Abstract
BACKGROUND
The incidence, severity, and duration of postoperative oxygen desaturation in the general surgical population are poorly characterized. We therefore used continuous pulse oximetry to quantify arterial oxygen saturation (SpO2) in a cross-section of patients having noncardiac surgery.
METHODS
Oxygen saturation, blinded to clinicians, was recorded at 1-minute intervals in patients >45 years old for up to 48 hours after noncardiac surgery in 1250 patients from Cleveland Clinic Main Campus and 250 patients from the Juravinski Hospital. We determined (1) the cumulative minutes of raw minute-by-minute values below various hypoxemic thresholds; and (2) the contiguous duration of kernel-smoothed (sliding window) values below various hypoxemic thresholds. Finally, we compared our blinded continuous values with saturations recorded during routine nursing care.
RESULTS
Eight hundred thirty-three patients had sufficient data for analyses. Twenty-one percent had ≥10 min/h with raw SpO2 values <90% averaged over the entire recording duration; 8% averaged ≥20 min/h <90%; and 8% averaged ≥5 min/h <85%. Prolonged hypoxemic episodes were common, with 37% of patients having at least 1 (smoothed) SpO2 <90% for an hour or more; 11% experienced at least 1 episode lasting ≥6 hours; and 3% had saturations <80% for at least 30 minutes. Clinical hypoxemia, according to nursing records, measured only in Cleveland Clinic patients (n = 594), occurred in 5% of the monitored patients. The nurses missed 90% of smoothed hypoxemic episodes in which saturation was <90% for at least one hour.
CONCLUSIONS
Hypoxemia was common and prolonged in hospitalized patients recovering from noncardiac surgery. The SpO2 values recorded in medical records seriously underestimated the severity of postoperative hypoxemia.
Respiratory arrests, especially in unmonitored settings, are among the most serious complications after noncardiac surgery.1 Respiratory arrests are difficult to study2 because they are fortunately rare. It is likely, however, that respiratory arrests are preceded by respiratory insufficiency3 as might be indicated by reduced oxyhemoglobin saturation (SpO2).4 Even if hypoxemia does not lead to respiratory arrest, it is a strong indicator of patient instability, compromises wound healing,5–7 and promotes other serious complications, including brain dysfunction,8,9 dysrhythmias,10 and myocardial ischemia.11–13
In a typical postoperative ward, vital signs are recorded at 4- to 6-hour intervals.2 This process usually involves waking patients, recording blood pressure oscillometrically, determining oral temperature, and obtaining a “spot” oxygen saturation measurement with a pulse oximeter. The difficulty is that many patients who breathe inadequately at rest or during sleep may present normal or near-normal oxygen saturation after they are awakened. Moreover, in some cases, nurses respond to poor saturation values by encouraging patients to breathe deeply until a near-normal value is obtained, with that value being the one recorded in the medical record.14,15 The consequence is that oxygen saturation values recorded in medical records may seriously underestimate the severity of postoperative hypoxemia; furthermore, recording at approximately 4- to 6-hour intervals precludes determining the duration of hypoxemic events.
To accurately determine the incidence and severity of postoperative hypoxemia, it is necessary to continuously record saturation while blinding clinicians to the values. Using this approach in a prospective cohort study of non-cardiac surgical patients, we tested the hypotheses that (1) desaturation after inpatient noncardiac surgery is common and often prolonged; and (2) oxygen saturation recorded during routine nursing care underestimates the magnitude and duration of postoperative desaturation.
METHODS
We analyzed oximetry data collected from a subgroup of patients enrolled in the study of Vascular events In Surgery patIents cOhort evaluatioN (VISION), a 40,000-patient prospective cohort study that enrolled a representative sample of adults undergoing noncardiac surgery and focused on vascular complications (NCT00512109). We published results of the first 15,000 patients included in the VISION study16,17; however, none of the patients reported here has been previously described. This study was conducted at the Cleveland Clinic Main Campus, Cleveland, Ohio, and Juravinski Hospital of the Hamilton Health Sciences, Hamilton, Ontario. This study was observational, and routine care was not influenced.
With IRB approval from each institution and written informed patient consent, we enrolled 1250 patients at the Cleveland Clinic and 250 patients at the Juravinski Hospital, all at least 45 years of age who were scheduled for noncardiac inpatient surgery with general and/or regional anesthesia. We deliberately selected a cross-section of elective and nonelective noncardiac surgical patients, ensuring that enrollment for each surgical service was approximately proportionate to their contribution to the overall surgical load. We excluded patients not expected to stay at least 1 night in the hospital, who received only local or topical anesthesia, or who had previously participated in the VISION study.
Measurements
Patient demographic and morphometric characteristics were recorded, along with comorbidities and surgical and anesthetic details. We extracted routine nurse-recorded SpO2, typically at 4- to 6-hour intervals, for the 1250 Cleveland Clinic patients, but these data were not available for the 250 Juravinski Hospital patients.
