Abstract
Equal distribution of the genetic material during cell division relies on efficient congression of chromosomes to the metaphase plate. Prior to their alignment, the Dynein motor recruited to kinetochores transports a fraction of laterally-attached chromosomes along microtubules toward the spindle poles. By doing that, Dynein not only contributes to chromosome movements, but also prevents premature stabilization of end-on kinetochore-microtubule attachments. This is achieved by 2 parallel mechanisms: 1) Dynein-mediated poleward movement of chromosomes counteracts opposite polar-ejection forces (PEFs) on chromosome arms by the microtubule plus-end-directed motors chromokinesins. Otherwise, they could stabilize erroneous syntelic kinetochore-microtubule attachments and lead to the random ejection of chromosomes away from the spindle poles; and 2) By transporting chromosomes to the spindle poles, Dynein brings the former to the zone of highest Aurora A kinase activity, further destabilizing kinetochore-microtubule attachments. Thus, Dynein plays an important role in keeping chromosome segregation error-free by preventing premature stabilization of kinetochore-microtubule attachments near the spindle poles.
Keywords: Aurora A, Dynein, error correction, kinetochore, spindle pole
Introduction
In order to maintain genome integrity chromosomes must be accurately distributed during mitosis. This is achieved after chromosome bi-orientation, in which sister kinetochores become attached to microtubules from opposite poles of the mitotic spindle and chromosomes align at the metaphase plate prior to sister chromatid separation in anaphase. Chromosome movement toward the spindle equator is initiated immediately after nuclear envelope breakdown (NEB) in prometaphase and is known as chromosome congression.1,2 Chromosome movements during congression are driven by 2 main mechanisms: microtubule polymerization/depolymerization-based motion3 and motor-dependent transport along microtubules.4-6 The latter is mainly achieved through the coordinated activities of cytoplasmic Dynein, CENP-E and chromokinesins.5
Cytoplasmic Dynein (from here on referred as Dynein) is a large protein complex (1.6 MDa) consisting of several subunits: 2 heavy chains containing ATPase motor domains, 2 intermediate chains, 2 light intermediate chains and 3 different types of light chains.7 Dynein interacts with many different proteins that regulate its activity and localization, enabling Dynein to perform its various cellular tasks.8 Dynactin, another large multi-protein complex (1 MDa) is involved in most Dynein functions, both by targeting it to specific locations and by increasing its processivity.9 Lis1, NudE and NudEL are important for Dynein function at kinetochores, nuclear and spindle positioning, as well as organelle and mRNA transport.8 Bicaudal D also plays a role in Dynein-mediated organelle transport and, together with NudE and NudEL, targets Dynein to the nuclear envelope where it contributes to the separation of spindle poles in early mitosis.10 The Rod, ZW10 and Zwilch (RZZ) complex and Spindly target Dynein specifically to kinetochores, coupling its function to kinetochore-microtubule attachments and the spindle assembly checkpoint.11 Thus, a number of different binding partners, together with a diverse distribution of specific subunits12 and their phospho-regulation,13 allow a single motor protein - Dynein - to be involved in numerous independent cellular functions, including chromosome congression.14-16
Another kinetochore-localized motor protein involved in chromosome movements is the kinesin-7 CENP-E. Although it localizes at the kinetochore fibrous corona,17,18 just like Dynein,19 CENP-E works in opposite direction, having the ability to move chromosomes toward the plus-ends of microtubules. By doing this, CENP-E directly supports the alignment of chromosomes to the metaphase plate.20-23
In an elegant, laser microsurgery-based study, it was shown that polar ejection forces (PEFs) on chromosome arms (also known as “polar winds”) actively contribute to chromosome movement away from the pole.24 Additional studies revealed that these forces are mainly generated by the microtubule plus-end-directed motor proteins chromokinesins.25-32
During the coordinated action of all these motor activities that mediate chromosome congression it is crucial to prevent and/or correct potential erroneous kinetochore-microtubule attachments. Syntelic attachments occur when both sister kinetochores become attached by microtubules originated from a single pole, while merotelic attachments arise when a single kinetochore becomes attached to both spindle poles. The best-studied error correction system is based on the action of the centromere-localized kinase Aurora B, which destabilizes kinetochore-microtubule attachments through phosphorylation of microtubule depolymerases (such as the kinesin-13 proteins MCAK and Kif2B)33-35 and Ndc80, a protein required for the stabilization of end-on kinetochore-microtubule attachments.36-38 Once chromosomes become bi-oriented and tension is applied between sister kinetochores, Ndc80 moves away from Aurora B, resulting in kinetochore-microtubule attachment stabilization.39,40
Here we elaborate on recent studies that demonstrated a role of Dynein,5 chromokinesins5,41 and Aurora A kinase5,41,42 in the prevention and correction of erroneous kinetochore-microtubule attachments during chromosome congression.
