Abstract
Objective
To evaluate the association between baseline olfaction and both cross-sectional and longitudinal cognitive assessments, motor symptoms, non-motor symptoms (NMS), and CSF biomarkers in early Parkinson's disease (PD).
Methods
Parkinson's Progression Marker's Initiative (PPMI) participants underwent baseline olfactory testing with the University of Pennsylvania Smell Identification Test (UPSIT). Serial assessments included measures of motor symptoms, NMS, neuropsychological assessment, and CSF biomarkers. Up to three years follow-up data were included.
Results
At baseline, worse olfaction (lowest tertile) was associated with more severe NMS, including anxiety and autonomic symptoms. Those in the lowest olfactory tertile were more likely to report cognitive impairment (37.4%) compared to those in the middle (24.4%) and highest olfactory tertiles (14.2%, p<0.001). Aβ1-42 was significantly lower, and tau/Aβ1-42 ratio was higher in those with worse olfaction. In longitudinal analyses, lower UPSIT score was associated with greater decline in MoCA score (β=0.02 [.01, 0.03], p=0.001) over time, as were composite measures of UPSIT score and either Aβ1-42 or tau/ Aβ1-42 ratio. In a Cox proportional hazards model, a composite measure of olfaction and Aβ1-42 was a significant predictor of conversion from normal cognition to mild cognitive impairment (MCI; i.e., MoCA<26), with subjects most impaired on both measures being 87% more likely to develop incident MCI (HR=1.87 [1.16, 3.01], p=0.01).
Conclusions
Worse baseline olfaction is associated with long-term cognitive decline. The addition of AD CSF biomarkers to olfactory testing may increase the likelihood of identifying those at highest risk for cognitive decline and progression to MCI.
Keywords: Parkinson's disease, Olfaction, Mild cognitive impairment, Non-motor symptoms, Cerebrospinal fluid
INTRODUCTION
Defining biomarkers for the diagnosis and prognosis of Parkinson's disease (PD) remains a major unmet need. Olfactory impairment is common in PD, with a prevalence ranging from 50-90% [1,2]. It is often one of the first manifestations of the disease [3], and pathologically, the olfactory bulb and lower brainstem are involved with synuclein pathology early on, with later spread through the rostral brainstem and eventually to the cerebral cortex [4]. These observations, along with the ease and low cost of assessment, make it an attractive biomarker.
Several studies have shed light on the relationship between olfaction and other non-motor symptoms (NMS) in PD, which are important causes of morbidity. Cross-sectional studies have shown associations between olfactory impairment and depression, anxiety, apathy, REM behavior sleep disorder (RBD) and autonomic symptoms [5-7]. General measures of cognition, including Mini-Mental Status Examination, were not associated with hyposmia in some studies [8], while others have demonstrated associations between olfaction and specific cognitive domains, including episodic verbal learning and verbal memory [9-12]. Worse baseline olfaction predicted self-reported cognitive impairment several years later in a retrospective cohort study [13], and a small longitudinal study identified severe hyposmia as an independent risk factor for development of dementia within 3 years [14].
While a marker of high sensitivity in PD, olfaction lacks specificity, and how it might be used in combination with other putative biomarkers is of interest. Cerebrospinal fluid biomarkers, including CSF tau (higher) and Aβ1-42 (lower) have been associated with cognitive impairment in PD and dementia with Lewy bodies (DLB) in cross sectional studies [15-16], and reduced CSF Aβ1-42 was an independent predictor of cognitive decline in two mixed stage PD cohorts [17-18].
In the present longitudinal, exploratory study, we aim to characterize olfaction in an early PD cohort, examine the association between baseline olfaction and measures of disease severity and other NMS, and determine if olfaction alone or in combination with CSF biomarkers predicts cognitive decline and conversion to mild cognitive impairment (MCI).
