Abstract
c-Jun is a proto-oncoprotein that is commonly overexpressed in many types of cancer and is believed to regulate cell proliferation, the cell cycle, and apoptosis by controlling AP-1 activity. Understanding the c-Jun regulation is important to develop treatment strategy for cancer. The COP9 signalosome subunit 6 (CSN6) plays a critical role in ubiquitin-mediated protein degradation. MEKK1 is a serine/threonine kinase and E3 ligase containing PHD/RING domain involved in c-Jun ubiquitination. Here, we show that CSN6 associates with MEKK1 and reduces MEKK1 expression level by facilitating the ubiquitin-mediated degradation of MEKK1. Also we show that CSN6 overexpression diminishes MEKK1-mediated c-Jun ubiquitination, which is manifested in mitigating osmotic stress-mediated c-Jun downregulation. Thus, CSN6 is involved in positively regulating the stability of c-Jun. Overexpression of CSN6 correlates with the upregulation of c-Jun target gene expression in cancer. These findings provide new insight into CSN6-MEKK1-c-Jun axis in tumorigenesis.
Keywords: COP9 signalosome, CSN6, c-Jun, MEKK1
Introduction
c-Jun is a proto-oncoprotein that regulates cell proliferation, cell cycle, and apoptosis by controlling AP-1 activity.1 The cellular activity of the AP-1 transcriptional factor depends on dimer formation between the Jun and Fos proteins. After the homo-dimerization or hetero-dimerization of these proteins, AP-1 dimers recognize the TPA response element (TRE: TGACTCA) or cyclic AMP response element (CRE:TGACGTCA) and begin the transcription of target genes that are involved in tumorigenesis.2 c-Jun is an essential component of the AP-1 dimer; its activation enhances AP-1-mediated cyclin D but suppresses p53 expression levels to promote carcinogenesis. Moreover, c-Jun overexpression is commonly observed in many cancers, such as breast cancer,3 glioblastoma,4 and colorectal adenocarcinoma.5 Thus, the cellular level and activity of c-Jun proteins are important in cancer regulation.
The regulation of c-Jun is very complex. c-Jun protein stability is controlled by E3 ubiquitin ligase proteins, such as MEKK16,7 and FBW7,8 and its activity is regulated by Casein kinase II9 and JNK2.10,11 Among the known regulators of c-Jun, MEK kinase 1 (MEKK1) has a dual function: it either activates c-Jun NH2-terminal kinases (JNKs), which are upstream kinases of c-Jun, or it degrades and ubiquitinates the c-Jun protein through the plant homeo-domain (PHD)/RING domain.6 MEKK1 was originally identified as a serine/threonine protein kinase that activates JNK and extracellular signal-regulated protein kinase under different extracellular stimuli, such as heat shock, ultraviolet, or osmotic stress.12 However, the direct upstream regulator of MEKK1-mediated c-Jun ubiquitination and degradation is not well understood.
The COP9 signalosome (CSN) is a protein complex that is evolutionarily conserved from plants to mammals and consists of 8 subunits (CSN1-8).13 CSN is a protein complex involved in protein degradation,14-17 transcriptional activation,18,19 signal transduction,20-23 and tumorigenesis.15,22,24-26 The role of the CSN's subunits in cancer has not been well characterized. Mammalian CSN subunits engage in developmental processes: targeted disruptions of mammalian Csn2, Csn3, Csn5, and Csn8 caused defective embryo development.27-30 We previously performed targeted disruption of the Csn6 gene in mice and found that Csn6−/− mice developed until 7.5 days post-coitus but not beyond this time.15 Also, Csn6+/− mouse tumor experiments showed that Csn6 haplo-insufficiency helps impede the development of cancer,15 suggesting that CSN6 signaling regulation is critical for tumor development. However, the mechanism and biological consequence of CSN6 expression in cancer remain not well studied.
Here, we demonstrate that CSN6 is involved in regulating c-Jun stability. The downstream target of the CSN6 axis—MEKK1 is a critical E3 ligase involved in this process. CSN6 antagonizes MEKK1 expression to regulate c-Jun stability. Our studies characterize the signaling of the CSN6-MEKK1-c-Jun axis in osmotic stress response. These results define a new mechanism for posttranslational regulation of MEKK1. Our findings also implicate a specific mechanism by which c-Jun is deregulated in human cancers.
Results
CSN6 interacts with MEKK1
It has been reported that MEKK1 regulates c-Jun stability 6 and that COP9 signalosome is known to regulate c-Jun stability.27 It is possible that there is a relationship between MEKK1 or CSN6 and c-Jun. To investigate the physical interaction between them, we first tested endogenous binding between CSN6 and MEKK1 and c-Jun. Cell lysates were subjected to immunoprecipitation with antibody of CSN6 or MEKK1. Indeed, endogenously expressed CSN6, MEKK1, and c-Jun were associated with each other (Fig. 1A). To verify exogenous binding, we transfected cells with Flag-CSN6 and examined MEKK1 binding. An association between exogenous CSN6 and endogenous MEKK1 was confirmed (Fig. 1B). In vitro binding was investigated using CSN6 and MEKK1 TNT products. Consistently, we confirmed in vitro binding between CSN6 and MEKK1 (Fig. 1C).
Figure 1.
