within the wound basal layer increases between 9-16 weeks after injury.14 We observed a similar increase; however, by examining earlier time-points, we also noticed that the gain in HF-derived cells displayed a biphasic pattern, with a sharp increase in basal layer cells soon after injury, followed by a more gradual long-term gain (Fig. 2A–B). This increase was unlikely to be due to cells which upregulated Shh and acquired reporter expression subsequent to wounding, since Hedgehog signaling is not known to play a role in IFE homeostasis or regeneration.
Figure 2.
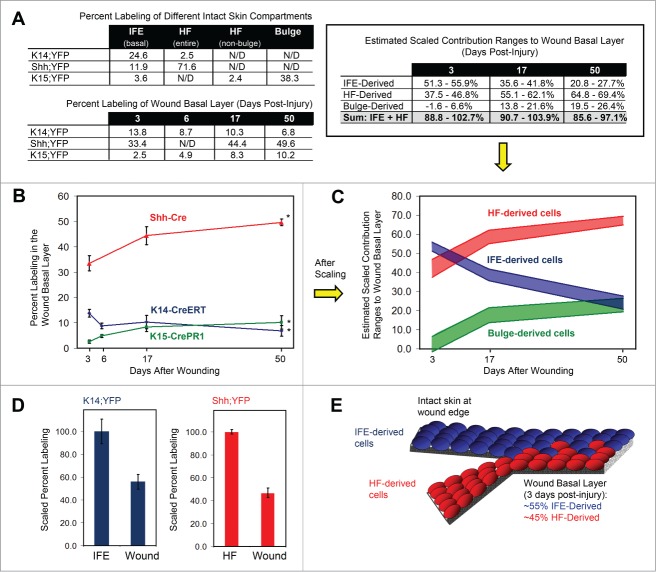
Wound healing contributions by different skin compartments. (A) Top left, observed percent labeling in different intact skin compartments, by mouse strain. Bottom left, observed percent labeling of wound basal layer at different times after injury. Top right, estimated scaled contribution ranges to wound basal layer, by compartment. N/D, not determined. (B) Graph showing percent labeled cells in the wound basal layer at different times after injury. Red line, Shh;YFP. Blue line, K14;YFP. Green line, K15;YFP. (*), p < 0.05 for comparisons between 3 and 50 day wounds within each lineage. (C) Scaled contribution ranges of different skin compartments to the regenerating wound basal layer over time. Each colored bar delineates the maximum and minimum estimated scaled contributions for each compartment. The actual contribution of each compartment at each timepoint lies somewhere between these maximum and minimum estimated values (see Supplementary Methods). Red, HF. Blue, IFE. Green, bulge. (D) Left, percent labeling in the wound basal layer in K14;YFP mice, upon scaling labeling in intact IFE basal layer to 100%. Right, percent labeling in the wound in Shh;YFP mice, upon scaling labeling in intact HFs to 100%. In both cases, there is a roughly 2-fold dilution of labeled cells in the wound versus the intact compartment. (E) Depiction of IFE (blue)- and HF (red)-derived cells converging in the wound and mutually diluting one other by roughly 2-fold.
In contrast to the gain in HF-derived cells, we observed that the percentage of labeled IFE-derived cells in the regenerating basal layer decreased sharply between 3-6 days post-injury, with a more gradual decline at later time-points (Fig. 2A-B). Indeed, this biphasic decline in labeled cells in K14;YFP mice coincided with the biphasic gain of labeled cells in wounds from Shh;YFP animals. To determine whether the arrival of bulge-derived cells into the wound might account for some of these changes, we quantitated the abundance of labeled cells in the regenerating epithelium in wounded K15;YFP mice. Contrary to previous reports, the proportion of YFP+ cells derived from the bulge actually increased in the wound over time when only basal layer cells were considered (Fig. 2A–B). Furthermore, a greater increase in bulge-derived cells was observed during earlier time-points after wounding, suggesting that the appearance of these cells may at least partially account for the initial overall gain in HF-derived cells in the wound. Together, these findings suggest that IFE-derived cells in the wound basal layer are either diluted or lost as HF-derived cells, including bulge-derived cells, appear in the wound during the early stages of regeneration.
The HF and IFE make nearly equivalent early contributions to regeneration
One of the initial goals of this study was to determine the relative overall contributions of the IFE, HF and bulge compartments to the wound. Thus far, we have quantitated the relative abundance of labeled cells derived from K14+ progenitors in the IFE, Shh+ progenitors in the HF, and K15+ progenitors in the bulge. The abundance of these labeled cells, however, under-estimates the actual cellular contributions made by each compartment, since none of these Cre strains displays complete labeling in either the IFE, HF or bulge. In addition, these strains do not exhibit labeling confined solely to one compartment.