SpO2 was continuously monitored with a pulse oximeter (Nellcor OxiMax N-600x, Covidien, Dublin, Ireland). We started monitoring patients upon arrival on a nursing floor or step-down unit after discharge from the postanesthesia care unit or intensive care unit (ICU). Patients who remained in the ICU for >72 hours were excluded from monitoring. Monitoring continued while patients remained hospitalized for up to 48 hours or the morning thereafter.
The pulse oximeter was mounted on a wheeled rack that included an oscillometric blood pressure monitor, computer, and battery; total weight of the system was about 33 kg. Patients were encouraged to remain connected to the monitor but were allowed to disconnect during transport, attending to personal hygiene, or receiving interventions such as imaging or dialysis. Study personnel visited each patient at least twice daily, including nights and weekends, to promote compliance with continuous SpO2 monitoring.
Continuous pulse oximetry data were not displayed or available to caregivers who monitored routine vital signs per routine. Study data were recorded internally and subsequently transferred to a secure database. Pulse oximeter waveforms were recorded but were averaged in 1-minute increments for analysis. Values <60% were discarded as artifact (only 0.09% of all values). We excluded patients who had <12 hours of continuous monitoring, gaps in monitor records exceeding 4 hours, or overall unrecorded time (cumulative duration of gaps in monitor records lasting at least 30 minutes) comprising >30% of total monitoring time.
Data Analysis
Pulse oximetry data are difficult to analyze because of the high degree of variability in saturation that occurs within each patient. Patients might, for example, experience frequent, short episodes of more severe hypoxemia; but in others, average SpO2 may linger only slightly below potentially harmful SpO2 thresholds for hours. We therefore considered both raw and smoothed (i.e., filtered) SpO2 data.
For the raw data, we conducted 2 analyses. First, to assess the overall exposure to hypoxemia for each patient, we summarized the distribution of hypoxemic minutes per hour of monitoring using the incidence curves. Various thresholds defining hypoxemia were used to generate the incidence curves. Second, we used quantile regression18 to characterize the median, quartiles, 10th and 90th percentiles, and 5th and 95th percentiles of the distribution of SpO2 across patients as a function of postoperative time. Nonlinearities in these quantile curves were allowed by incorporating restricted cubic splines in the quantile regression models.
For the analyses of filtered SpO2 data, we smoothed each individual patient's SpO2-versus-time profile using a Gaussian kernel algorithm (i.e., the smoothed estimate of SpO2 for a specific time point was a weighted average of the surrounding SpO2 values, where the weights were determined according to a Gaussian distribution centered at that time point with an interquartile range of 3 hours). Using the smoothed profiles, we then estimated the incidence of hypoxemic episodes of varying duration under a range of SpO2 thresholds characterizing hypoxemia, along with 95% confidence intervals based on normal approximation theory.
For the comparison of oxygen saturation between our data and nurse-recorded data, we used only Cleveland Clinic patients because the nursing flow sheet record was readily available in electronic form for those patients. Analyses of the nursing records were restricted to the period of continuous SpO2 monitoring. The number of measurements made and the incidence of hypoxemia (SpO2 < 90%) were calculated using nursing records. Among the remaining patients (for whom no observed hypoxemia occurred according to the nurses’ records), we calculated the incidence of contiguous SpO2 <90% and 85% lasting 1 hour and 2 hours using the smoothing algorithm previously described.
All data analysis was performed using R statistical software version 3.0.0 for 64-bit Unix operating system (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Figure 1 shows the enrollment, exclusions, and patients available for analysis among 1500 consenting patients. The most common reason for disconnections appeared to be that patients simply declined to continue with the study and remain tethered to the monitoring equipment, especially because they felt better and became more mobile.
Figure 1.
Study diagram, showing enrollment, exclusions, and patients available for analysis. VISION = Vascular events In Surgery patIents cOhort evaluatioN; ICU = intensive care unit; PACU = post-anesthesia care unit; DNR = do not resuscitate.
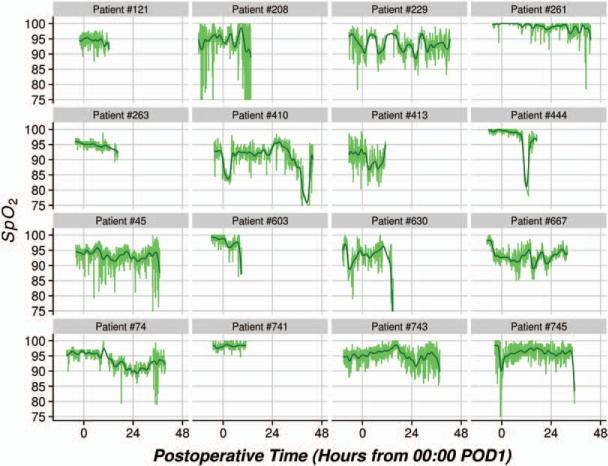
Eight hundred thirty-three patients with adequate SpO2 data were analyzed. Demographic and morphometric characteristics, comorbidities, as well as surgical and anesthetic details are presented in Table 1. Figure 2 displays samples of 16 individual patient's SpO2-versus-time profiles, along with overlaid smooth curves obtained from the Gaussian kernel estimator. The figure indicates that hypoxemic episodes as expressed by the raw data were frequent, whereas general trends expressed by the smoothed curves reveal the occurrence of prolonged episodes of generally low SpO2 values for some patients.