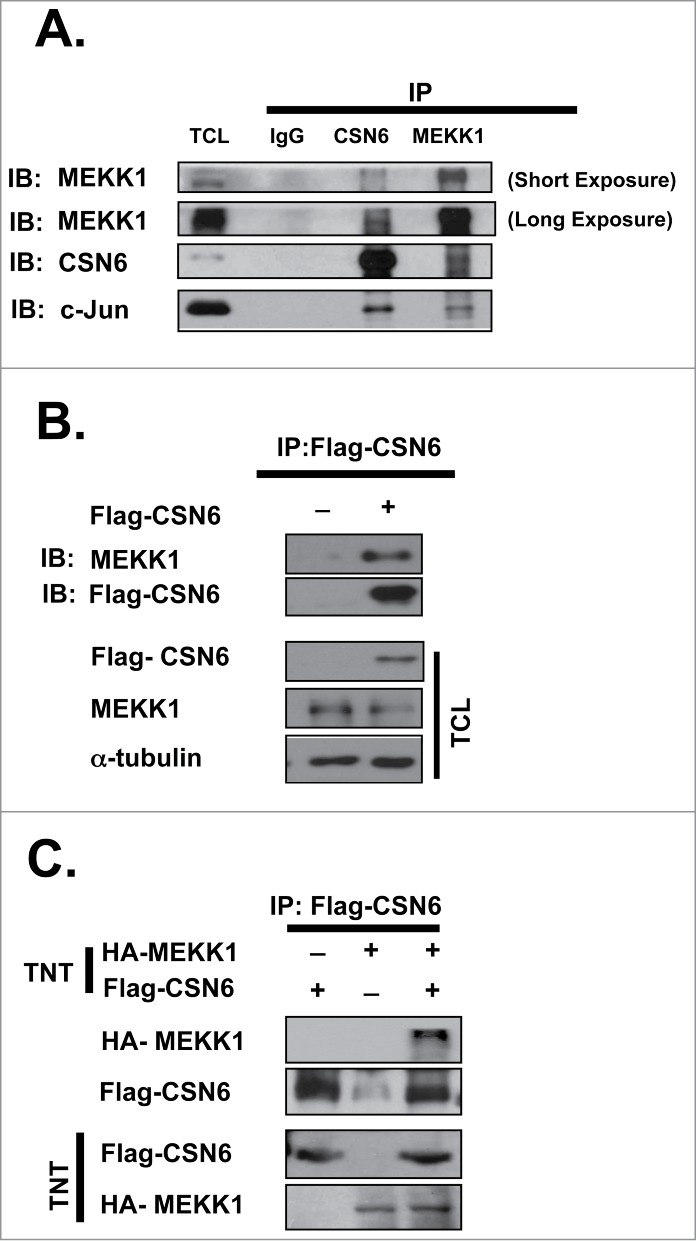
In vivo and in vitro binding between CSN6 and MEKK1. (A) Endogenous CSN6 interacts with MEKK1. HEK 293T cells were lysed and subjected to immunoprecipitation with IgG-, CSN6-, and MEKK1-specific antibodies. Both immunoprecipitation and total cell lysate (TCL) were immunoblotted with the indicated antibodies. (B) Exogenous CSN6 interacts with endogenous MEKK1. HEK 293T cells were transfected with Flag-CSN6 plasmid and then subjected to immunoprecipitation (anti-Flag) and Western blot analysis with MEKK1 antibody. (C) CSN6 binds with MEKK1 in vitro. Flag-CSN6 and HA-MEKK1 proteins were created using an in vitro transcription/translation system (TNT). Proteins were immunoprecipitated with M2 Flag beads and subjected to an SDS-PAGE analysis. (A, B) All cells were treated with 10 μM MG132 for 6 hrs before being harvested.
CSN6 negatively regulates MEKK1 steady-state expression
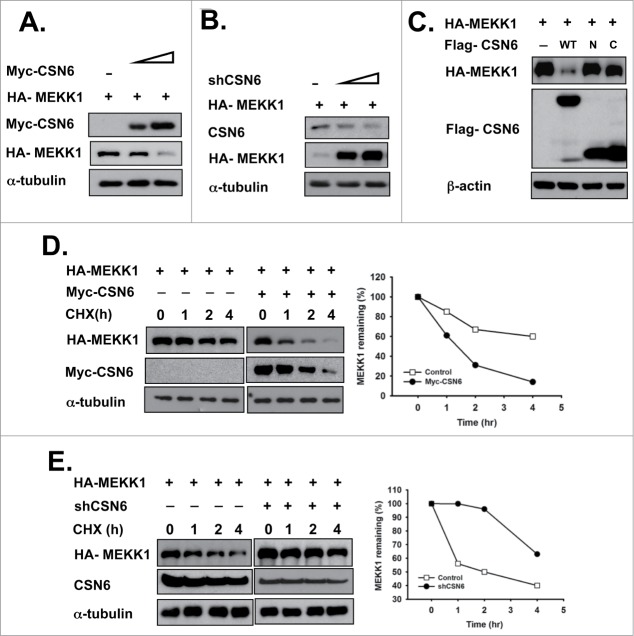
It was reported that MEKK1 functions as an E3 ligase to destabilize c-Jun protein.6 In addition, we previously observed that CSN6 can regulate the stability of several E3 ligases.15,23,31 On the basis of these findings, we determined whether CSN6 regulates MEKK1 to stabilize c-Jun proteins. We found that high expression levels of CSN6 suppressed MEKK1 expression levels in a dose-dependent manner (Fig. 2A). In contrast, MEKK1 levels were stabilized by knocking down CSN6 (Fig. 2B). We also characterized the functional domain of CSN6 that regulates MEKK1 stability. Full-length, N-terminus (aa 1–184 containing MPN motif) only, or C-terminus (aa 185–327) only CSN6 plasmid DNA was co-transfected with MEKK1. Only full-length CSN6 regulated MEKK1, not truncated forms (Fig. 2C). To confirm that regulation of MEKK1 by CSN6 occurs at the post-transcriptional level, we examined the turnover rate of MEKK1 protein in the presence of the de novo protein synthesis inhibitor cycloheximide (CHX). The rate was higher in the CSN6 overexpression group than in the control group (Fig. 2D). In contrast, in the CSN6 knockdown group, the MEKK1 protein turnover rate was significantly lower (Fig. 2E). Together, these results suggest that CSN6 is reducing the stability of MEKK1.
Figure 2.
CSN6 negatively regulates MEKK1 steady-state expression. (A) Overexpression of CSN6 downregulates MEKK1 levels. HEK293T cells were co-transfected with Myc-CSN6 and HA-MEKK1 plasmid. A Western blot analysis was performed with the same amount of cell lysate. (B) MEKK1 was stabilized under CSN6 knockdown conditions. HEK293T cells were co-transfected with shCSN6 and HA-MEKK1 plasmid and then subjected to immunoblotting with the indicated antibodies. (C) Full-length CSN6 decreased MEKK1 expression. Cells were co-transfected with Flag-WT CSN6 or the truncated form of CSN6 (N terminal or C-terminal) and HA-MEKK1 plasmid. Cell lysates were immunoblotted with the indicated antibodies. (D) Overexpression of CSN6 increased the turnover rate of MEKK1. Myc-CSN6- and HA-MEKK1-co-transfected HEK 293T cells were treated with CHX for the indicated times and then subjected to Western blot analysis. The density of MEKK1 was measured, and the integrated optical density (IOD) was measured. The turnover of MEKK1 was indicated graphically. (E) Knockdown of CSN6 decreased the turnover rate of MEKK1. shCSN6 and HA-MEKK1-co-transfected HEK 293T cells were treated with CHX for the indicated times and then subjected to immunoblotting analysis. The density of MEKK1 was measured, and the integrated optical density (IOD) was measured. The turnover of MEKK1 was indicated graphically.