With these considerations in mind, we attempted to estimate the overall relative contributions of the HF and IFE to the wound by first determining how efficiently the different Cre recombinases induced labeling in their respective compartments in intact skin near the start of the experiment. In K14;YFP mice, for instance, we determined that 24.6% of basal cells in the intact IFE were YFP+, and that 2.5% of HF cells were also labeled (Fig. 2A). In Shh;YFP animals, 71.6% of HF cells were YFP+, although labeling was also observed in 11.9% of basal IFE cells (Fig. 2A).
To generate a rough estimate of the overall IFE contribution to the wound basal layer, we scaled the IFE labeling efficiency in K14;YFP mice to 100%. We also scaled the observed percentage of labeled cells in the wound by the same factor (see Supplemental Materials and Methods). This estimate is predicated on the important assumption that there does not exist a functionally distinct sub-population of unlabeled cells in the IFE of K14;YFP mice that contributes disproportionately to the wound. It is important to note that this scaling approach does not alter the overall shape of the curves derived from simply counting labeled cells (compare Fig. 2B and 2C). Rather, this approach attempts to normalize the data to reflect the initial labeling efficiencies seen in each compartment, such that more direct comparisons between IFE- and HF-derived cells can be made.
We therefore estimated that 3 days after injury, had 100% of basal IFE cells been labeled in K14;YFP animals, about 56% of the basal layer cells within the regenerating wound margin would also have been YFP+ (Fig. 2A). These data suggest that IFE-derived cells are diluted approximately 2-fold early on by non-IFE-derived cells at the site of injury (Fig. 2D). We visually confirmed this dilution effect by examining whole-mounts from mice expressing K14-CreERT coupled with a ROSA26R promoter-driven β-Galactosidase reporter allele (K14;LacZ). As shown in Fig. 3A, the amount of labeled cells within the regenerated epidermis is diluted relative to intact adjacent epidermis from the same animal.
Figure 3.
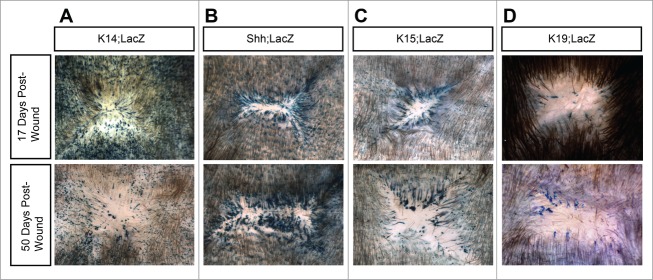
Persistence of labeled cells in regenerated skin. (A–D) β-gal whole-mounts of re-epithelialized wounds, 17 days (top) or 50 days (bottom) after injury. Results from additional mice for each strain and timepoint are shown in Figure S1.
This 2-fold dilution of IFE-derived cells suggests that cells from the HF may account for the remaining cells in the wound, and that HF cells, in turn, are also initially diluted about 2-fold by IFE-derived cells. To test this prediction, we scaled the HF labeling efficiency in Shh;YFP mice to 100%. We next scaled the observed percent labeling in the wound by the same factor, yielding a rough estimate that, 3 days after wounding, approximately 47% of regenerating basal cells are HF-derived (Fig. 2A). This result is consistent with our prediction, and suggests that 3 days after injury, IFE- and HF-derived cells mutually dilute one another and make nearly equivalent contributions to the regenerating basal epithelium (Fig. 2D–E).
As mentioned above, cell labeling in K14;YFP and Shh;YFP mice was not absolutely confined to either the IFE or HF compartment, respectively. While this precluded us from determining the exact wound healing contributions made by either the IFE or HF, we were able instead to calculate a range of possible contributions for each compartment by subtracting the estimated scaled contributions of labeled HF cells in K14;YFP mice and labeled IFE cells in Shh;YFP mice (see Supplemental Materials and Methods). From this we deduced that, 3 days after wounding, the IFE contributes between 51-56%, and the HF contributes between 37-47%, of cells to the regenerating basal epithelium (Fig. 2A, 2C). It is important to emphasize that, although labeling in both mouse strains was not exclusively confined to one compartment, the estimated range of cellular contributions to the wound is relatively narrow even when compensating for all potential contributions made by other labeled cell lineages. Thus, our findings suggest that while both the IFE and HF initially make early sizeable contributions to the wound basal layer, a slight plurality of cells is likely IFE-derived.