Congression of Peripheral Chromosomes Depends on Motor Proteins
Chromosomes can congress to the metaphase plate either using polymerization/depolymerization of kinetochore microtubules or motor protein-mediated transport along microtubules. However, it remained unclear why some chromosomes prefer one pathway over the other. Chromosomes that depend on motor proteins first move to the spindle pole and only after initiate alignment to the spindle equator. This initial poleward movement depends on the microtubule minus-end-directed motor Dynein at unattached kinetochores,14-16,43,44 whereas the subsequent motion toward the equator depends on the microtubule plus-end-directed motor proteins CENP-E at kinetochores20 and chromokinesins on chromosome arms.25,27,28 We sought to investigate whether the dependence on motor proteins for chromosome congression correlates with chromosome positioning at NEB. To address this, we generated a stable U2OS cell line expressing GFP-CENP-A and mCherry-α-tubulin, which allowed us to track kinetochore positions relative to the mitotic spindle using high-resolution live-cell imaging. Then we inhibited CENP-E, which resulted in Dynein-mediated accumulation of few chromosomes (∼15%) near spindle poles. By backtracking these polar chromosomes to their original positions at NEB, we found that they initially occupied a peripheral region outside the interpolar spindle ellipsoid, and were always significantly closer to one of the poles.5 In contrast, chromosomes that were already positioned in the interpolar region at NEB were easily accessible by microtubules from both spindle poles and became bi-oriented soon after NEB. Importantly, these chromosomes completed alignment in the absence of all 3 motor activities.5 Thus, we concluded thatonly peripheral chromosomes that cannot bi-orient soon after NEB require motor proteins to congress.
Dynein Prevents Premature Kinetochore-Microtubule Attachments by Counteracting Polar Ejection Forces
Dynein-dependent poleward transport of peripheral chromosomes plays an important role in preventing premature and potentially erroneous stabilization of end-on kinetochore-microtubule attachments.5 Dynein achieves this by counteracting PEFs generated mainly by the microtubule plus-end directed motor proteins chromokinesins.25-32 Co-depletion of CENP-E and Dynein in live U2OS cells stably expressing H2B-GFP and mCherry-α-tubulin to simultaneously visualize chromosomes and microtubules, respectively, led to the ejection of polar chromosomes from spindle poles and stabilization of their kinetochore-microtubule attachments.5 This was opposite from the behavior of chromosomes that remained locked at the poles and lacked stable end-on kinetochore-microtubule attachments in CENP-E inhibited cells, in which Dynein function was left intact.5,45,46 Importantly, chromosome ejection and attachment stabilization in the absence of Dynein were both repressed after simultaneous depletion of the 2 human chromokinesins (Kid and Kif4A),5 in line with a recently reported role of PEFs in the stabilization of syntelic attachments in Drosophila S2 cells.47
To dissect the functions of motor proteins at distinct chromosomal loci, as well as their respective contribution to chromosome movements, we used laser microsurgery to physically separate the chromosome arms from the kinetochore-containing chromosome body.5 To specifically investigate polar chromosomes we inhibited CENP-E prior to laser microsurgery, which locked a group of chromosomes at the spindle poles. After laser microsurgery, acentric (i.e. without kinetochore) chromosomal fragments moved in random directions (not only toward the spindle equator, but also toward the cell cortex) in a chromokinesin-dependent manner, while kinetochore-containing fragments remained stationary at the poles.5 Thus, kinetochore-mediated forces are dominant over PEFs acting on the arms of polar chromosomes.