CSN6 decreases MEKK1 stability through enhancing the ubiquitination of MEKK1
We found that CSN6-mediated MEKK1 downregulation was rescued by the proteasome inhibitor MG132 (Figure 3A). These results suggest that CSN6 reduced the steady-state expression of MEKK1 in a proteasome-dependent manner. We further confirmed that CSN6 induces MEKK1 poly-ubiquitination via the 26S proteasome. First, we examined both CSN6+/+ and CSN6+/− mouse embryonic fibroblasts (MEFs) and observed decreased MEKK1 ubiquitination levels in CSN6+/− MEFs (Fig. 3B). Next, we examined the effect of CSN6 on MEKK1 ubiquitination under denaturing conditions. Overexpression of CSN6 induced MEKK1 poly-ubiquitination (Fig. 3C). However, poly-ubiquitination of MEKK1 was decreased when CSN6 was knocked down (Fig. 3D). These results indicate that CSN6 can negatively affect the steady-state expression of MEKK1 via enhancing MEKK1 ubiquitination.
Figure 3.
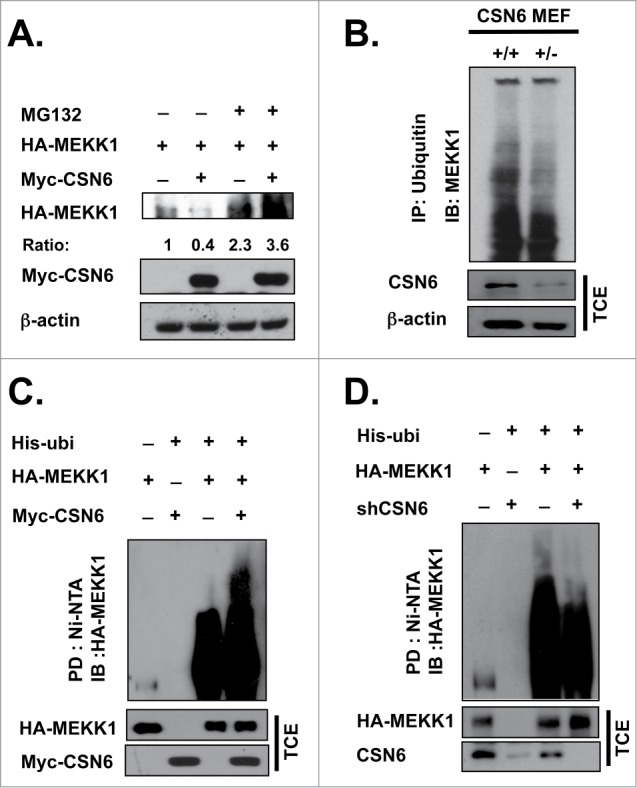
CSN6 induces MEKK1 ubiquitination. (A) HEK293T cells were co-transfected with Myc-CSN6 and HA-MEKK1 plasmid for 48 hrs and then treated with DMSO or MG132 for 6 hrs before being harvested. A Western blot analysis was performed using the same amount of cell lysate. (B) Loss of CSN6 decreased MEKK1 ubiquitination levels. csn6+/+ and csn6+/− MEFs were lysed and subjected to immunoprecipitation with ubiquitin antibody and then immunoblotted with MEKK1 antibody. (C, D) CSN6 efficiently ubiquitinates MEKK1. HEK 293T cells were co-transfected with the indicated plasmids, followed by nickel bead purification for an in vivo ubiquitination assay. An immunoblot analysis was performed. (B-D) All cells were treated with 10 μM MG132 for 6 hrs before being harvested.
CSN6 hinders MEKK1-mediated c-Jun destabilization
MEKK1 functions as an E3 ligase for c-Jun, and we observed CSN6-induced MEKK1 degradation. Thus, we further investigated whether CSN6 can rescue MEKK1-mediated c-Jun degradation. Indeed, MEKK1 reduced the steady-state expression of c-Jun, but overexpression of CSN6 reversed MEKK1-mediated c-Jun degradation (Fig. 4A). We also determined whether CSN6 rescued c-Jun protein degradation by MEKK1 under sorbitol-induced osmotic stress conditions. Indeed, sorbitol decreased both phosphorylated and non-phosphorylated c-Jun protein expression over time. Importantly, c-Jun expression levels were stabilized when CSN6 was overexpressed (Fig. 4B). To determine whether MEKK1 is a major mediator of c-Jun regulation in the CSN6-MEKK1-c-Jun axis, we examined both the WT and C433A RING mutant (lost E3 ligase function) forms of MEKK1.33 We found that c-Jun protein expression levels were not affected by C433A MEKK1. Importantly, CSN6 rescued c-Jun degradation in WT MEKK1 but not in C433A MEKK1 (Fig. 4C). These data suggest that CSN6 regulates c-Jun stability by antagonizing MEKK1's activity.