HF and IFE contributions vary in the wound basal layer over time
As noted above, upon extending our analyses beyond 3 days after wounding, the percentage of labeled HF-derived cells increased in the regenerating basal epithelium, whereas the percentage of IFE-derived cells decreased. While IFE-derived cells comprised a slight majority in the early wound basal layer, by 50 days after injury, we estimated that only about 21-28% of cells originated from the IFE, using data from K14;YFP mice. By contrast, based on our analyses of Shh;YFP animals, we estimated that about 65-69% of cells now originated from HFs (Fig. 2A, 2C).
Altogether, these observations suggest that IFE-derived cells become diluted or lost as HF-derived cells appear in the wound early on, and that IFE-derived cells may be at a slight long-term disadvantage relative to HF-derived cells. Notably, it is important to mention that, regardless of how the proportion of different cell lineages shifted, the estimated HF and IFE wound contributions calculated independently from separate mouse strains totaled to approximately 90-100% for all 3 time points after injury (estimates from Shh;YFP + K14;YFP mice, or HF + IFE = ∼89–103% at 3 days after wounding; ∼91–104% at 17 days; and ∼86–97% at 50 days) (Fig. 2A). While it is important to keep in mind the assumptions that were incorporated into our analyses, these results suggest that our model can account for the vast majority of cells in the wound at all times.
Bulge-derived cells make permanent, but delayed, contributions to the wound basal layer
To estimate the overall contribution of the bulge compartment to the wound basal layer, we performed similar analyses as above using K15;YFP mice and determined that 38.3% of bulge cells were labeled in intact skin near the start of the experiment (Fig. 2A). In addition, about 2.4% of non-bulge HF cells and 3.6% of IFE cells were also YFP+. We next scaled the percentage of labeled bulge cells to 100%, and scaled the amount of labeled cells in the wound by the same factor. After subtracting the scaled contributions of labeled non-bulge-derived cells in these animals, we estimated that 3 days after injury, almost no YFP+ cells in the wound basal layer originated from the bulge (Supplemental Materials and Methods) (Fig. 2A).
Between 3-6 days after injury, the percentage of labeled basal layer cells in the wound nearly doubled in K15;YFP mice, and during the 6-50 day time-frame, the abundance of these cells continued to increase (Fig. 2B). After performing similar calculations as above, we determined that only after 3 days post-injury did bulge-derived cells contribute meaningfully to the wound basal layer (Fig. 2A). By 50 days after wounding, we estimated that bulge-derived cells constituted 19.5-26.4% of all basal cells in the regenerated epithelium. Surprisingly, these calculations suggest that the proportion of bulge-derived cells in the wound at this point may be nearly equivalent to that of all cells derived from the entire IFE (Fig. 2C).
To confirm the persistence of bulge-derived cells in regenerated skin, we performed whole-mount analyses using mice expressing K15-CrePR1 and the LacZ reporter allele (K15;LacZ mice). Although whole-mount analysis does not distinguish between labeled basal and suprabasal cells in the skin, the overall abundance of labeled cells visible in later-stage, homeostatic regenerated skin (after 17 days post-injury) is largely correlated with the abundance of labeled cells in the basal layer (Fig. 1F). Similar to previous studies,9 we observed labeled cells streaming radially toward the center of the wound (Fig. 3C). Concordant with our findings using the YFP reporter allele, we also noted that these streams persisted at least 50 days after wounding. Moreover, we observed that the majority of labeled cells were confined to the periphery of the regenerated epithelium, with fewer labeled cells in the middle of the wound. By contrast, labeled cells in both K14;LacZ and Shh;LacZ mice were readily observed near the wound center (Fig. 3A–B). These findings are consistent with the interpretation that bulge-derived cells typically enter the wound subsequent to other lineages in the skin. As a result, these cells are largely restricted to the wound periphery.
Since our findings in K15;YFP mice are complicated by the fact that non-bulge cells are occasionally labeled, we sought to further confirm our results using mice expressing an inducible Keratin 19 promoter-driven Cre recombinase (K19-CreERT2).25 When coupled with the LacZ reporter allele, K19;LacZ mice displayed fewer labeled bulge cells compared to K15-CrePR1 mice (Figure S2); however, labeling was more specific to the bulge than in K15-CrePR1 animals, since labeled non-bulge cells were rarely observed in K19;LacZ mice. When we wounded these animals, labeled cells were also retained in regenerated skin up to 50 days after injury (Fig. 3D). Thus, our results using both K15-CrePR1 and K19-CreERT2 mice and 2 different reporter alleles suggest that bulge-derived cells which specifically enter the wound basal layer can make permanent, albeit delayed, contributions to the wound epithelium.