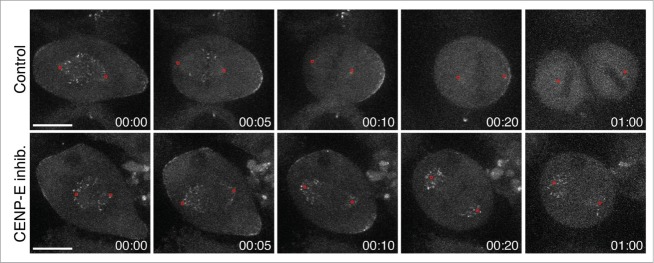
Previous electron microscopy studies have shown that polar chromosomes in CENP-E inhibited cells lacked stable end-on kinetochore-microtubule attachments.45,46 As so, we reasoned that Dynein must accumulate on these unattached kinetochores and prevent chromokinesins to move chromosomes away from the spindle poles after CENP-E inhibition. To directly test this, we used high-resolution live-cell imaging of HeLa cells stably expressing GFP-labeled Dynein Heavy Chain (DHC).48 While DHC-GFP was stripped off the kinetochores during chromosome congression in control cells, polar kinetochores from CENP-E-inhibited cells were highly enriched of DHC-GFP (Fig. 1 and Video S1). Overall, these data leads to the conclusion that Dynein-mediated poleward force is dominant over chromokinesin-generated PEFs, thereby preventing premature and erroneous stabilization of kinetochore-microtubule attachments and random ejection of chromosomes from spindle poles.
Figure 1.
Dynein “locks” peripheral chromosomes at the spindle poles after CENP-E inhibition. High-resolution live-cell imaging (eleven 1μm-separated z-planes; 10 sec time interval) of HeLa cells stably expressing DHC-GFP (kind gift from Iain Cheeseman, Whitehead Institute, MIT, USA). Images were taken with an iXonEMC electron-multiplying CCD camera (Andor Technology), using a 100x 1.4 NA Plan-Apochromatic DIC objective on an inverted microscope (TE2000U; Nikon) equipped with a CSU-X1 spinning-disc confocal head (Yokogawa Corporation of America). Note that DHC-GFP is highly expressed at unattached kinetochores from polar chromosomes after CENP-E inhibition with 20 nM GSK92329 (MedChemexpress). Red circles indicate the position of the spindle poles. Scale bar: 10 μm. Time: hrs:mins
Dynein Prevents Premature Kinetochore-Microtubule Attachments by Bringing Peripheral Chromosomes Closer to Aurora A Kinase at the Spindle Poles
To further examine the stability of kinetochore-microtubule attachments of polar chromosomes in CENP-E-inhibited cells lacking Dynein activity, we performed immunofluorescence analysis with antibodies against Mad1.5 Mad1 is a spindle assembly checkpoint protein that is removed from kinetochores upon end-on attachment to microtubules, and therefore can be used as an attachment sensor. Contrary to CENP-E-inhibited cells in which Dynein activity remains present, kinetochores from chromosomes that were ejected from spindle poles in the absence of Dynein and CENP-E inhibition showed reduced Mad1 levels, confirming the premature stabilization of kinetochore-microtubule attachments. As expected, a similar effect was observed in CENP-E-inhibited cells after co-inhibition of Aurora B kinase, which we used as a positive control. Surprisingly, Mad1 levels were also reduced on polar chromosomes after inhibition of Aurora A kinase, indicating a role of Aurora A in the spatial regulation of kinetochore-microtubule attachments near the spindle poles. Indeed, Aurora A had been previously implicated in the stabilization of kinetochore-microtubule attachments,49,50 which might be explained by the fact that Aurora A shares 70% identity of its catalytical domain with Aurora B51 and that both kinases recognize almost identical consensus target motifs.52 Thus, Aurora A and Aurora B might have overlapping functions in the correction of kinetochore-microtubule attachments that are spatially regulated by their different localization (Aurora A being placed at the centrosomes and Aurora B at the centromeres), which is defined by their different binding partners.51 Additionally, Aurora A might play other important roles in chromosome congression by regulating proteins directly involved in this process.53,54