Figure 4.
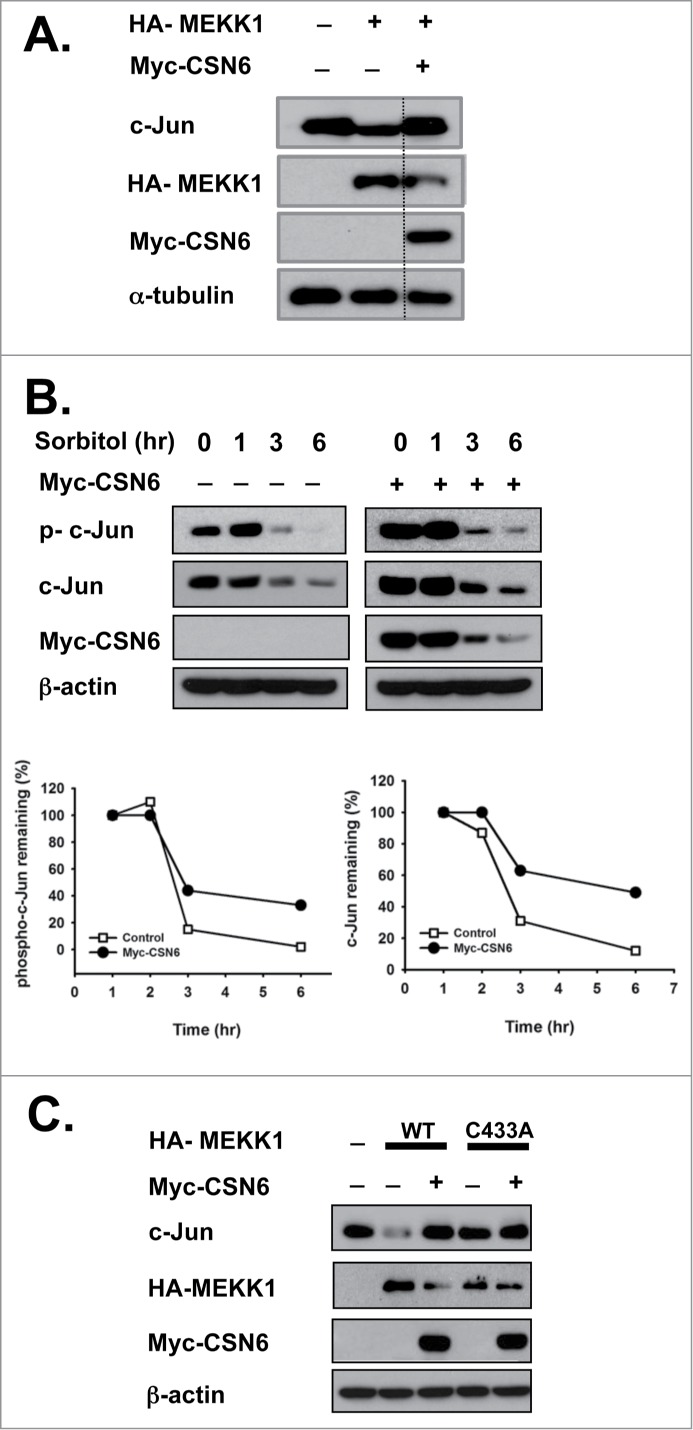
CSN6 rescues MEKK1-mediated c-Jun destabilization. (A) CSN6 inhibits c-Jun destabilization by MEKK1. HEK293T cells were co-transfected with the indicated plasmids, and an immunoblot analysis was performed with c-Jun antibody. (B) CSN6 rescues osmotic stress-induced c-Jun degradation. Cells were transfected with Myc-CSN6 and then treated with 500 mM sorbitol for the indicated times. A Western blot analysis was performed with the same amount of cell lysates. The density of phospho-c-Jun or MEKK1 was measured, and the integrated optical density (IOD) was measured. The turnover of phospho-c-Jun or MEKK1 was indicated graphically. (C) CSN6 hampered WT MEKK1-induced c-Jun degradation. Cells were co-transfected with HA-WT or the PHD/RING finger domain mutant form of MEKK1 (C433A) and Myc-CSN6 and then subjected to immunoblotting with c-Jun antibody.
CSN6 reduces MEKK1-mediated c-Jun ubiquitination
Although CSN6 rescues c-Jun protein degradation by downregulating MEKK1 expression, the effect of CSN6 on MEKK1-induced c-Jun ubiquitination needs to be verified. As expected, MEKK1 increased c-Jun poly-ubiquitination, but overexpression of CSN6 hindered MEKK1-mediated c-Jun ubiquitination (Fig. 5A). Moreover, C433A MEKK1 had no functional effect on c-Jun ubiquitination (Fig. 5A). We then determined whether CSN6 rescued sorbitol-induced c-Jun ubiquitination. As expected, sorbitol increased c-Jun poly-ubiquitination, but CSN6 overexpression significantly suppressed ubiquitination levels of c-Jun (Fig. 5B). We further verified whether MEKK1 is a major mediator of c-Jun ubiquitination in the CSN6-MEKK1-c-Jun axis by examining both MEKK1+/+ and MEKK1−/− MEFs. C-Jun is not ubiquitinated in MEKK1−/− MEFs (Fig. 5C). We confirmed that CSN6 reduced c-Jun poly-ubiquitination only in MEKK1+/+ MEFs (Fig. 5C), indicating that CSN6 mediates c-Jun ubiquitination is through regulating MEKK1.
Figure 5.
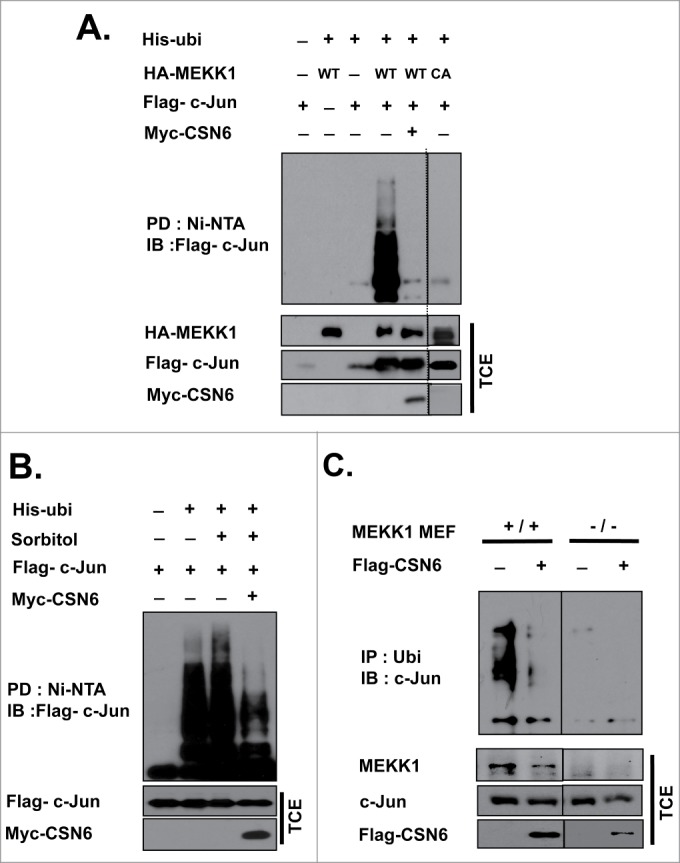
CSN6 inhibits MEKK1-mediated c-Jun ubiquitination. (A) HEK 293T cells were transfected with the expression vector for His-ubi, Flag-c-Jun, HA-MEKK1, and Myc-CSN6, followed by nickel bead purification and a Western blot analysis. (B) HEK 293T cells were co-transfected with the indicated expression vectors and then treated with 500 mM sorbitol for 6 hrs before being harvested. Lysates were subjected to nickel bead purification for an in vivo ubiquitination assay. (C) MEKK1+/+ and MEKK1−/− MEFs were transfected with Flag-CSN6, lysed and subjected to immunoprecipitation with ubiquitin antibody, and then immunoblotted with c-Jun antibody. All cells were treated with 10 μM MG132 for 6 hrs before being harvested.