Notch inhibition enables IFE-derived cells to out-compete HF-derived cells
Given that the proportion of IFE-derived cells declines both initially, possibly due to an influx of HF-derived cells into the wound, and also later perhaps due to a slight competitive disadvantage relative to HF-derived cells, we wondered whether we could genetically alter the relative contributions of these 2 lineages to healing skin. In both the IFE and HF, Notch signaling is critical for promoting cell differentiation.26 In the presence of ligand, the Notch receptor is cleaved, releasing an intracellular domain that complexes with nuclear co-factors including CSL/RBP-Jk and a Mastermind-like (MAML) coactivator to induce target genes. Expression of a GFP-tagged, dominant negative form of MAML (dnMAML) inhibits Notch signaling and can cause epidermal hyperplasia and cyst formation.27
To determine whether suppressing Notch can reverse the loss of IFE-derived cells in the wound, we generated mice expressing K14-CreERT and a ROSA26R promoter-driven inducible allele of dnMAML-GFP (K14;dnMAML mice).28 After inducing expression of dnMAML primarily in the IFE, we wounded these animals and assessed the proportion of GFP+ cells at the site of injury over time (Fig. 4A). Although expression of dnMAML did not affect the overall rate of wound closure (data not shown), GFP+ IFE-derived cells quickly out-competed other cells in the wound area. Strikingly, within 17 days after injury, nearly the entire regenerated epithelium was labeled, and these cells were maintained up to 50 days after wounding (Fig. 4A–C and data not shown). The proportion of GFP+ cells within intact skin also increased steadily over time, but not as quickly as within the wound (Fig. 4C). By contrast, non-GFP-expressing cells, including most HF-derived cells, were completely eliminated.
Figure 4.
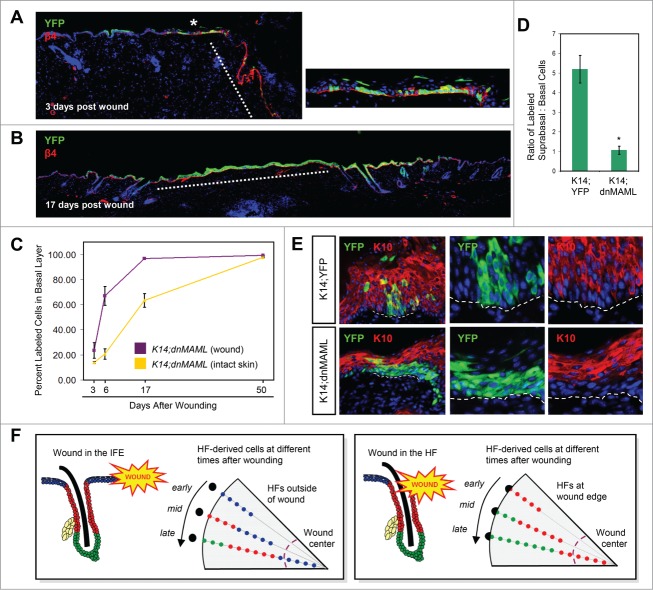
Notch inhibition promotes cell persistence in the wound. (A) Labeled cells (green) in K14;dnMAML mice, 3 days after injury. Red, β4+ basal layer. Dotted line, wound margin. Right, magnified view of (*), showing early expansion of labeled cells at the wound periphery, where extensive cell proliferation is typically observed. (B) Complete replacement of unlabeled cells in the wound (dotted line) by those expressing dnMAML (green), 17 days after injury. (C) Percentage of basal layer cells that express dnMAML inside the wound (purple) and in intact IFE (gold), over time. (D) Ratio of labeled suprabasal:basal cells, 6 days after wounding, in control K14;YFP and K14;dnMAML mice. (*), p = 0.002. For K14;YFP, the same data are shown in Figure 1F. (E) Co-localization of K10 (red) with dnMAML or control labeled cells (green), 6 days after wounding. (F) Left, model showing that in wounds which slice across the IFE (blue), IFE-derived cells closest to the injury enter the wound first (early), followed by more distal HF-derived cells (red), and finally by bulge-derived cells (green) (late). Since bulge-derived cells enter the wound last, these cells are confined to the wound periphery, whereas IFE-derived cells reach the center. Right, in instances where the wound shears the top of a HF, HF-derived infundibular or isthmus cells enter the wound first, followed by bulge-derived cells. Here, bulge-derived cells can migrate closer to the wound center. Lineage-specific differences in cell proliferation (not illustrated) might also affect the appearance of cells in the wound. Black circles represent hair follicle openings and gray areas represent the wound area (overhead view). Purple dotted lines are zones where HF neogenesis occur near the wound center, largely inaccessible to bulge-derived cells.