Two recent studies further confirmed our observation about the role of Aurora A in error correction near the poles in mitosis and also in meiosis I. Ye and colleagues41 demonstrated the existence of an Aurora A activity gradient centered on the spindle poles in Drosophila S2 cells using a Fluorescence Resonance Energy Transfer sensor bound to microtubules that was sensitive to Aurora A phosphorylation. They also showed that Aurora A reduces the stability of kinetochore-microtubule attachments and that it counteracts the attachment stabilization effect of chromokinesin-generated PEFs. Finally, they confirmed the role of Aurora A in error correction also in human and PtK1 cells and defined Ser-55 of Ndc80 as an Aurora A-specific phosphorylation site at kinetochores, shedding light on the molecular mechanism of Aurora A-based error correction. Chmatal and colleagues42 investigated the role of Aurora A in error correction using oocytes from mice generated by crossing a strain containing Robertsonian chromosomes (chromosomes created by fusion of 2 telocentric chromosomes at the centromeres) with a standard laboratory strain, containing all telocentric chromosomes. This resulted in oocytes containing trivalents, which were placed off the center of the spindle equator, toward one of the spindle poles. Kinetochore-microtubule attachments on chromosomes closer to the pole were always less stable than the attachments on chromosomes near the spindle equator, unless Aurora A kinase was inhibited, demonstrating its role in attachment destabilization near the spindle poles. Moreover, they used live-cell imaging to show that Mad1-GFP signal accumulated on kinetochores near the spindle pole, both in trivalents and syntelically-attached bivalents that became efficiently corrected as they approached the spindle pole. Altogether, these studies in 4 different systems5,41,42 demonstrate that, in addition to a centromeric-based error correction mechanism mainly driven by Aurora B, an Aurora A-regulated error correction mechanism exists in the vicinity of the spindle poles to prevent the premature stabilization of end-on kinetochore microtubule attachments. This is likely to be important for subsequent CENP-E-mediated congression of polar chromosomes along pre-existing spindle microtubules.20
Fighting the “polar winds” to see the Aurora (A): an integrated model for how Dynein prevents incorrect kinetochore-microtubule attachments at the spindle poles
Here we propose an integrated mechanism for how Dynein prevents the premature stabilization of (erroneous) kinetochore-microtubule attachments near the spindle poles (Fig. 2). This is achieved by moving peripheral chromosomes toward one of the spindle poles before congression to the spindle equator. By transporting peripheral chromosomes to the vicinity of the spindle poles, Dynein counteracts the stabilizing effect of chromokinesin-mediated PEFs, while bringing these chromosomes to the zone of highest Aurora A activity, which further prevents stabilization of end-on kinetochore-microtubule attachments.
Figure 2.
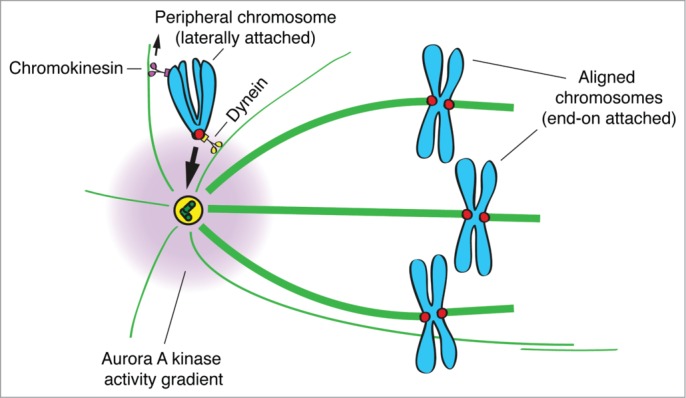
Dynein prevents premature stabilization of erroneous kinetochore-microtubule attachments by counteracting PEFs and by bringing chromosomes closer to Aurora A activity at the spindle poles. Illustrative model depicting the dominant role of Dynein in bringing peripheral chromosomes toward the center of an Aurora A activity gradient at the spindle poles, against chromokinesin-mediated PEFs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Work in the laboratory of H.M. is funded by FLAD Life Science 2020, The Louis-Jeantet YICA 2015 and PRECISE grant from the European Research Council.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Walczak CE, Cai S, Khodjakov A. Mechanisms of chromosome behaviour during mitosis. Nat Rev Mol Cell Biol 2010; 11:91-102; PMID:20068571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kops GJ, Saurin AT, Meraldi P. Finding the middle ground: how kinetochores power chromosome congression. Cell Mol Life Sci 2010; 67:2145-61; PMID:20232224; http://dx.doi.org/ 10.1007/s00018-010-0321-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchison T, Evans L, Schulze E, Kirschner M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell 1986; 45:515-27; PMID:3708686; http://dx.doi.org/ 10.1016/0092-8674(86)90283-7 [DOI] [PubMed] [Google Scholar]