Table 1.
Summary of Baseline and Intraoperative Characteristics
| Statistics (n = 831) | |
|---|---|
| Center | |
| Hamilton | 22% |
| Cleveland Clinic | 78% |
| Age (y) | 64 ± 10 |
| Female sex | 46% |
| Weight (kg) | 86 [73, 98] |
| Height (cm) | 170 [163, 178] |
| History of DVT/PE | 7% |
| Current atrial fibrillation | 3% |
| History of atrial fibrillation | 6% |
| History of CVA | 4% |
| History of CAD | 16% |
| Recent high-risk CAD | 1% |
| History of CHF | 4% |
| Cardiocerebral resuscitation (<1 y) | 3% |
| Cardiocerebral resuscitation (>1 y) | 14% |
| Aortic stenosis | 2% |
| PVD | 6% |
| History of diabetes | 19% |
| History of peptic ulcer | 3% |
| History of tobacco use | 55% |
| Sleep apnea | 16% |
| History of HTN | 61% |
| History of COPD | 8% |
| Dialysis | 0% |
| History of chronic pain | 20% |
| Active cancer | 22% |
| Hemoglobin (g/dL) | 14 [12, 15] |
| Creatinine (mg/dL) | 0.9 [0.7, 1.0] |
| Systolic blood pressure (mm Hg) | 142 ± 24 |
| Diastolic blood pressure (mm Hg) | 76 ± 12 |
| Heart rate (bpm) | 75 ± 13 |
| Stomach surgery | 9% |
| Intraabdominal surgery | 14% |
| (Complex) Visceral resection | 11% |
| Radical prostatectomy | 4% |
| Major hip/pelvic surgery | 13% |
| Knee arthroplasty | 18% |
| Craniotomy | 3% |
| Major spine surgery | 14% |
| Minimally invasive surgery | 3% |
| Other type of surgery | 10% |
| Endoscopic approach | 22% |
| Open approach | 81% |
| General anesthesia | 76% |
| Spinal anesthesia | 23% |
| Epidural anesthesia | 10% |
| Nerve block | 7% |
| Nitrous oxide administration | 7% |
These data were unavailable for 2 patients of the 833 analyzed. Results presented as a percentage, means ± standard deviations, or medians (first quartile, third quartile).
CAD = coronary artery disease; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; DVT = deep vein thrombosis; HTN = hypertension; PE = pulmonary embolism; PVD = peripheral vascular disease.
Figure 2.
Sample of 16 patients’ raw SpO2 data versus postoperative time (green lines), along with overlaid kernel smooth estimates depicting general trends (black lines). POD = postoperative day.
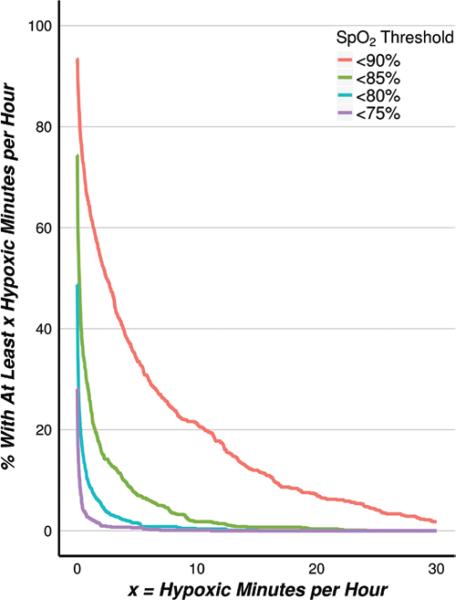
Twenty-one percent (95% confidence interval, 18%–24%] of patients averaged at least 10 minutes per hour with raw SpO2 values <90%, and 8% (6%–10%) of patients averaged at least 20 minutes per hour. Likewise, approximately 8% (6%–10%) of patients averaged at least 5 minutes per hour with raw SpO2 <85% (Fig. 3).
Figure 3.
(Raw SpO2 data) Incidence of patients with an average number of minutes per hour in hypoxemia > X during monitoring, according to progressive SpO2 thresholds characterizing hypoxemia.
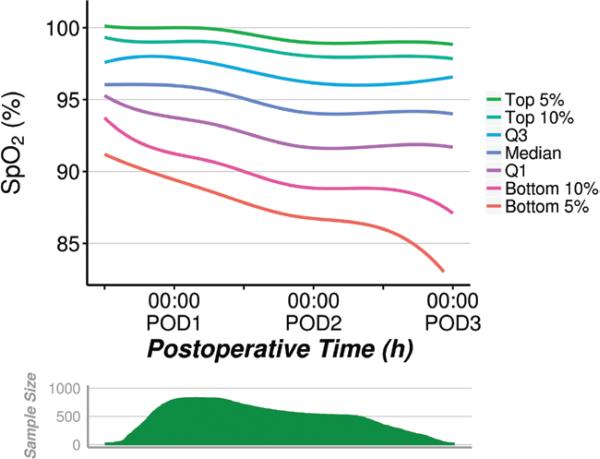
The distribution of SpO2 as a function of postoperative time is displayed in Figure 4. Generally, measured SpO2 values decreased as postoperative time increased, although this corresponded with a decline in sample size as patients were discharged.