We next examined the location of labeled K14;dnMAML keratinocytes that had initially moved into the site of injury, compared with control labeled K14;YFP cells. Six days after wounding, we observed that GFP+ cells from K14;dnMAML mice displayed a strong bias toward occupying the basal layer of the wound, whereas YFP+ cells from control animals were more frequently observed suprabasally, similar to results recently reported in esophageal epithelium29 (Fig. 4D). Consistent with a role for Notch in promoting differentiation, we also observed that dnMAML-expressing cells were less likely than control cells to express the early stratification marker Keratin 10 (K10) (Fig. 4E). Although this may be partially attributable to the strong tendency of these cells to remain within the basal layer, we also frequently did not detect K10 expression in dnMAML-expressing cells positioned within the second and third suprabasal layers (Fig. 4E), suggesting that Notch promotes cell differentiation during wound healing. Together, these findings indicate that suppressing Notch signaling can provide a competitive advantage that enables IFE-derived cells to overcome factors that limit their persistence during regeneration.
Discussion
HF- and IFE-derived cells contribute to wound healing in both humans and mice.30 In superficial wounds, cells originating from the tops of remnant HFs and eccrine sweat ducts form islands of regenerated epithelia that expand and converge over time.31 In deeper wounds, re-epithelialization typically occurs from the periphery and is mediated by the recruitment of cells from wound-adjacent IFE, HFs and glands.32 Consistent with a role for HFs in regeneration, the hair cycle can affect the rate of wound closure in mice,33 while in Edaradd mutant mice lacking primary HFs, wound healing in tail skin is delayed.34 In spite of these and other findings, however, it has recently been proposed that IFE-derived cells, but not HF-derived cells, are the major long-term contributors to wound healing.18
While previous studies have focused on whether keratinocyte stem cell sub-populations differ in their innate ability to persist in the wound, the potential influence of extrinsic, non-genetic factors affecting wound healing contributions have been largely overlooked. In this study, we speculate that the population shifts observed during regeneration can be largely explained by several such factors, including basal versus suprabasal cell position, spatial constraint and distance from the wound.
During the early phases of regeneration, the wound epithelium becomes thickened due to an influx of cells as well as increased proliferation. As regeneration proceeds, the suprabasal layers of this epithelium are lost as the skin reverts to a more normal IFE-like phenotype. Consistent with this, we observed early overall decreases in both bulge- and IFE-derived cells from the suprabasal layers of the wound. Similar early declines have also been noted in tail skin, suggesting that this effect is not site- or lineage-specific.10 Indeed, once we focused our analyses on the wound basal layer, we observed that HF-derived cells, including bulge-derived cells, actually increased in the wound over time. Altogether, these findings suggest that cellular position within the wound epithelium—basal vs. suprabasal—may affect whether a cell persists.
As wounds healed, we also observed shifts in the proportion of IFE-, HF- and bulge-derived cells in the regenerative basal layer. These shifts largely occurred soon after wounding, suggesting that the arrival of HF-derived cells diluted IFE-derived cells already in the wound, or caused them to be lost. We cannot rule out the possibility, however, that these early changes might also be due to intrinsic differences in the ability of these cells to undergo proliferation, especially at the wound margin,4,35 which we also quantitated here (Figure S3). Nonetheless, the reciprocal population changes observed over time suggest that spatial constraint in the wound may be a critical extrinsic factor: As the number of HF-derived cells, including bulge-derived cells, increases in the wound, the proportion of IFE-derived cells exhibits a compensatory decline until a homeostatic balance is reached. This balance, nonetheless, can be over-ridden by conferring a competitive advantage, such as Notch suppression, to one cell population.