- 4.McIntosh JR, Molodtsov MI, Ataullakhanov FI. Biophysics of mitosis. Q Rev Biophys 2012; 45:147-207; PMID:22321376; http://dx.doi.org/ 10.1017/S0033583512000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barisic M, Aguiar P, Geley S, Maiato H. Kinetochore motors drive congression of peripheral polar chromosomes by overcoming random arm-ejection forces. Nat Cell Biol 2014; 16:1249-56; PMID:25383660; http://dx.doi.org/ 10.1038/ncb3060 [DOI] [PubMed] [Google Scholar]
- 6.Cross RA, McAinsh A. Prime movers: the mechanochemistry of mitotic kinesins. Nat Rev Mol Cell Biol 2014; 15:257-71; PMID:24651543; http://dx.doi.org/ 10.1038/nrm3768 [DOI] [PubMed] [Google Scholar]
- 7.Allan VJ. Cytoplasmic dynein. Biochem Soc Trans 2011; 39:1169-78; PMID:21936784; http://dx.doi.org/ 10.1042/BST0391169 [DOI] [PubMed] [Google Scholar]
- 8.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol 2009; 10:854-65; PMID:19935668; http://dx.doi.org/ 10.1038/nrm2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroer TA. Dynactin. Annu Rev Cell Dev Biol 2004; 20:759-79; PMID:15473859; http://dx.doi.org/ 10.1146/annurev.cellbio.20.012103.094623 [DOI] [PubMed] [Google Scholar]
- 10.Raaijmakers JA, Medema RH. Function and regulation of dynein in mitotic chromosome segregation. Chromosoma 2014; 123:407-22; PMID:24871939; http://dx.doi.org/ 10.1007/s00412-014-0468-7 [DOI] [PubMed] [Google Scholar]
- 11.Barisic M, Geley S. Spindly switch controls anaphase: spindly and RZZ functions in chromosome attachment and mitotic checkpoint control. Cell Cycle 2011; 10:449-56; PMID:21252629; http://dx.doi.org/ 10.4161/cc.10.3.14759 [DOI] [PubMed] [Google Scholar]
- 12.Pfister KK. Distinct functional roles of cytoplasmic dynein defined by the intermediate chain isoforms. Exp Cell Res 2015; 334:54-60; PMID:25576383; http://dx.doi.org/ 10.1016/j.yexcr.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whyte J, Bader JR, Tauhata SB, Raycroft M, Hornick J, Pfister KK, Lane WS, Chan GK, Hinchcliffe EH, Vaughan PS, et al.. Phosphorylation regulates targeting of cytoplasmic dynein to kinetochores during mitosis. J Cell Biol 2008; 183:819-34; PMID:19029334; http://dx.doi.org/ 10.1083/jcb.200804114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vorozhko VV, Emanuele MJ, Kallio MJ, Stukenberg PT, Gorbsky GJ. Multiple mechanisms of chromosome movement in vertebrate cells mediated through the Ndc80 complex and dynein/dynactin. Chromosoma 2008; 117:169-79; PMID:18057949; http://dx.doi.org/ 10.1007/s00412-007-0135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Tulu US, Wadsworth P, Rieder CL. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr Biol 2007; 17:973-80; PMID:17509882; http://dx.doi.org/ 10.1016/j.cub.2007.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Yu W, Liang Y, Zhu X. Kinetochore dynein generates a poleward pulling force to facilitate congression and full chromosome alignment. Cell Res 2007; 17:701-12; PMID:17680027; http://dx.doi.org/ 10.1038/cr.2007.65 [DOI] [PubMed] [Google Scholar]
- 17.Cooke CA, Schaar B, Yen TJ, Earnshaw WC. Localization of CENP-E in the fibrous corona and outer plate of mammalian kinetochores from prometaphase through anaphase. Chromosoma 1997; 106:446-55; PMID:9391217; http://dx.doi.org/ 10.1007/s004120050266 [DOI] [PubMed] [Google Scholar]