CSN6 regulates c-Jun target genes through MEKK1
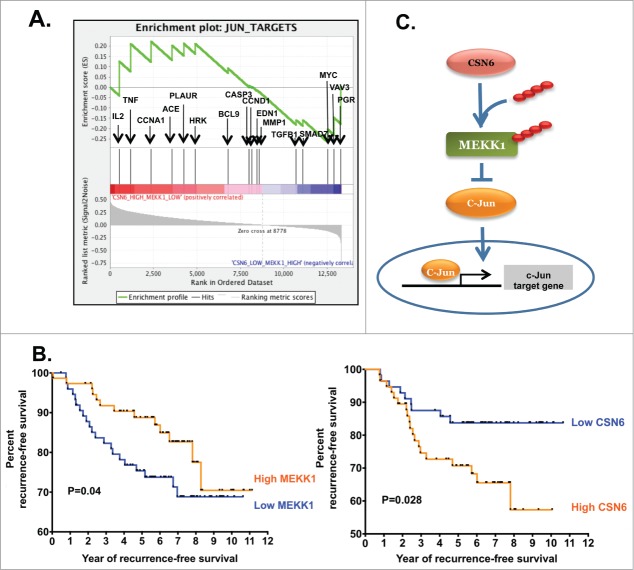
To understand the contribution of the CSN6-MEKK1-c-Jun axis to human tumorigenesis, we analyzed the gene expression profiles of 278 stage I–III breast cancer patients; we found a positive correlation between CSN6 expression and c-Jun target genes in tumors (Fig. 6A). Thus CSN6-c-Jun link in cell studies can be recapitulated in breast cancer samples. Importantly, we also show that low level of MEKK1 is correlated with poor survival of breast cancer patients (Fig. 6B), confirming that deregulation of MEKK1 is involved in tumorigenesis. Here, we introduced a novel mechanism by which CSN6-MEKK1 signaling regulates c-Jun ubiquitination and degradation. In conclusion, CSN6 increased c-Jun stability by suppressing MEKK1 stability (Fig. 6C).
Figure 6.
CSN6 regulates the c-Jun target gene through MEKK1. (A) An enrichment score graph and ranked list metric graph show the upregulation of c-Jun target genes in breast cancer patients with high CSN6 and low MEKK1 expression. (B) Kaplan-Meier survival curves of overall survival duration based on MEKK1 and CSN6 expression in gene expression profiles of breast cancer patient cohort GSE20194. Data were downloaded from the Gene Expression Omnibus database and matched with corresponding clinical data in analysis. Log-rank analysis was used to test for significance. P = 0.04 and 0.028 respectively. (C) Model of CSN6-MEKK1-c-Jun axis in regulating c-Jun transcriptional activity.
Discussion
CSN6 is overexpressed/amplified in cancers, which leads to poor survival,22,34-37 indicating that abnormal CSN6 overexpression allows cancer to have growth advantages. However, its role in cancer remains not well characterized. In the present study, we found that CSN6 deregulation is causing destabilization of MEKK1, a PHD/RING domain containing serine/threonine kinase involved in c-Jun regulation. c-Jun is a part of the transcription factor AP-1 that is regulated by a wide variety of extracellular signaling. We found that CSN6 has unprecedented biological activity in downregulating MEKK1, thereby inducing c-Jun elevation. This study demonstrates how CSN6 has a role in promoting cancer development by influencing MEKK-c-Jun signaling.
MEKK1, a MAPK kinase kinase, is activated in response to stimuli that can alter the cytoskeleton and cell migration. MEKK1 is an upstream signal mediator of MKK1 and MKK4, leading to the activation of ERK1/2 and JNK. JNK signaling is an important mediator of the cellular stress response. MEKK1 activates JNK and phosphorylation of c-Jun and also stimulates c-Jun ubiquitination and degradation. The regulatory mechanism of MEKK1 is not well characterized. Our CSN6 studies show a new layer of MEKK1 regulation. Given that CSN6 participates in enhancing MEKK1 ubiquitination/degradation, CSN6 has the potential role in multiple biological functions, as MEKK1 is an important signal mediator in deregulations of cancer hallmarks,38 including cell growth, apoptosis, cancer invasion39,40 and tumorigenesis.41-44 Our studies open a new avenue to address these issues.
In COP9 signalosome, CSN6 shared with CSN5 the Mpr1-Pad1-N-terminal (MPN) domain,45 which is involved in controlling Cullin deneddylation activity.31,46 The biological function of the MPN domain remains not clear, but the domain has polar residues that resemble the active site residues of metalloproteases 47 and is critical in a proteasome-associated activity.48 The N-terminal CSN6 includes CSN6's MPN domain, which is conserved in the N-terminus of yeast Mpr1 and Pad1 proteins. Our data show that N-terminal CSN6 alone is not able to mediate MEKK1 downregulation, while full-length CSN6 can destabilize MEKK1. This is somewhat of a surprise as CSN6's MPN domain is the major domain involved in targeting protein stability regulation such as Myc.31 The data suggest that every domain of CSN6 is participated in MEKK1 downregulation, which warrants further investigation.
MEKK1 is a195 kDa protein and has a N-terminal plant homeobox domain (PHD) with E3 ligase activity. MEKK1 kinase activity is required for ubiquitination of MEKK1.33 MEKK1 demonstrates autoubiquitination and is inhibited by mutation of cysteine 443 to alanine (C443A) within the PHD domain. We show that CSN6 enhances MEKK1 ubiquitination and induces subsequent downregulation. We also show that C433A MEKK1 is resistant to CSN6-mediated downregulation, suggesting that CSN6 is involved in enhancing autoubiquitination of MEKK1. MEKK1 is a major activator for the JNK pathway,49 while MEKK1 can also regulate the ERK pathway.50,51 Importantly, MEKK1 is critical for ERK1/2 ubiquitination. It is shown that both kinase activity and E3 ligase activity are required to regulate ERK1 /2 ubiquitination.33 Given that CSN6 is affecting the stability of MEKK1, it is possible that CSN6 will have impact on ERK1/2 signaling.
Our data show that CSN6 overexpression leads to enhanced expression of c-Jun target genes in cancer. Also we show that low level of MEKK1 expression correlates with poor survival in breast cancer patients. This is consistent with the observation that MEKK1 can be a tumor suppressor.41-44 CSN6's negative impact on MEKK1 and positive impact on c-Jun stabilization allow tumor cells to have a growth advantage; hence, reversing this process will be a good strategic design for the treatment of cancers. A strategy to antagonize CSN6-mediated MEKK1 degradation will be effective. Our study indicates that Akt activation is stabilizing CSN6.22 Thus, inhibiting Akt signal pathway and subsequent destabilization of CSN6 may have a potential for clinical treatment of cancer patients with c-Jun deregulation.
In summary, our findings show that CSN6 overexpression can facilitate MEKK1 degradation in cancer and provide important insight into upstream regulation of MEKK1-mediated c-Jun deregulation in cancers. The fact that CSN6 functions as a negative regulator of MEKK1 suggests that hindering the CSN6 signaling axis is an efficient therapeutic approach in cancer treament.