Figure 4.
(Raw SpO2 data) Distribution of SpO2 across the patients in the sample, over postoperative time. Curves estimated using quantile regression with restricted cubic splines. POD = postoperative day.
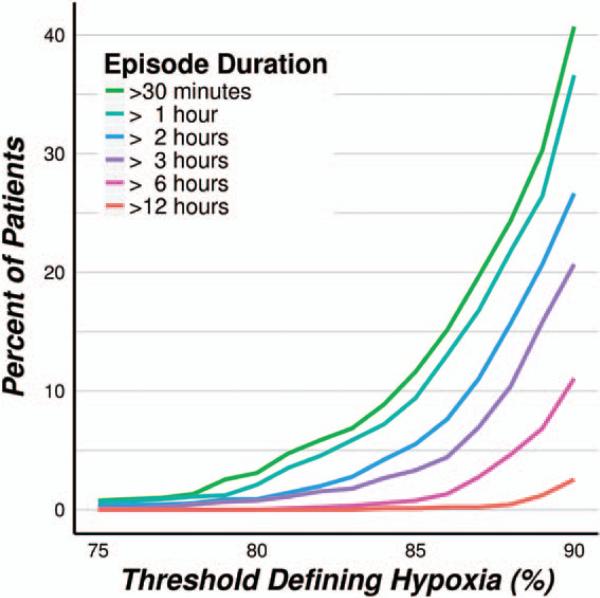
Analysis of the smoothed SpO2 profiles revealed that prolonged hypoxemic episodes were common, according to a threshold of SpO2 <90%, 37% (34%–40%) of patients had at least 1 episode lasting an hour or more, while 11% (9%–13%) experienced at least 1 episode lasting 6 hours or more. Severely hypoxemic episodes, those <80% for at least 30 minutes, were observed in 3% (2%–4%) of patients (Fig. 5).
Figure 5.
(Smoothed SpO2 data) Incidence of at least 1 single hypoxic episode of varying minimal duration under progressive SpO2 thresholds characterizing hypoxemia.
Among the 646 Cleveland Clinic patients included in the final analysis (78% of the total analyzed sample of 833 patients), 630 had available SpO2 measurements from the nursing flow sheet recorded from >10 noncardiac surgical specialty wards. Among these, 594 had nurse-recorded SpO2 observations occurring within the continuous monitoring study period. The median [Q1, Q3] number of observations made from the nurses during the continuous monitoring study period was 9 [6, 11], corresponding to a median [Q1, Q3] of 5.6 [5.0, 6.2] measurements per 24 hours.
Only 30 of 594 (5%) patients had any hypoxemia (SpO2 <90%) according to the nursing flow sheets. Among the remaining 564 patients with no clinical record of hypoxemia, 214 (38%) had at least 1 contiguous episode of smoothed SpO2 <90% lasting >1 hour and 154 (27%) had at least 1 episode of SpO2 <90% lasting >2 hours (according to the smoothed monitor data). The nurses missed 90% (214 of 237, 95% confidence interval 86%–94%) of the hypoxemic events during which smoothed saturation was <90% for at least 1 hour.
Of the 564 patients without nurse-recorded hypoxemia, 68 (12%) had at least 1 episode of SpO2 <90% lasting >6 hours. Using a more severe criterion of SpO2 <85%, 54 patients (10%) had at least 1 episode lasting >1 hour and 34 (6%) had at least 1 episode lasting >2 hours, and 6 (1%) had at least 1 episode lasting >6 hours.
Data on postoperative oxygen and postoperative continuous positive airway pressure/bilevel positive airway pressure were unavailable for 2 patients of the 833 included in the analysis. Five hundred fifty-two of these patients (66% of the analyzed sample) received supplemental oxygen (either nasal or mask) at some time on the first postoperative day, 286 (34%) received oxygen at some time on the second postoperative day, and 162 (19%) received oxygen at some time on the third postoperative day. Continuous positive airway pressure/bilevel positive airway pressure was given to 42 patients (5.0% of the analyzed sample) at some time on the first postoperative day, 39 patients (4.7%) on the second postoperative day, and 34 patients (4.1%) on the third postoperative day.
DISCUSSION
The prevalence of postoperative hypoxemia is often underestimated. In our study, we deliberately assessed postoperative hypoxemia in a cross-section of noncardiac surgical patients. Our methodology stands in contrast to many previous studies that targeted at-risk populations such as patients with obstructive sleep apnea, patients recovering from cardiothoracic operations,19 or patients given standardized postoperative opioid treatments including patient-controlled analgesia and continuous epidural infusion.20–22 Hypoxemia was common in our patients. Twenty-one percent had ≥10 min/h with raw SpO2 values <90% averaged over the entire recording duration; 8% averaged ≥20 min/h <90%; and 8% averaged ≥5 min/h <85%. Prolonged hypoxemic episodes were common, with 37% of patients having at least 1 (smoothed) SpO2 <90% for an hour or more; 11% experienced at least 1 episode lasting ≥6 hours; and 3% had saturations <80% for at least 30 minutes. Postoperative patients, even those without specific risk factors, thus experience far more hypoxemia than is generally appreciated.