- 18.Yao X, Anderson KL, Cleveland DW. The microtubule-dependent motor centromere-associated protein E (CENP-E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules. J Cell Biol 1997; 139:435-47; PMID:9334346; http://dx.doi.org/ 10.1083/jcb.139.2.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wordeman L, Steuer ER, Sheetz MP, Mitchison T. Chemical subdomains within the kinetochore domain of isolated CHO mitotic chromosomes. J Cell Biol 1991; 114:285-94; PMID:1830054; http://dx.doi.org/ 10.1083/jcb.114.2.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, McEwen BF, Khodjakov A. Chromosomes can congress to the metaphase plate before biorientation. Science 2006; 311:388-91; PMID:16424343; http://dx.doi.org/ 10.1126/science.1122142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaar BT, Chan GK, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol 1997; 139:1373-82; PMID:9396744; http://dx.doi.org/ 10.1083/jcb.139.6.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood KW, Sakowicz R, Goldstein LS, Cleveland DW. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 1997; 91:357-66; PMID:9363944; http://dx.doi.org/ 10.1016/S0092-8674(00)80419-5 [DOI] [PubMed] [Google Scholar]
- 23.Barisic M, Silva e Sousa R, Tripathy SK, Magiera MM, Zaytsev AV, Pereira AL, Janke C, Grishchuk EL, Maiato H. Mitosis. Microtubule detyrosination guides chromosomes during mitosis. Science 2015; 348:799-803; PMID:25908662; http://dx.doi.org/ 10.1126/science.aaa5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieder CL, Davison EA, Jensen LC, Cassimeris L, Salmon ED. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J Cell Biol 1986; 103:581-91; PMID:3733881; http://dx.doi.org/ 10.1083/jcb.103.2.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonio C, Ferby I, Wilhelm H, Jones M, Karsenti E, Nebreda AR, Vernos I. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell 2000; 102:425-35; PMID:10966105; http://dx.doi.org/ 10.1016/S0092-8674(00)00048-9 [DOI] [PubMed] [Google Scholar]
- 26.Brouhard GJ, Hunt AJ. Microtubule movements on the arms of mitotic chromosomes: polar ejection forces quantified in vitro. Proc Natl Acad Sci U S A 2005; 102:13903-8; PMID:16174726; http://dx.doi.org/ 10.1073/pnas.0506017102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funabiki H, Murray AW. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 2000; 102:411-24; PMID:10966104; http://dx.doi.org/ 10.1016/S0092-8674(00)00047-7 [DOI] [PubMed] [Google Scholar]
- 28.Wandke C, Barisic M, Sigl R, Rauch V, Wolf F, Amaro AC, Tan CH, Pereira AJ, Kutay U, Maiato H, et al.. Human chromokinesins promote chromosome congression and spindle microtubule dynamics during mitosis. J Cell Biol 2012; 198:847-63; PMID:22945934; http://dx.doi.org/ 10.1083/jcb.201110060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levesque AA, Compton DA. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J Cell Biol 2001; 154:1135-46; PMID:11564754; http://dx.doi.org/ 10.1083/jcb.200106093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stumpff J, Wagenbach M, Franck A, Asbury CL, Wordeman L. Kif18A and chromokinesins confine centromere movements via microtubule growth suppression and spatial control of kinetochore tension. Dev Cell 2012; 22:1017-29; PMID:22595673; http://dx.doi.org/ 10.1016/j.devcel.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bieling P, Kronja I, Surrey T. Microtubule motility on reconstituted meiotic chromatin. Curr Biol 2010; 20:763-9; PMID:20399095; http://dx.doi.org/ 10.1016/j.cub.2010.02.067 [DOI] [PubMed] [Google Scholar]
- 32.Mazumdar M, Sundareshan S, Misteli T. Human chromokinesin KIF4A functions in chromosome condensation and segregation. J Cell Biol 2004; 166:613-20; PMID:15326200; http://dx.doi.org/ 10.1083/jcb.200401142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell 2004; 6:253-68; PMID:14960279; http://dx.doi.org/ 10.1016/S1534-5807(04)00025-5 [DOI] [PubMed] [Google Scholar]
- 34.Bakhoum SF, Thompson SL, Manning AL, Compton DA. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat Cell Biol 2009; 11:27-35; PMID:19060894; http://dx.doi.org/ 10.1038/ncb1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol 2004; 14:273-86; PMID:14972678; http://dx.doi.org/ 10.1016/j.cub.2004.01.055 [DOI] [PubMed] [Google Scholar]
- 36.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 2006; 127:983-97; PMID:17129783; http://dx.doi.org/ 10.1016/j.cell.2006.09.039 [DOI] [PubMed] [Google Scholar]