Materials and Methods
Cell culture and reagents
HEK293T cells were purchased from ATCC, and mekk1−/− MEFs were obtained from Dr. Zhimin Lu (The University of Texas MD Anderson Cancer Center, Houston, Texas). csn6+/+ and csn6+/− MEFs were collected as described previously.15 Cells were cultured in DMEM/F12 media with 10% (HEK 293T) or 20% (MEFs) fetal bovine serum (Gemini), 2 mM L-glutamine (Cellgro), and 1% antibiotic-antimycotic solution (Invitrogen). MG132, CHX, and sorbitol were obtained from Sigma. Ni-NTA agarose was obtained from Invitrogen. We used the following antibodies: Flag (M2 monoclonal antibody, Sigma), actin (Sigma), hemagglutinin (HA, 12CA5, Roche), ubiquitin (Zymed Laboratories, Inc.), tubulin (Sigma), CSN6 (BIOMOL International), c-Myc (9E10, Santa Cruz), MEKK1 (Bethyl), c-Jun, and phospho-c-Jun (Cell Signaling).
Immunoblotting and immunoprecipitation
Cells were lysed with lysis buffer (1 L contained 15 g of 1 M Tris [Fisher], 30 mL of 5 M NaCl [Fisher], 1 mL of Nonidet P-40 [USB Corp.], 1 mL of Triton X-100 [Sigma], and 2 mL of 0.5 M EDTA [Fisher]) and a mixture of protease and phosphatase inhibitors (5 mM NaV, 1 mM NaF, 1 μM DTT, 0.1 mg/mL Pepstatin A, 1 mM PMSF, and 1,000× complete mixture protease inhibitor [Roche]). Protein lysates were standardized, and equal amounts of proteins were subjected to an immunoblotting analysis. For immunoprecipitation, cells were lysed with lysis buffer, and the same amount of protein was immunoprecipitated with specific antibody overnight at 4°C. The antibody was pulled down with protein A/G beads (Santa Cruz) for 3 hrs at 4°C, and a Western blot analysis was performed as previously described.7,52
Protein turnover assay
Cells were transfected with the indicated plasmids for 48 hrs and then treated with 200 μg/ml CHX for the indicated times as previously described.16,53 Cells were lysed and standardized and then subjected to an immunoblotting analysis with the indicated antibodies.
In vivo ubiquitination assay
Ubiquitination assay was performed as previously described.7,54,55 Non-denaturing condition: csn6+/+, csn6+/−, mekk1+/+, and mekk1−/− MEFs were transfected with the indicated plasmids and then treated with 100 μg/ml of MG132 for 6 hrs. Cell lysates were immunoprecipitated with ubiquitin antibody overnight at 4°C, and a Western blot analysis was performed. Denaturing condition: HEK 293T cells were co-transfected with His-tag-contained plasmid for 48 hrs and lysed with denaturing buffer (6 M guanidine-HCl, 0.1 M Na2HPO4/NaH2PO4, and 10 mM imidazole). After sonication, cell lysates were incubated with Ni-NTA agarose beads for 3 hrs at ambient temperature, and a Western blot analysis was performed with the indicated antibodies.
Human tumor tissue samples
High CSN6 and low MEKK1 quartile levels were compared with low CSN6 and high MEKK1 quartile levels using Gene Set Enrichment Analysis (Gene Set Enrichment Analysis, Broad Institute, MIT). Tissue samples from cohort GSE-20194, which consisting of 255 untreated stage I–stage III breast cancer patients at MD Anderson, was used for the analysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by grants from the National Institutes of Health (R01CA089266 to M.H.L.), Directed Medical Research Programs (DOD SIDA BC062166 to S.J.Y. and M.H.L.), Susan G. Komen Breast Cancer Foundation (KG081048 to S.J.Y. and M.H.L.), and the Fidelity foundation to M.H.L. The University of Texas MD Anderson Cancer Center is supported by NIH core grant CA16672.
References
- 1.Wisdom R, Johnson RS, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J 1999; 18:188-97; PMID:9878062; http://dx.doi.org/ 10.1093/emboj/18.1.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakiri L, Matsuo K, Wisniewska M, Wagner EF, Yaniv M. Promoter specificity and biological activity of tethered AP-1 dimers. Mol Cell Biol 2002; 22:4952-64; PMID:12052899; http://dx.doi.org/ 10.1128/MCB.22.13.4952-4964.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith LM, Wise SC, Hendricks DT, Sabichi AL, Bos T, Reddy P, Brown PH, Birrer MJ. cJun overexpression in MCF-7 breast cancer cells produces a tumorigenic, invasive and hormone resistant phenotype. Oncogene 1999; 18:6063-70; PMID:10557095; http://dx.doi.org/ 10.1038/sj.onc.1202989 [DOI] [PubMed] [Google Scholar]
- 4.Blau L, Knirsh R, Ben-Dror I, Oren S, Kuphal S, Hau P, Proescholdt M, Bosserhoff AK, Vardimon L. Aberrant expression of c-Jun in glioblastoma by internal ribosome entry site (IRES)-mediated translational activation. Proc Natl Acad Sci U S A 2012; 109:E2875-84; PMID:23027969; http://dx.doi.org/ 10.1073/pnas.1203659109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Birkenbach M, Hart J. Expression of Jun family members in human colorectal adenocarcinoma. Carcinogenesis 2000; 21:1313-7; PMID:10874008; http://dx.doi.org/ 10.1093/carcin/21.7.1313 [DOI] [PubMed] [Google Scholar]
- 6.Xia Y, Wang J, Xu S, Johnson GL, Hunter T, Lu Z. MEKK1 mediates the ubiquitination and degradation of c-Jun in response to osmotic stress. Mol Cell Biol 2007; 27:510-7; PMID:17101801; http://dx.doi.org/ 10.1128/MCB.01355-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao R, Yang HY, Shin J, Phan L, Fang L, Che TF, Su CH, Yeung SC, Lee MH. CDK inhibitor p57 (Kip2) is downregulated by Akt during HER2-mediated tumorigenicity. Cell Cycle 2013; 12:935-43; PMID:23421998; http://dx.doi.org/ 10.4161/cc.23883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nateri AS, Riera-Sans L, Da Costa C, Behrens A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 2004; 303:1374-8; PMID:14739463; http://dx.doi.org/ 10.1126/science.1092880 [DOI] [PubMed] [Google Scholar]
- 9.Lin A, Frost J, Deng T, Smeal T, al-Alawi N, Kikkawa U, Hunter T, Brenner D, Karin M. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell 1992; 70:777-89; PMID:1516134; http://dx.doi.org/ 10.1016/0092-8674(92)90311-Y [DOI] [PubMed] [Google Scholar]