A critical distinction between our report and published reports is that previous studies were unblinded. Consequently, initial desaturation in previous studies presumably triggered interventions by unblinded clinicians in an effort to ameliorate desaturation and reduce subsequent hypoxemia. In contrast, the saturations we recorded were unavailable to the clinical team and therefore could not trigger interventions. The frequent incidence and substantial degree of desaturation we report better reflects the true severity of hypoxemic exposure in patients not continuously monitored with SpO2 after noncardiac surgery.
In striking contrast, the nursing records, which were generally recorded at 4-hour intervals, showed little hypoxemia. Only 5% of patients had even a single saturation <90% according to the nursing record. Among the remaining 95%, continuous episodes of hypoxemia lasting ≥2 hours were common. In an extreme case, 1 patient had 2026 total monitored minutes with SpO2 <90%, an average of 36 min/h of monitoring, while the lowest SpO2 value among the 12 measurements in the nursing flow sheet was 92%.
Taenzer et al.23 similarly demonstrated that manually charted oxygen saturations were, on average, 6.5% (95% confidence interval, 4.0%–9.0%) higher than those recorded electronically. They postulated that inaccurate recording was the primary cause rather than the arousal consequent to a nurse visit. On our surgical wards, vital signs (heart rate, oxygen saturation, arterial blood pressure, and temperature) are typically evaluated every 4 hours by nurse assistants using a process that deliberately arouses patients. We suspect that patient arousal truly increases SpO2 measurements but that the arousal-induced increases are unsustained once the stimulus is removed. However, for whatever reason, ventilation was often suboptimal during the long unmonitored intervals.
Continuous monitoring proved challenging with the equipment we used that was mounted on an IV pole and had a total weight near 33 kg. Consequently, patients often disconnected the system when they felt well enough to ambulate or attend to their personal hygiene. In addition, some patients became frustrated with the number of attached monitors and lines and subsequently chose to discontinue our monitoring. Modern battery-powered, wrist-mounted monitors that communicate wirelessly with hospital systems have the potential to eliminate these problems.
Monitoring was presumably most often discontinued by patients who were relatively healthy and ambulatory and presumably also less likely to experience much hypoxemia. To the extent that monitoring was nonrandomly discontinued, the desaturation we report may overestimate exposure in the general inpatient postoperative population. Stone et al.24 reported substantial nocturnal hypoxemia in half of the patients using patient-controlled analgesia while breathing room air, a result that is generally consistent with our finding of substantial hypoxemia.
Patients who had smaller operations and recovered well were discharged earlier than sicker ones. For example, by the second postoperative day, only 62% remained hospitalized. Attrition bias is thus the presumed explanation for saturations decreasing over time. In other words, saturations (as a percentile of the remaining population) likely worsened over time because the healthier patients either discontinued monitoring or were discharged rather than because individual patients worsened over time.
Supplemental oxygen, which was overwhelmingly used during the initial postoperative hours, but less on subsequent days, may have contributed as well by maintaining early saturation. Lack of more precise data on the use of supplemental oxygen and its impact on preventing desaturations create uncertainties interpreting these data. Another limitation of the study is that preoperative SpO2 was not recorded; we thus do not know what fraction of the observed postoperative hypoxemia might have been routine in our population. Furthermore, we summarized the morbidity as baseline for enrolled patients. But we did not correlate it with hypoxemia events individually. This uncertainty limits determining what may be causal of desaturation or worse outcomes.
Our study was not powered for major respiratory complications nor did we attempt to associate hypoxemia with other adverse outcomes. However, hypoxemia is widely thought to be harmful independent of major respiratory complications. For example, respiratory compromise is a more common cause of postoperative admission and read-mission to critical care units than cardiac dysrhythmias.25,26 Saturation monitoring clearly facilitates diagnosis of hypoxemia,27 and treatment enhances saturation.28
In a randomized unblinded trial in 1219 patients, Ochroch et al.29 found that continuous pulse oximeter monitoring in patients recovering from cardiac and thoracic surgery reduced neither critical care unit readmission nor mortality but did reduce the number of patients transferred to a critical care unit for pulmonary reasons.29 That study had substantial methodologic limitations including questionable concealment of randomization that led to a misbalance in randomization of 589 patients in the pulse oximeter monitoring group and 630 patients in the normal care group. Furthermore, the study was conducted at 2 large tertiary care facilities, and patients treated at these facilities may not be representative of the broader surgical inpatient population.
A similar phenomenon also was observed in orthopedic patients in whom surveillance monitoring (pulse oximetry with nursing notification for violation of alarm limits via wireless pager) reduced the need for rescue intervention and ICU transfers.30 Whether preventing hypoxemia improves patient outcomes remains unclear. Supplemental oxygen may reduce the severity of the hypoxemia.24 It remains possible that hypoxemia is largely an indicator of underlying disease rather than a treatable mechanism; however, there are thresholds of hypoxemia that all clinicians believe warrant treatment (e.g., SpO2 <85%). Whether saturation monitoring and consequent clinical interventions, such as prolongation of supplemental oxygen, improve patient outcomes can only be determined by large, randomized trials.