- 37.Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, et al.. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 2008; 133:427-39; PMID:18455984; http://dx.doi.org/ 10.1016/j.cell.2008.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 2006; 127:969-82; PMID:17129782; http://dx.doi.org/ 10.1016/j.cell.2006.09.047 [DOI] [PubMed] [Google Scholar]
- 39.Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P, Brautigan DL, Stukenberg PT, Kapoor TM. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 2008; 453:1132-6; PMID:18463638; http://dx.doi.org/ 10.1038/nature06923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 2009; 323:1350-3; PMID:19150808; http://dx.doi.org/ 10.1126/science.1167000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye AA, Deretic J, Hoel CM, Hinman AW, Cimini D, Welburn JP, Maresca TJ. Aurora A Kinase Contributes to a Pole-Based Error Correction Pathway. Curr Biol 2015; 25:1842-51; PMID:26166783; http://dx.doi.org/ 10.1016/j.cub.2015.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chmatal L, Yang K, Schultz RM, Lampson MA. Spatial Regulation of Kinetochore Microtubule Attachments by Destabilization at Spindle Poles in Meiosis I. Curr Biol 2015; 25:1835-41; PMID:26166779; http://dx.doi.org/ 10.1016/j.cub.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharp DJ, Rogers GC, Scholey JM. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nat Cell Biol 2000; 2:922-30; PMID:11146657; http://dx.doi.org/ 10.1038/35046574 [DOI] [PubMed] [Google Scholar]
- 44.Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol 1990; 110:81-95; PMID:2295685; http://dx.doi.org/ 10.1083/jcb.110.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell 2001; 12:2776-89; PMID:11553716; http://dx.doi.org/ 10.1091/mbc.12.9.2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Putkey FR, Cramer T, Morphew MK, Silk AD, Johnson RS, McIntosh JR, Cleveland DW. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev Cell 2002; 3:351-65; PMID:12361599; http://dx.doi.org/ 10.1016/S1534-5807(02)00255-1 [DOI] [PubMed] [Google Scholar]
- 47.Cane S, Ye AA, Luks-Morgan SJ, Maresca TJ. Elevated polar ejection forces stabilize kinetochore-microtubule attachments. J Cell Biol 2013; 200:203-18; PMID:23337118; http://dx.doi.org/ 10.1083/jcb.201211119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiyomitsu T, Cheeseman IM. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat Cell Biol 2012; 14:311-7; PMID:22327364; http://dx.doi.org/ 10.1038/ncb2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katayama H, Sasai K, Kloc M, Brinkley BR, Sen S. Aurora kinase-A regulates kinetochore/chromatin associated microtubule assembly in human cells. Cell Cycle 2008; 7:2691-704; PMID:18773538; http://dx.doi.org/ 10.4161/cc.7.17.6460 [DOI] [PubMed] [Google Scholar]
- 50.Bakhoum SF, Kabeche L, Murnane JP, Zaki BI, Compton DA. DNA-damage response during mitosis induces whole-chromosome missegregation. Cancer Discov 2014; 4:1281-9; PMID:25107667; http://dx.doi.org/ 10.1158/2159-8290.CD-14-0403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hochegger H, Hegarat N, Pereira-Leal JB. Aurora at the pole and equator: overlapping functions of Aurora kinases in the mitotic spindle. Open biology 2013; 3:120185; PMID:23516109; http://dx.doi.org/ 10.1098/rsob.120185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR 3rd, Chan CS, Drubin DG, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 2002; 111:163-72; PMID:12408861; http://dx.doi.org/ 10.1016/S0092-8674(02)00973-X [DOI] [PubMed] [Google Scholar]
- 53.Hegarat N, Smith E, Nayak G, Takeda S, Eyers PA, Hochegger H. Aurora A and Aurora B jointly coordinate chromosome segregation and anaphase microtubule dynamics. J Cell Biol 2011; 195:1103-13; PMID:22184196; http://dx.doi.org/ 10.1083/jcb.201105058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim Y, Holland AJ, Lan W, Cleveland DW. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell 2010; 142:444-55; PMID:20691903; http://dx.doi.org/ 10.1016/j.cell.2010.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.