- 10.Luo Y, Yang C, Ye M, Jin C, Lee MH, Yeung SY, McKeehan WL. Deficiency of metabolic regulator FGFR4 delays breast cancer progression through systemic and microenvironmental metabolic alterations. Cancer Metab 2013; 1(1):21; PMID:24279986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeschke A, Karasarides M, Ventura JJ, Ehrhardt A, Zhang C, Flavell RA, Shokat KM, Davis RJ. JNK2 is a positive regulator of the cJun transcription factor. Mol Cell 2006; 23:899-911; PMID:16973441; http://dx.doi.org/ 10.1016/j.molcel.2006.07.028 [DOI] [PubMed] [Google Scholar]
- 12.Yujiri T, Sather S, Fanger GR, Johnson GL. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 1998; 282:1911-4; PMID:9836645; http://dx.doi.org/ 10.1126/science.282.5395.1911 [DOI] [PubMed] [Google Scholar]
- 13.Wei N, Deng XW. COP9: a new genetic locus involved in light-regulated development and gene expression in arabidopsis. Plant cell 1992; 4:1507-18; PMID:1467650; http://dx.doi.org/ 10.1105/tpc.4.12.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao S, Fang L, Qdaisat A, Yeung SC, Lee MH. COP9 signalosome subunit 6 (CSN6) regulates E6AP/UBE3A in cervical cancer. Oncotarget 2015; in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao R, Yeung SC, Chen J, Iwakuma T, Su CH, Chen B, Qu C, Zhang F, Chen YT, Lin YL, et al.. Subunit 6 of the COP9 signalosome promotes tumorigenesis in mice through stabilization of MDM2 and is upregulated in human cancers. J Clin Invest 2011; 121:851-65; PMID:21317535; http://dx.doi.org/ 10.1172/JCI44111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi J, Guma S, Fang L, Phan F, Ivan C, Baggery K, Sood A, Lee MH. Regulating the ubiquitination of CDK inhibitor p27 Kip1 via CSN6-COP1 axis. Cell Cycle 2015; 14(14):2265-73; PMID:25945542; http://dx.doi.org/ 10.1080/15384101.2015 in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi HH, Fang LK, Chen JS, Chou PC, Phan L, Su CH, Ivan C, Baggery K, Sood A, Yeung SC, et al.. COP1 enhances ubiquitin-mediated degradation of p27Kip1 to promote cancer cell growth. Oncotarget 2015; in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong WJ, Yoon J, Park JC, Lee SH, Lee SH, Kaduwal S, Kim H, Yoon JB, Choi KY. Ras stabilization through aberrant activation of Wnt/beta-catenin signaling promotes intestinal tumorigenesis. Science signal 2012; 5:ra30; PMID:22494971; http://dx.doi.org/ 10.1126/scisignal.2002242 [DOI] [PubMed] [Google Scholar]
- 19.Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell 2012; 151:1443-56; PMID:23245942; http://dx.doi.org/ 10.1016/j.cell.2012.11.027 [DOI] [PubMed] [Google Scholar]
- 20.Zhang XC, Chen J, Su CH, Yang HY, Lee MH. Roles for CSN5 in control of p53/MDM2 activities. J Cell Biochem 2008; 103:1219-30; PMID:17879958; http://dx.doi.org/ 10.1002/jcb.21504 [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Zhao R, Su CH, Linan M, Tseng C, Phan L, Fang L, Yang HY, Yang H, Wang W, et al.. CDK inhibitor p57 (Kip2) is negatively regulated by COP9 signalosome subunit 6. Cell Cycle 2012; 11:4633-41; PMID:23187808; http://dx.doi.org/ 10.4161/cc.22887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue Y, Chen J, Choi HH, Phan L, Chou PC, Zhao R, Yang H, Santiago J, Liu M, Yeung GE, et al.. HER2-Akt signaling in regulating COP9 signalsome subunit 6 and p53. Cell Cycle 2012; 11:4181-90; PMID:23095642; http://dx.doi.org/ 10.4161/cc.22413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi HH, Gully C, Su CH, Velazquez-Torres G, Chou PC, Tseng C, Zhao R, Phan L, Shaiken T, Chen J, et al.. COP9 signalosome subunit 6 stabilizes COP1, which functions as an E3 ubiquitin ligase for 14-3-3sigma. Oncogene 2011; 30:4791-801; PMID:21625211; http://dx.doi.org/ 10.1038/onc.2011.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al.. YAP/TAZ Incorporation in the beta-Catenin Destruction Complex Orchestrates the Wnt Response. Cell 2014; 158:157-70; PMID:24976009; http://dx.doi.org/ 10.1016/j.cell.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 25.Zhao R, Phan L, Chen B, Yang HY, Chen J, Che TF, Qiao Y, Zhang J, Yeung SC, Lee MH. Ubiquitination-mediated p57Kip2 Degradation by CSN5 Confers Cancer Cell Proliferation. Cancer Hallmarks 2013; 1:133-44; http://dx.doi.org/ 10.1166/ch.2013.1013 [DOI] [Google Scholar]
- 26.Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev 1999; 13:270-83; PMID:9990852; http://dx.doi.org/ 10.1101/gad.13.3.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lykke-Andersen K, Schaefer L, Menon S, Deng XW, Miller JB, Wei N. Disruption of the COP9 signalosome Csn2 subunit in mice causes deficient cell proliferation, accumulation of p53 and cyclin E, and early embryonic death. Mol Cell Biol 2003; 23:6790-7; PMID:12972599; http://dx.doi.org/ 10.1128/MCB.23.19.6790-6797.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J, Walz K, Nakamura H, Carattini-Rivera S, Zhao Q, Vogel H, Wei N, Justice MJ, Bradley A, Lupski JR. COP9 signalosome subunit 3 is essential for maintenance of cell proliferation in the mouse embryonic epiblast. Mol Cell Biol 2003; 23:6798-808; PMID:12972600; http://dx.doi.org/ 10.1128/MCB.23.19.6798-6808.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomoda K, Yoneda-Kato N, Fukumoto A, Yamanaka S, Kato JY. Multiple functions of Jab1 are required for early embryonic development and growth potential in mice. J Biol Chem 2004; 279:43013-8; PMID:15299027; http://dx.doi.org/ 10.1074/jbc.M406559200 [DOI] [PubMed] [Google Scholar]