METHODS
Data used in this study came from the Parkinson's Progression Markers initiative (PPMI), a multicenter, observational cohort study following de novo (untreated at enrollment) PD patients and healthy controls. Participants underwent clinical assessments, imaging and blood and CSF collection at predetermined time points. The aims and methodology have been previously published [19]. Up-to-date information and further details on the study are available on-line (http://www.ppmi-info.org/). To be included in the PD arm, subjects had to meet clinical criteria for PD with a diagnosis within two years, confirmed by DAT imaging deficit. Enrollment was complete at the time of data acquisition and included a total of 423 patients with PD. Written informed consent was obtained from all study participants, and the study was approved by the institutional review board at each PPMI site.
Clinical Characteristics
Disease duration was calculated in months from the date of diagnosis. Education was reported in years by the study participant. Sub scores of the Movement Disorder Society – Unified Parkinson's Disease Rating Scale (MDS-UPDRS) were collected at each visit, including MDS-UPDRS part III to capture severity of motor symptoms [20].
Olfaction
Odor identification was assessed using the University of Pennsylvania Smell Identification Test (UPSIT) with lower scores reflecting poorer olfactory function [21]. Published normative values adjusted for age and sex were used to determine the percentile and olfactory classification for each subject. In order to group subjects into clinically meaningful categories, the PD patients were divided into olfactory tertiles based on raw UPSIT score. In doing so, the lowest olfactory tertile contained all participants classified as anosmic (based on published normative values adjusted for age and sex), while the middle tertile contained all participants with severe microsmia (figure e-2).
NMS assessments
Assessments of NMS included (i) the REM Sleep Behavior screening questionnaire, in which a positive screen was defined as a score >5 (consistent with possible RBD) [22], (ii) the Scales for Outcomes in Parkinson's Disease – Autonomic Questionnaire (SCOPA-AUT) to assess autonomic symptoms [23], (iii) the 15-item Geriatric Depression Scale (GDS-15) (cutoff ≥5 to indicate clinically significant depression) [24], and (iv) the State-Trait Anxiety Inventory (STAI; total of State and Trait subscales). Global cognition was assessed with the Montreal Cognitive Assessment (MoCA), and the recommended cutoff score of <26 was used to define MCI (PD-MCI level I category, abbreviated assessment) [25]. Question 1 on part 1 of the MDS-UPDRS was used to screen for patient reported cognitive impairment. Individual neuropsychological tests for specific domains have been previously described [26] and included the Benton Judgement of Line Orientation, Hopkins Verbal Learning Test-Revised delayed recall and recognition, Letter-Number Sequencing Test, Semantic Fluency and Symbol Digit Modalities Test.
CSF Biomarkers
CSF was collected at each study site as described in the PPMI biologics manual (http://www.ppmi-info.org/). Aβ1-42, total tau (t-tau) and phosphorylated tau (p-tau) were measured using the xMAP luminex platform (Luminex Corp) with INNO-BIA AlzBio3 immunoassay kit-based reagents (research use-only; Immunogenetics Inc) with a mean coefficient of variation below 10% between runs [27].