In summary, hypoxemia was common and prolonged in patients recovering from noncardiac surgery. The SpO2 values recorded in medical records markedly underestimated the incidence and severity of postoperative hypoxemia.
Acknowledgments
Funding: Funded by Covidien (Dublin, Ireland). None of the authors has any personal financial interest in this research.
Footnotes
The authors declare no conflicts of interests.
DISCLOSURES
Name: Zhuo Sun, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Zhuo Sun has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Daniel I. Sessler, MD.
Contribution: This author helped design the study, conduct the study, and write the manuscript.
Attestation: Daniel I. Sessler reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Jarrod E. Dalton, PhD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Jarrod E. Dalton has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: PJ Devereaux, MD, PhD.
Contribution: This author helped write the manuscript.
Attestation: PJ Devereaux approved the final manuscript.
Name: Aram Shahinyan, MD.
Contribution: This author helped conduct the study and write the manuscript.
Attestation: Aram Shahinyan has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Amanda J. Naylor, BA.
Contribution: This author helped conduct the study and write the manuscript.
Attestation: Amanda J. Naylor reviewed the analysis of the data and approved the final manuscript.
Name: Matthew T. Hutcherson, BS.
Contribution: This author helped conduct the study and write the manuscript.
Attestation: Matthew T. Hutcherson reviewed the analysis of the data and approved the final manuscript.
Name: Patrick S. Finnegan, BA, NREMT-B.
Contribution: This author helped conduct the study.
Attestation: Patrick S. Finnegan has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Vikas Tandon, MD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Attestation: Vikas Tandon approved the final manuscript.
Name: Saeed Darvish-Kazem, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Saeed Darvish-Kazem has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Shaan Chugh, MD.
Contribution: This author helped write the manuscript.
Attestation: Shaan Chugh has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Hussain Alzayer, BSc, MD.
Contribution: This author helped write the manuscript and is a chart reviewer.
Attestation: Hussain Alzayer reviewed the analysis of the data and approved the final manuscript.
Name: Andrea Kurz, MD.
Contribution: This author helped design the study, conduct the study, and write the manuscript.
Attestation: Andrea Kurz has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
This manuscript was handled by: Sorin Brull, MD.
REFERENCES
- 1.Hodari A, Tsiouris A, Eichenhorn M, Horst M, Rubinfeld I. Exploring National Surgical Quality Improvement Program respiratory comorbidities: developing a predictive understanding of postoperative respiratory occurrences, Clavien 4 complications, and death. J Surg Res. 2013;183:663–7. doi: 10.1016/j.jss.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Leuvan CH, Mitchell I. Missed opportunities? An observational study of vital sign measurements. Crit Care Resusc. 2008;10:111–15. [PubMed] [Google Scholar]
- 3.Brindley PG, Markland DM, Mayers I, Kutsogiannis DJ. Predictors of survival following in-hospital adult cardiopulmo-nary resuscitation. CMAJ. 2002;167:343–8. [PMC free article] [PubMed] [Google Scholar]
- 4.MacIntyre NR. Supporting oxygenation in acute respiratory failure. Respir Care. 2013;58:142–50. doi: 10.4187/respcare.02087. [DOI] [PubMed] [Google Scholar]
- 5.Abdelmalak BB, Cata JP, Bonilla A, You J, Kopyeva T, Vogel JD, Campbell S, Sessler DI. Intraoperative tissue oxygenation and postoperative outcomes after major non-cardiac surgery: an observational study. Br J Anaesth. 2013;110:241–9. doi: 10.1093/bja/aes378. [DOI] [PubMed] [Google Scholar]
- 6.Govinda R, Kasuya Y, Bala E, Mahboobi R, Devarajan J, Sessler DI, Akça O. Early postoperative subcutaneous tissue oxygen predicts surgical site infection. Anesth Analg. 2010;111:946–52. doi: 10.1213/ANE.0b013e3181e80a94. [DOI] [PubMed] [Google Scholar]
- 7.Greif R, Akça O, Horn EP, Kurz A, Sessler DI. Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161–7. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg J, Kehlet H. Postoperative mental confusion—association with postoperative hypoxemia. Surgery. 1993;114:76–81. [PubMed] [Google Scholar]