- 30.Menon S, Chi H, Zhang H, Deng XW, Flavell RA, Wei N. COP9 signalosome subunit 8 is essential for peripheral T cell homeostasis and antigen receptor-induced entry into the cell cycle from quiescence. Nat Immunol 2007; 8:1236-45; PMID:17906629; http://dx.doi.org/ 10.1038/ni1514 [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Shin JH, Zhao R, Phan L, Wang H, Xue Y, Post SM, Ho Choi H, Chen JS, Wang E, et al.. CSN6 drives carcinogenesis by positively regulating Myc stability. Nat Commun 2014; 5:5384; PMID:25395170; http://dx.doi.org/ 10.1038/ncomms6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi HH, Su CH, Fang L, Zhang J, Yeung SC, Lee MH. CSN6 deregulation impairs genome integrity in a COP1-dependent pathway. Oncotarget 2015; 6:11779-93; PMID:25957415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Z, Xu S, Joazeiro C, Cobb MH, Hunter T. The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell 2002; 9:945-56; PMID:12049732; http://dx.doi.org/ 10.1016/S1097-2765(02)00519-1 [DOI] [PubMed] [Google Scholar]
- 34.Lee MH, Zhao R, Phan L, Yeung SC. Roles of COP9 signalosome in cancer. Cell Cycle 2011; 10:3057-66; PMID:21876386; http://dx.doi.org/ 10.4161/cc.10.18.17320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmena L, Hakem R. From photomorphogenesis to cancer: a CSN journey. Cell Cycle 2013; 12:205-6; PMID:23287466; http://dx.doi.org/ 10.4161/cc.23422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang F, Lu W, Choi HH, Yeung SC, Tung JY, Hsiao CD, Fuentes-Mattei E, Menter D, Wang L, Chen C, et al.. ERK2-Dependent Phosphorylation of CSN6 Is Critical in Colorectal Cancer Development Cancer Cell 2015; in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang L, Yang Z, Zhou J, Tung JY, Hsiao CD, Wang L, Deng Y, Wang P, Wang J, Lee MH. Circadian Clock Gene CRY2 Degradation Is Involved in Chemoresistance of Colorectal Cancer. Mol Cancer Ther 2015; 14:1476-87; PMID:25855785; http://dx.doi.org/ 10.1158/1535-7163.MCT-15-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 39.Ho MY, Liang CM, Liang SM. MIG-7 and phosphorylated prohibitin coordinately regulate lung cancer invasion/metastasis. Oncotarget 2015; 6:381-93; PMID:25575814 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Wang Y, Zhou BP. Epithelial-Mesenchymal Transition—A Hallmark of Breast Cancer Metastasis. Cancer Hallmarks 2013; 1:38-49; PMID:24611128; http://dx.doi.org/ 10.1166/ch.2013.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L, et al.. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 2012; 486:405-9; PMID:22722202; http://dx.doi.org/ 10.1038/nature11154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, Van Tine BA, Hoog J, Goiffon RJ, Goldstein TC, et al.. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 2012; 486:353-60; PMID:22722193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cancer Genome. Atlas N. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490:61-70; PMID:23000897; http://dx.doi.org/ 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pham TT, Angus SP, Johnson GL. MAP3K1: Genomic Alterations in Cancer and Function in Promoting Cell Survival or Apoptosis. Genes Cancer 2013; 4:419-26; PMID:24386504; http://dx.doi.org/ 10.1177/1947601913513950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinaldi T, Bolotin-Fukuhara M, Frontali L. A Saccharomyces cerevisiae gene essential for viability has been conserved in evolution. Gene 1995; 160:135-6; PMID:7628709; http://dx.doi.org/ 10.1016/0378-1119(95)00212-O [DOI] [PubMed] [Google Scholar]
- 46.Saha A, Deshaies RJ. Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol Cell 2008; 32:21-31; PMID:18851830; http://dx.doi.org/ 10.1016/j.molcel.2008.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maytal-Kivity V, Reis N, Hofmann K, Glickman MH. MPN+, a putative catalytic motif found in a subset of MPN domain proteins from eukaryotes and prokaryotes, is critical for Rpn11 function. BMC Biochem 2002; 3:28; PMID:12370088; http://dx.doi.org/ 10.1186/1471-2091-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyapina S, Cope G, Shevchenko A, Serino G, Tsuge T, Zhou C, Wolf DA, Wei N, Deshaies RJ. Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science 2001; 292:1382-5; PMID:11337588; http://dx.doi.org/ 10.1126/science.1059780 [DOI] [PubMed] [Google Scholar]
- 49.Yan M, Dai T, Deak JC, Kyriakis JM, Zon LI, Woodgett JR, Templeton DJ. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature 1994; 372:798-800; PMID:7997270; http://dx.doi.org/ 10.1038/372798a0 [DOI] [PubMed] [Google Scholar]
- 50.Karandikar M, Xu S, Cobb MH. MEKK1 binds raf-1 and the ERK2 cascade components. J Biol Chem 2000; 275:40120-7; PMID:10969079; http://dx.doi.org/ 10.1074/jbc.M005926200 [DOI] [PubMed] [Google Scholar]
- 51.Xu S, Robbins D, Frost J, Dang A, Lange-Carter C, Cobb MH. MEKK1 phosphorylates MEK1 and MEK2 but does not cause activation of mitogen-activated protein kinase. Proc Natl Acad Sci U S A 1995; 92:6808-12; PMID:7624324; http://dx.doi.org/ 10.1073/pnas.92.15.6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuentes-Mattei E, Velazquez-Torres G, Phan L, Zhang F, Chou PC, Shin JH, Choi HH, Chen JS, Zhao R, Chen J, et al.. Effects of obesity on transcriptomic changes and cancer hallmarks in estrogen receptor-positive breast cancer. J Natl Cancer Inst 2014; 106; PMID:24957076; http://dx.doi.org/ 10.1093/jnci/dju158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phan L, Shin JH, Zhou Z, Gully C, Phan L, Velazquez-Torres T, Fuentes-Mattei E, Yeung G, Su CH, Wang H, et al.. The cell cycle regulator 14-3-3σ opposes and reverses cancer metabolic reprogramming Nature Commun 2015; 6:7530; PMID:26179207; http://dx.doi.org/ 10.1038/ncomms8530 in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen YY, Chou PC, Pham L, Su CH, Chen J, Hsieh YC, Xue Y-W, Qu C-J, Gully C, Parreno K, et al.. DNA damage-mediated c-Myc degradation requires 14-3-3 sigma. Cancer Hallmarks 2013; 1:3-17; http://dx.doi.org/ 10.1166/ch.2013.1002 [DOI] [Google Scholar]
- 55.Gully CP, Velazquez-Torres G, Shin JH, Fuentes-Mattei E, Wang E, Carlock C, Chen J, Rothenberg D, Adams HP, Choi HH, et al.. Aurora B kinase phosphorylates and instigates degradation of p53. Proc Natl Acad Sci U S A 2012; 109:E1513-22; PMID:22611192; http://dx.doi.org/ 10.1073/pnas.1110287109 [DOI] [PMC free article] [PubMed] [Google Scholar]