The centrosome is the major microtubule (MT)-organizing center (MTOC) in animal cells. It comprises a pair of centrioles and the surrounding pericentriolar materials (PCM), and is duplicated once per cell cycle in a highly spatiotemporally regulated manner. Because of the intricate links between centrosomal functions, mitotic spindle assembly and cell division, defects in the centrosome structure and function often lead to malformation of mitotic spindles and genomic instabilities, which result in a range of human diseases including tumorigenesis, ciliopathy, microcephaly, and dwarfism.1 Previous studies indicated that the proximal end of the mother centriole plays instructive roles in the biogenesis of the PCM and the procentriole and that the PCM is essential for centriole stability. However, the molecules and the mechanism that control centriole-PCM interaction are poorly understood. In our recent study, published in the current issue of Cell,2 we provided evidence showing that the activating transcription factor 5 (ATF5) acts unexpectedly as an essential structural PCM protein that bridges the PCM and the proximal end of the mother centriole. By interacting with both pericentrin (PCNT) in the PCM and the polyglutamylated tubulin (PGT) at the proximal end of the mother centriole, ATF5 controls the formation of the PCM and the integrity of the centrioles in a cell cycle- and centriole age-dependent manner.(Fig. 1)
Figure 1.
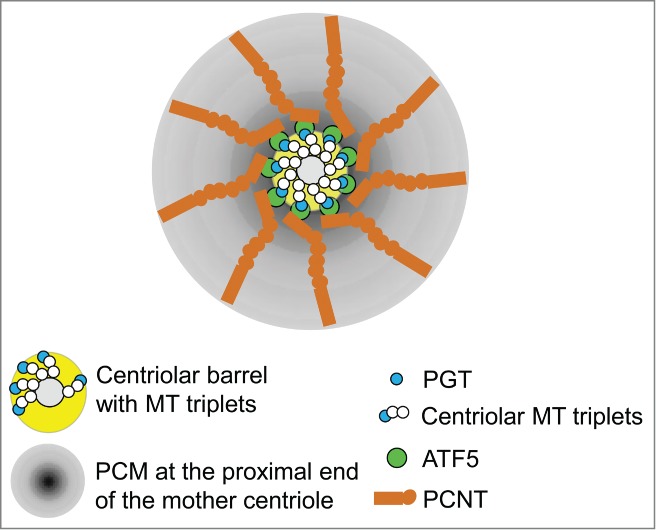
Simultaneous interactions between ATF5 and PCNT and between ATF5 and PGT promote PCM accumulation at the proximal end of the mother centriole.
ATF5 is a member of the ATF/CREB family of transcription factors and has been found to regulate a number of genes that control cell proliferation and survival.3,4 However, we showed in the new study that ATF5 interacts with multiple centrosomal proteins and is highly enriched at the centrosome (and the midbody) in a cell cycle-dependent manner, that ATF5 forms a characteristic 9-fold symmetrical ring structure in the inner layer of the PCM outfitting the proximal end of the mother centriole, that ATF5 binds to the mother centriole via PGT whose enrichment at the centriole is known to be a part of centrosome maturation, and that ATF5 interacts with PCNT in the PCM that secures PCM attachment to the mother centriole. Functionally, ATF5 depletion blocks PCM accumulation at the centrosome, induces fragmentation of centrioles, and causes the formation of multi-polar mitotic spindles and genomic instability. These results significantly extended previous findings that PGT.5 and PCNT.6 are important for holding the centrosome together and delineated a plausible mechanism by which PGT-ATF5-PCNT tripartite acts as the linchpin between the mother centriole and the PCM and is essential for maintaining the integrity of the centrioles.
Our study also revealed the mechanism of the protein-protein interactions within the PGT-ATF5-PCNT tripartite. We showed that the interaction between ATF5 and PGT is precipitated by the electrostatic interaction between the DNA binding domain (DBD) of ATF5, which is stacked with positively charged amino acids, and the heavily negatively charged PGT. This finding identified an essential function for a previously puzzling observation that increased tubulin polyglutamylation takes place at the proximal end the mother centriole during centrosome maturation process. The discovery also explained the intriguing property of the proximal end of the mother centriole as the initiation site for the PCM, solving a long-standing question in centrosome biology. The notion that an electrostatic force to be essential for holding centriole-PCM together is highly consistent with another previous discovery that the PCM can be stripped from the centrosome by high salt.7 On the other hand, the ATF5 DBD and its downstream basic leucine zipper (bZIP) region, which together form an extended α-helix coil, interact with PCNT by coiled-coil protein-protein interaction. The ability of ATF5 to participate in coiled-coil protein-protein interactions will likely make ATF5 a suitable partner for additional cytoskeleton-related coiled-coil proteins. Simultaneous interaction between ATF5 and PGT and between ATF5 and PCNT, i.e., PGT and PCNT sandwich ATF5 in a PGT-ATF5-PCNT tripartite, can be achieved as long as the electrostatic interaction between the positively charged ATF5 DBD α-helix and PGT and the coiled-coil protein-protein interaction between the ATF5 DBD and PCNT do not interfere with each other.
Notably, ATF5 over-expression elicits a completely different set of defects in the centrosome and the MT cytoskeleton, including gross exo-centrosomal aggregation of γ-tubulin in the nucleus and severe deformation of the MT cytoskeleton, that are not a mirror-image of those induced by ATF5 depletion.2 It is therefore reasonable to expect that regulation of the centrosome and the cytoskeleton by ATF5 is likely more complicated than currently known. Important future research will include investigation of the roles and mechanisms of ATF5 in the regulation of the midbody and the cytokinesis, in ATF5 involvement in the maintenance and differentiation of stem cells, and in the sensing and response to cellular stress.
References
- 1.Bettencourt-Dias M, et al.. Trends Genet 2011; 27:307-15; PMID:21680046; http://dx.doi.org/ 10.1016/j.tig.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madarampalli B, et al.. Cell 2015; 162:580-92. PMID:26213385; http://dx.doi.org/ 10.1016/j.cell.2015.06.055 [DOI] [PubMed] [Google Scholar]
- 3.Liu DX, et al.. Mol Cell Biol 2011; 31:3906-16; PMID:21791614; http://dx.doi.org/ 10.1128/MCB.05887-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dluzen D, et al.. J Biol Chem 2011; 286:7705-13; PMID:21212266; http://dx.doi.org/ 10.1074/jbc.M110.207639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobinnec Y, et al.. J Cell Biol 1998; 143:1575-89; PMID:9852152; http://dx.doi.org/ 10.1083/jcb.143.6.1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmerman WC, et al.. Mol Biol Cell 2004; 15:3642-57; PMID:15146056; http://dx.doi.org/ 10.1091/mbc.E03-11-0796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stearns T, et al.. Cell 1994; 76:623-37. PMID:8124706; http://dx.doi.org/ 10.1016/0092-8674(94)90503-7 [DOI] [PubMed] [Google Scholar]