Statistical Analysis
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) statistics (version 22, SPSS, Inc., Chicago, IL). All statistical tests were two-sided. Statistical significance was set at p<0.05. Since our study was exploratory rather than confirmatory, multiple testing adjustment was not performed [28]. Normality assumptions were checked where appropriate. Demographic characteristics and baseline motor, non-motor and CSF biomarkers were compared using chi-square, one way ANOVA and analysis of linear trend or Kruskal-Wallis tests for non-normally distributed data where appropriate. Because age and sex differed among the three olfactory tertiles, linear and logistic regressions were used to control for age and sex when comparing baseline clinical characteristics. Age and sex were also controlled for in all longitudinal analyses. Education was included as a covariate for analyses involving cognition. Longitudinal analysis over a three year follow-up period was performed using linear mixed effects models, which accounts for correlations among repeated measures and for missing data (due to differing durations of follow up) [29]. Variables of interest based on published data, biologic plausibility, and/or identified as being significantly associated with olfaction at baseline were included in linear mixed effects models. Subject-specific random intercepts were used to account for the correlation between repeated measures. Fixed-effects in each mixed-effects model were UPSIT score, age, sex, time and baseline test score. Education was also included for the models involving the MoCA and neuropsychiatric tests. The relationship between olfaction and CSF biomarkers and decline in MoCA was first analyzed using the UPSIT and CSF biomarkers as continuous variables. There was no association between p-tau and olfaction or any of the cognitive endpoints, so it was not included in further analyses. Since CSF biomarkers Aβ1-42 and T-Tau/ Aβ1-42 and olfaction were independently associated with decline in MoCA, we created a composite score as a way to classify risk for decline in MoCA based on a combination of olfactory status and CSF values. Because olfaction was divided into tertiles, we also divided the CSF biomarkers into tertiles. Those in the lowest olfactory tertile had a higher risk of cognitive decline than the middle and highest olfactory tertile and those in the lowest Aβ1-42 (or highest t-tau/Aβ1-42 ratio) had a higher risk for cognitive decline than the middle and highest tertile. Therefore, those who were considered at higher risk in both groups were combined in a composite measure as follows, to determine if the addition of CSF biomarkers to olfactory testing would improve the predictive value:
-
(i)
Abeta-Group 1=lower olfaction tertiles + lower Aβ1-42 tertiles
-
(ii)
Abeta-Group 2=middle olfaction tertiles + middle Aβ1-42 tertiles and
-
(iii)
Abeta-Group 3=better olfaction tertiles + higher Aβ1-42 tertiles
The dependent variable for these mixed effects models was MoCA score, and the covariates included age, sex, time (years), education and baseline MoCA score. For each model, the coefficient (β) represents the difference in annual rate of change in the MoCA for each 1 point increase in the parameter (dependent variable) for the continuous variables. For the models involving categorical variables, each coefficient represents the difference in annual rate of change of the MoCA between the group specified in that row and the reference group (as defined in Table 3). Cox proportional hazards regression was used to estimate the age, sex and education adjusted effects of olfaction alone and olfaction combined with CSF biomarkers on the progression to MCI. Participants with baseline MCI were excluded from this analysis.
Table 3.
Olfaction and CSF biomarkers as predictors of decline in cognitive measures.
| Continuous Variables (MoCA as dependent variable) | Estimatea | p-value |
|---|---|---|
| UPSIT | 0.02 (0.01, 0.03) | 0.001 |
| Aβ1-42 | 0.002 (0.001, 0.003) | <0.001 |
| T-Tau/ Aβ1-42 | −5.10 (−6.45, −3.75) | <0.001 |
| Categorical Variables (MoCA as dependent variable) | ||
| Aβ1-42 group 1 | −0.21 (−0.441, 0.012) | 0.064 |
| Aβ1-42 group 2 | −0.14 (−0.0359, 0.088) | 0.24 |
| T-Tau/ Aβ1-42 group 1 | −0.33 (−0.557, −0.103) | 0.004 |
| T-Tau/ Aβ1-42 group 2 | 0.05 (−0.176, 0.269) | 0.68 |
| UPSIT, Aβ1-42 composite group 1 | −0.27 (−0.489, −0.055) | 0.014 |
| UPSIT, Aβ1-42 composite group 2 | −0.25 (−0.478, −0.013) | 0.038 |
| UPSIT, T-Tau/Aβ1-42 composite group 1 | −0.48 (−0.698, −0.258) | <0.001 |
| UPSIT, T-Tau/Aβ1-42 composite group 2 | −0.045 (−0.272, 0.181) | 0.7 |
Abbreviations: MoCA = Montreal Cognitive Assessment; UPDRS = Unified Parkinson's Disease Rating Scale
Data are shown as β (95% Confidence Interval). Each coefficient (β) represents the difference in annual rate of change in the MoCA for each 1 point increase in the parameter listed. Decline in MoCA as a function of UPSIT, Aβ1-42 and T-Tau/ Aβ1-42 as continuous variables are shown in the first half of the table. For the remainder of the table, each coefficient represents the difference in annual rate of change of the MoCA between the group specified in that row and the reference group (defined below). The group assignments for Aβ1-42 and UPSIT composite measure are as follows: Group 1 is lower olfaction and lower Aβ1-42 and Group 2 is Middle olfaction and middle Aβ1-42. Group 3 serves as the reference group and is comprised of higher olfaction and higher Aβ1-42. The groups are defined as follows for T-Tau/Aβ1-42 ratio and UPSIT composite measure: Group 1 is lower olfaction and higher Tau/Aβ1-42 ratio and Group 2 is middle olfaction and middle T-Tau/Aβ1-42 ratio. Group 3 serves as the reference group and is comprised of higher olfaction and lower T-Tau/Aβ1-42 ratio.