- 9.Aakerlund LP, Rosenberg J. Postoperative delirium: treatment with supplementary oxygen. Br J Anaesth. 1994;72:286–90. doi: 10.1093/bja/72.3.286. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg-Adamsen S, Lie C, Bernhard A, Kehlet H, Rosenberg J. Effect of oxygen treatment on heart rate after abdominal surgery. Anesthesiology. 1999;90:380–4. doi: 10.1097/00000542-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg J, Rasmussen V, von Jessen F, Ullstad T, Kehlet H. Late postoperative episodic and constant hypoxaemia and associated ECG abnormalities. Br J Anaesth. 1990;65:684–91. doi: 10.1093/bja/65.5.684. [DOI] [PubMed] [Google Scholar]
- 12.Goldman MD, Reeder MK, Muir AD, Loh L, Young JD, Gitlin DA, Casey KR, Smart D, Fry JM. Repetitive nocturnal arterial oxygen desaturation and silent myocardial ischemia in patients presenting for vascular surgery. J Am Geriatr Soc. 1993;41:703–9. doi: 10.1111/j.1532-5415.1993.tb07457.x. [DOI] [PubMed] [Google Scholar]
- 13.Gill NP, Wright B, Reilly CS. Relationship between hypoxaemic and cardiac ischaemic events in the perioperative period. Br J Anaesth. 1992;68:471–3. doi: 10.1093/bja/68.5.471. [DOI] [PubMed] [Google Scholar]
- 14.Duff B, Gardiner G, Barnes M. The impact of surgical ward nurses practising respiratory assessment on positive patient outcomes. Aust J Adv Nurs. 2007;24:52–6. [PubMed] [Google Scholar]
- 15.McGain F, Cretikos MA, Jones D, Van Dyk S, Buist MD, Opdam H, Pellegrino V, Robertson MS, Bellomo R. Documentation of clinical review and vital signs after major surgery. Med J Aust. 2008;189:380–3. doi: 10.5694/j.1326-5377.2008.tb02083.x. [DOI] [PubMed] [Google Scholar]
- 16.Botto F, Alonso-Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, Guyatt G, Cruz P, Graham M, Wang CY, Berwanger O, Pearse RM, Biccard BM, Abraham V, Malaga G, Hillis GS, Rodseth RN, Cook D, Polanczyk CA, Szczeklik W, Sessler DI, Sheth T, Ackland GL, Leuwer M, Garg AX, Lemanach Y, Pettit S, Heels-Ansdell D, Luratibuse G, Walsh M, Sapsford R, Schünemann HJ, Kurz A, Thomas S, Mrkobrada M, Thabane L, Gerstein H, Paniagua P, Nagele P, Raina P, Yusuf S, Devereaux PJ, Devereaux PJ, Sessler DI, Walsh M, Guyatt G, McQueen MJ, Bhandari M, Cook D, Bosch J, Buckley N, Yusuf S, Chow CK, Hillis GS, Halliwell R, Li S, Lee VW, Mooney J, Polanczyk CA, Furtado MV, Berwanger O, Suzumura E, Santucci E, Leite K, Santo JA, Jardim CA, Cavalcanti AB, Guimaraes HP, Jacka MJ, Graham M, McAlister F, McMurtry S, Townsend D, Pannu N, Bagshaw S, Bessissow A, Bhandari M, Duceppe E, Eikelboom J, Ganame J, Hankinson J, Hill S, Jolly S, Lamy A, Ling E, Magloire P, Pare G, Reddy D, Szalay D, Tittley J, Weitz J, Whitlock R, Darvish-Kazim S, Debeer J, Kavsak P, Kearon C, Mizera R, O'Donnell M, McQueen M, Pinthus J, Ribas S, Simunovic M, Tandon V, Vanhelder T, Winemaker M, Gerstein H, McDonald S, O'Bryne P, Patel A, Paul J, Punthakee Z, Raymer K, Salehian O, Spencer F, Walter S, Worster A, Adili A, Clase C, Cook D, Crowther M, Douketis J, Gangji A, Jackson P, Lim W, Lovrics P, Mazzadi S, Orovan W, Rudkowski J, Soth M, Tiboni M, Acedillo R, Garg A, Hildebrand A, Lam N, Macneil D, Mrkobrada M, Roshanov PS, Srinathan SK, Ramsey C, John PS, Thorlacius L, Siddiqui FS, Grocott HP, McKay A, Lee TW, Amadeo R, Funk D, McDonald H, Zacharias J, Villar JC, Cortés OL, Chaparro MS, Vásquez S, Castañeda A, Ferreira S, Coriat P, Monneret D, Goarin JP, Esteve CI, Royer C, Daas G, Chan MT, Choi GY, Gin T, Lit LC, Xavier D, Sigamani A, Faruqui A, Dhanpal R, Almeida S, Cherian J, Furruqh S, Abraham V, Afzal L, George P, Mala S, Schünemann H, Muti P, Vizza E, Wang CY, Ong GS, Mansor M, Tan AS, Shariffuddin II, Vasanthan V, Hashim NH, Undok AW, Ki U, Lai HY, Ahmad WA, Razack AH, Malaga G, Valderrama-Victoria V, Loza-Herrera JD, De Los Angeles Lazo M, Rotta-Rotta A, Szczeklik W, Sokolowska B, Musial J, Gorka J, Iwaszczuk P, Kozka M, Chwala M, Raczek M, Mrowiecki T, Kaczmarek B, Biccard B, Cassimjee H, Gopalan D, Kisten T, Mugabi A, Naidoo P, Naidoo R, Rodseth R, Skinner D, Torborg A, Paniagua P, Urrutia G, Maestre ML, Santaló M, Gonzalez R, Font A, Martínez C, Pelaez X, De Antonio M, Villamor JM, García JA, Ferré MJ, Popova E, Alonso-Coello P, Garutti I, Cruz P, Fernández C, Palencia M, Díaz S, Del Castillo T, Varela A, de Miguel A, Muñoz M, Piñeiro