Interestingly, both we and Ito et al., noted an initial delay in the appearance of bulge-derived cells in the wound margin.9 This observation might be explained if cells proliferate and file sequentially into the wound by order of proximity to the site of injury: In other words, cells closest to the wound enter first, followed by cells located farther from the site of trauma (Fig. 4F). In this scenario, the most proximal cells, typically located in the IFE or HF infundibulum, are expected to reach the center of the wound soonest, relegating later-arriving cells to the margins. Only in rare situations when the wound specifically slices through the top of a HF, do bulge-derived cells gain more direct access to the site of injury (Fig. 4F). Consistent with these findings, previous studies have indicated that mobilized bulge cells can be retained in the HF infundibulum,15,36 and that HF isthmus-derived cells appear to migrate farther into the wound than do lower bulge-derived cells.10 It is important to note that these observations might also be explained if bulge-derived cells exhibit innate differences in proliferation or migration rates that delay their entry into the site of injury. Nonetheless, the relative absence of bulge-derived cells in the middle of the wound might provide an extrinsic explanation for why these cells reportedly do not contribute to HF neogenesis, which occurs only at the wound center.22,37
Over the course of these studies, we also derived rough estimates in an attempt to answer the question we had originally posed: What is the overall HF and IFE contribution to wound healing? By our calculations, the answer is complicated and varies by time. Three days after injury, we estimated that both HF- and IFE-derived cells make nearly equal contributions to the regenerating wound margin. By 50 days after wounding, however, about 65-70% of cells within the regenerated epithelium originated from the HF. Interestingly, we estimated that bulge-derived cells by themselves comprised approximately 25% of all basal cells in the healed area—roughly equivalent to the entire proportion of IFE-derived cells. These calculations further suggest that specifically among HF-derived cells within the wound, roughly 1 in every 2.7 cells is derived from the bulge.
It is important to caution that these estimates were made on the assumption that the Cre recombinases used in this study labeled functionally representative cell populations within their respective compartments. If a distinct sub-population of cells, for instance in the IFE, exhibits enhanced persistence in the wound and is also disproportionately labeled in K14;YFP mice, however, this would skew our results. Indeed, the existence of both fast- and slow-cycling sub-populations in tail epidermis has recently been reported,38 although whether these different sub-populations exist in dorsal skin remains controversial.39 At the same time, other studies have argued that the epidermis is maintained by only one type of progenitor cell that stochastically undergoes either symmetric or asymmetric divisions.40,41 In the HF, molecularly distinct cell sub-populations clearly exist, but whether these lineages differ in their functional ability to contribute to regeneration has remained unclear. While bulge-derived cells have been reported to be lost from the wound over time, again, this may be due to a perceived overall loss of suprabasal cells from the thickened wound epithelium.
A prevailing view of regeneration is that upon entering the site of injury, all cells assimilate an IFE-like phenotype.9 But are these cells truly equivalent? It is interesting to note that, after the initial population shifts that occurred soon after wounding, we observed that HF-derived cells do appear to possess a slight long-term competitive advantage over IFE-derived cells. These findings suggest that even after assimilating an “IFE-like” phenotype, HF-derived cells may still differ subtly from true IFE-derived cells, perhaps in the degree of Notch activation. Further studies, potentially incorporating lineage tracing and live cell imaging,42 will be required to “flesh out” these and other interesting questions regarding wounding and cell fate.
Materials and Methods
Mice
The following strains were used: Tg(KRT14-cre/ERT)20Efu (K14-CreERT)21; Tg(Krt-15-cre/PGR)22Cot (K15-CrePR1)20; Shhtm1(EGFP/cre)Cjt (Shh-Cre)43; Krt19tm1(cre/ERT)Ggu (K19-CreERT)25; Gt(ROSA)26Sortm1(EYFP)Cos44; Gt(ROSA)26Sortm1Sor45; and ROSA26A-dnMAML1-GFP (dnMAML).28 All animals contained single copies of the transgene and reporter alleles.
Cre induction
K14-CreERT and K19-CreERT mice were induced by injection of tamoxifen (Sigma-Aldrich) diluted in corn oil. K14-CreERT mice were injected at 7.5 weeks of age with 1 mg of tamoxifen per 40 g of mouse weight for 3 days. K19-CreERT mice were injected at 4 weeks of age with 5 mg of tamoxifen per 40 g of mouse weight every other day for 4 total injections. K15-CrePR1 mice were induced at 7.5 weeks of age by topical RU486 (Sigma-Aldrich), as previously described.23
Mouse manipulations
For K14-CreERT and K15-CrePR1 mice, full thickness wounds were created 3 days after the final application of drug, as previously described.23 K19-CreERT and Shh-Cre animals were wounded at 7.5-8.0 weeks of age. Biopsies spanning the wound site as well as matched contralateral intact skin were collected between 3-50 days after wounding. All studies were performed in accordance with regulations established by the University of Michigan Unit for Laboratory Animal Medicine.
Histology, immunohistochemistry and quantitation
Descriptions of histological methods, antibodies, and detailed wound quantitation procedures for all data presented in Figs. 1–2 can be found in Supplemental Materials and Methods.