P, Cusati G, Del Barrio M, Membrillo MJ, Orozco D, Reyes F, Sapsford RJ, Barth J, Scott J, Hall A, Howell S, Lobley M, Woods J, Howard S, Fletcher J, Dewhirst N, Williams C, Rushton A, Welters I, Leuwer M, Pearse R, Ackland G, Khan A, Niebrzegowska E, Benton S, Wragg A, Archbold A, Smith A, McAlees E, Ramballi C, Macdonald N, Januszewska M, Stephens R, Reyes A, Paredes LG, Sultan P, Cain D, Whittle J, Del Arroyo AG, Sessler DI, Kurz A, Sun Z, Finnegan PS, Egan C, Honar H, Shahinyan A, Panjasawatwong K, Fu AY, Wang S, Reineks E, Nagele P, Blood J, Kalin M, Gibson D, Wildes T, Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Writing Group, on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators; Appendix 1. The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Study Investigators Writing Group; Appendix 2. The Vascular events In noncardiac Surgery patIents cOhort evaluatioN Operations Committee; Vascular events In non-cardiac Surgery patIents cOhort evaluatioN VISION Study Investigators Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564–78. doi: 10.1097/ALN.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 17.Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, Srinathan S, Biccard BM, Chow CK, Abraham V, Tiboni M, Pettit S, Szczeklik W, Lurati Buse G, Botto F, Guyatt G, Heels-Ansdell D, Sessler DI, Thorlund K, Garg AX, Mrkobrada M, Thomas S, Rodseth RN, Pearse RM, Thabane L, McQueen MJ, VanHelder T, Bhandari M, Bosch J, Kurz A, Polanczyk C, Malaga G, Nagele P, Le Manach Y, Leuwer M, Yusuf S. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 18.Koenker R, Bassett G., Jr Regression quantiles. Econometrica. 1978;46:33–50. [Google Scholar]
- 19.Bierman MI, Stein KL, Snyder JV. Pulse oximetry in the postoperative care of cardiac surgical patients. A randomized controlled trail. Chest. 1992;102:1367–70. doi: 10.1378/chest.102.5.1367. [DOI] [PubMed] [Google Scholar]
- 20.Gögenur I, Rosenberg-Adamsen S, Lie C, Carstensen M, Rasmussen V, Rosenberg J. Relationship between nocturnal hypoxaemia, tachycardia and myocardial ischaemia after major abdominal surgery. Br J Anaesth. 2004;93:333–8. doi: 10.1093/bja/aeh208. [DOI] [PubMed] [Google Scholar]
- 21.Overdyk FJ, Carter R, Maddox RR, Callura J, Herrin AE, Henriquez C. Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth Analg. 2007;105:412–8. doi: 10.1213/01.ane.0000269489.26048.63. [DOI] [PubMed] [Google Scholar]
- 22.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–23. doi: 10.1093/bja/aeh180. [DOI] [PubMed] [Google Scholar]
- 23.Taenzer AH, Pyke J, Herrick MD, Dodds TM, McGrath SP. A comparison of oxygen saturation data in inpatients with low oxygen saturation using automated continuous monitoring and intermittent manual data charting. Anesth Analg. 2014;118:326–31. doi: 10.1213/ANE.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 24.Stone JG, Cozine KA, Wald A. Nocturnal oxygenation during patient-controlled analgesia. Anesth Analg. 1999;89:104–10. doi: 10.1097/00000539-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg AL, Watts C. Patients readmitted to ICUs* : a systematic review of risk factors and outcomes. Chest. 2000;118:492–502. doi: 10.1378/chest.118.2.492. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg AL, Hofer TP, Hayward RA, Strachan C, Watts CM. Who bounces back? Physiologic and other predictors of intensive care unit readmission. Crit Care Med. 2001;29:511–8. doi: 10.1097/00003246-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Moller JT, Jensen PF, Johannessen NW, Espersen K. Hypoxaemia is reduced by pulse oximetry monitoring in the operating theatre and in the recovery room. Br J Anaesth. 1992;68:146–50. doi: 10.1093/bja/68.2.146. [DOI] [PubMed] [Google Scholar]
- 28.Canet J, Ricos M, Vidal F. Postanesthetic hypoxemia and oxygen administration. Anesthesiology. 1991;74:1161–2. doi: 10.1097/00000542-199106000-00039. [DOI] [PubMed] [Google Scholar]
- 29.Ochroch EA, Russell MW, Hanson WC III, Devine GA, Cucchiara AJ, Weiner MG, Schwartz SJ. The impact of continuous pulse oximetry monitoring on intensive care unit admissions from a postsurgical care floor. Anesth Analg. 2006;102:868–75. doi: 10.1213/01.ane.0000195583.76486.c4. [DOI] [PubMed] [Google Scholar]
- 30.Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010;112:282–7. doi: 10.1097/ALN.0b013e3181ca7a9b. [DOI] [PubMed] [Google Scholar]