Statistics
An unpaired Student's t-Test was used to assess significance. All error bars shown are SE. Calculations were performed at http://www.physics.csbsju.edu/stats/Index.html.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the Wong and Dlugosz labs at the University of Michigan for sharing reagents and insights.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
SYW acknowledges the support of the NIH/NIAMS (R00AR059796, R01AR065409); the University of Michigan Department of Dermatology; the Biological Sciences Scholars Program; the Center for Organogenesis; and the UM Comprehensive Cancer Center. JFR acknowledges the support of the NIH (R01AR054396, R01GM095941); the Packard Foundation; and the Program for Breakthrough Biomedical Research.
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008; 453:314-21; PMID:18480812; http://dx.doi.org/ 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- 2.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development 2004; 131:3021-34; PMID:15197160; http://dx.doi.org/ 10.1242/dev.01253 [DOI] [PubMed] [Google Scholar]
- 3.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol 2003; 37:219-30; http://dx.doi.org/ 10.1046/j.1523-1747.2003.12387.x [DOI] [PubMed] [Google Scholar]
- 4.Safferling K, Sütterlin T, Westphal K, Ernst C, Breuhahn K, James M, Jäger D, Halama N, Grabe N. Wound healing revised: a novel reepithelialization mechanism revealed by in vitro and in silico models. J Cell Biol 2013; 203:691-709; PMID:24385489; http://dx.doi.org/ 10.1083/jcb.201212020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schafer M, Werner S. Transcriptional control of wound repair. Annu Rev Cell Dev Biol 2007; 23:69-92; PMID:17474876; http://dx.doi.org/ 10.1146/annurev.cellbio.23.090506.123609 [DOI] [PubMed] [Google Scholar]
- 6.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 2007; 127:998-1008; PMID:17435785; http://dx.doi.org/ 10.1038/sj.jid.5700786 [DOI] [PubMed] [Google Scholar]
- 7.Schepeler T, Page ME, Jensen KB. Heterogeneity and plasticity of epidermal stem cells. Development 2014; 141:2559-67; PMID:24961797; http://dx.doi.org/ 10.1242/dev.104588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaks V, Kasper M, Toftgard R. The hair follicle - a stem cell zoo. Exp Cell Res 2010; 316:1422-8; PMID:20338163; http://dx.doi.org/ 10.1016/j.yexcr.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005; 11:1351-4; PMID:16288281; http://dx.doi.org/ 10.1038/nm1328 [DOI] [PubMed] [Google Scholar]
- 10.Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 2013; 13:1-12; PMID:23827700; http://dx.doi.org/ 10.1016/j.stem.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al.. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010; 327:1385-9; PMID:20223988; http://dx.doi.org/ 10.1126/science.1184733 [DOI] [PubMed] [Google Scholar]
- 12.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011; 8:552-65; PMID:21549329; http://dx.doi.org/ 10.1016/j.stem.2011.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 2008; 3:33-43; PMID:18593557; http://dx.doi.org/ 10.1016/j.stem.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J 2007; 21:1358-66; PMID:17255473; http://dx.doi.org/ 10.1096/fj.06-6926com [DOI] [PubMed] [Google Scholar]
- 15.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000; 102:451-61; PMID:10966107; http://dx.doi.org/ 10.1016/S0092-8674(00)00050-7 [DOI] [PubMed] [Google Scholar]
- 16.Howard JM, Nuguid JM, Ngole D, Nguyen H. Tcf3 expression marks both stem and progenitor cells in multiple epithelia. Development 2014; 141:3143-52; PMID:25038042; http://dx.doi.org/ 10.1242/dev.106989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doupe DP, Jones PH. Cycling progenitors maintain epithelia while diverse cell types contribute to repair. Bioessays 2013; 35:443-51; PMID:23463676; http://dx.doi.org/ 10.1002/bies.201200166 [DOI] [PubMed] [Google Scholar]
- 18.Blanpain C, Fuchs E. Plasticity of epithelial stem cells in tissue regeneration. Science 2014; 344:1242281; PMID:24926024; http://dx.doi.org/ 10.1126/science.1242281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 2005; 9:855-61; PMID:16326396; http://dx.doi.org/ 10.1016/j.devcel.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 20.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 2004; 22:411-7; http://dx.doi.org/ 10.1038/nbt950 [DOI] [PubMed] [Google Scholar]
- 21.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA 1999; 96:8551-6; PMID:10411913; http://dx.doi.org/ 10.1073/pnas.96.15.8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007; 447:316-20; PMID:17507982; http://dx.doi.org/ 10.1038/nature05766 [DOI] [PubMed] [Google Scholar]
- 23.Wong SY, Reiter JF. Wounding mobilizes hair follicular stem cells to form tumors. Proc Natl Acad Sci USA 2011; 108:4093-8; PMID:21321207; http://dx.doi.org/ 10.1073/pnas.1013098108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 2009; 5:1-12; PMID:19570504; http://dx.doi.org/ 10.1016/j.stem.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis 2008; 46:318-23; PMID:18543299; http://dx.doi.org/ 10.1002/dvg.20397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watt FM, Estrach S, Ambler CA. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol 2008; 20:171-9; PMID:18342499; http://dx.doi.org/ 10.1016/j.ceb.2008.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proweller A, Tu L, Lepore JJ, Cheng L, Lu MM, Seykora J, Millar SE, Pear WS, Parmacek MS. Impaired Notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res 2006; 66:7438-44; PMID:16885339; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-0793 [DOI] [PubMed] [Google Scholar]
- 28.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, Pear WS. Notch signaling is an important regulator of type 2 immunity. J Exp Med 2005; 202:1037-42; PMID:16230473; http://dx.doi.org/ 10.1084/jem.20050923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alcolea MP, Greulich P, Wabik A, Frede J, Simons BD, Jones PH. Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat Cell Biol 2014; 16:615-22; PMID:24814514; http://dx.doi.org/ 10.1038/ncb2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito M, Cotsarelis G. Is the hair follicle necessary for normal wound healing? J Invest Dermatol 2008; 128:1059-61; PMID:18408743; http://dx.doi.org/ 10.1038/jid.2008.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rittié L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol 2013; 182:163-71; http://dx.doi.org/ 10.1016/j.ajpath.2012.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop GH. Regeneration after experimental removal of skin in man. Am J Anat 1945; 76:153-81; http://dx.doi.org/ 10.1002/aja.1000760202 [DOI] [Google Scholar]
- 33.Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the “hair growth-wound healing connection:” anagen phase promotes wound re-epithelialization. J Invest Dermatol 2011; 131:518-28; PMID:20927125; http://dx.doi.org/ 10.1038/jid.2010.291 [DOI] [PubMed] [Google Scholar]
- 34.Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol 2008; 128:1311-8; PMID:18037901; http://dx.doi.org/ 10.1038/sj.jid.5701178 [DOI] [PubMed] [Google Scholar]
- 35.Matoltsy AG, Viziam CB. Further observations on epithelialization of small wounds: an autoradiographic study of incorporation and distribution of 3H-thymidine in the epithelium covering skin wounds. J Invest Dermatol 1970; 55:20-5; PMID:5425061; http://dx.doi.org/ 10.1111/1523-1747.ep12290488 [DOI] [PubMed] [Google Scholar]
- 36.Veniaminova NA, Vagnozzi AN, Kopinke D, Do TT, Murtaugh LC, Maillard I, Dlugosz AA, Reiter JF, Wong SY. Keratin 79 identifies a novel population of migratory epithelial cells that initiates hair canal morphogenesis and regeneration. Development 2013; 140:4870-80; PMID:24198274; http://dx.doi.org/ 10.1242/dev.101725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol 2012; 23:946-53; PMID:23085626; http://dx.doi.org/ 10.1016/j.semcdb.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mascré G, Dekoninck S, Drogat B, Youssef KK, Broheé S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012; 489:257-62; http://dx.doi.org/ 10.1038/nature11393 [DOI] [PubMed] [Google Scholar]
- 39.Gomez C, Chua W, Miremadi A, Quist S, Headon DJ, Watt FM. The Interfollicular Epidermis of Adult Mouse Tail Comprises Two Distinct Cell Lineages that Are Differentially Regulated by Wnt, Edaradd, and Lrig1. Stem Cell Reports 2013; 1:19-27; PMID:24052938; http://dx.doi.org/ 10.1016/j.stemcr.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature 2007; 446:185-9; PMID:17330052; http://dx.doi.org/ 10.1038/nature05574 [DOI] [PubMed] [Google Scholar]
- 41.Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 2013; 342:1226-30; PMID:24311688; http://dx.doi.org/ 10.1126/science.1239730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 2013; 502:513-8; PMID:24097351; http://dx.doi.org/ 10.1038/nature12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 2004; 118:517-28; PMID:15315763; http://dx.doi.org/ 10.1016/j.cell.2004.07.024 [DOI] [PubMed] [Google Scholar]
- 44.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001; 1:4; PMID:11299042; http://dx.doi.org/ 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999; 21:70-1; PMID:9916792; http://dx.doi.org/ 10.1038/5007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.