For example, a subject in Group 1 with a lower UPSIT and higher T-Tau/Aβ1-42 ratio would be expected to decline 0.5 points more rapidly per year on the MoCA compared to a subject in Group 3 with a higher UPSIT score and lower Tau/Aβ1-42 ratio. Estimates of rate of change are adjusted for age, sex, years of education and baseline variable score in a linear mixed effects model.
RESULTS
Participant baseline characteristics
A total of 423 PD patients were enrolled at the time of data acquisition. Of the PD participants enrolled, 389 had completed 1 year of follow up, 366 had completed 2 years of follow up and 196 had completed 3 years of follow up at the time of data download (June 30, 2015). The baseline characteristics of the participants stratified by olfactory tertiles are shown in Table 1. Age and sex differed among the groups, but other baseline characteristics, including education and disease duration, were similar. Baseline scores on tests of cognitive function in the cohort have been reported elsewhere [26].
Table 1.
PD Participant characteristics at baseline stratified by olfactory tertile
| Olfactory Tertile | |||||
|---|---|---|---|---|---|
| Lowest (N=147) | Middle (N=135) | Highest (N=141) | Unadjusted p-value | Adjusted p-valuea | |
| Age (years) | 65.0 (8.3) | 62.0 (9.2) | 58.0 (10.3) | <0.001b | - |
| Sex, % Male | 111 (75.5%) | 87 (64.4%) | 79 (56.0%) | 0.002c,3 | - |
| Education (years) | 15.6 (3.2) | 15.3 (3.0) | 15.7 (2.8) | 0.67b | - |
| Disease duration (months) | 6.6 (6.9) | 7.0 (6.9) | 6.2 (5.9) | 0.66b | - |
| UPSIT Total | 13.3 (3.6) | 22.5 (2.2) | 31.7 (3.4) | <0.001b | - |
| UPDRS Part 1A | 1.6 (1.8) | 1.1 (1.4) | 1.0 (1.4) | 0.003d,1,3 | <0.001 |
| UPDRS Part 1B | 4.6 (3.1) | 4.0 (2.9) | 4.5 (3.5) | 0.8 | 0.9 |
| UPDRS Part II | 6.3 (4.0) | 5.1 (4.0) | 5.5 (4.2) | 0.8 | 0.17 |
| UPDRS part III | 22.4 +/− 9.5 | 21.1 +/− 8.6 | 19.2 +/− 8.1 | 0.002b | 0.07 |
| Hoehn and Yahr Stage | 2 (1-2) | 2 (1-2) | 2 (1-2) | 0.011d,2,3 | 0.31 |
| RBD | 49 (33.3%) | 29 (21.5%) | 30 (21.3%) | 0.027c,1,3 | 0.052 |
| UPDRS1.1 Cognition, N (% positive) | 55 (37.4%) | 33 (24.4%) | 20 (14.2%) | <0.001c,1,2,3 | <0.001 |
| Montreal Cognitive Assessment (MoCA) | 26.7 (2.5) | 27.3 (2.2) | 27.5 (2.2) | 0.004b | 0.16 |
| Depression | 22 (15.0%) | 19 (14.1%) | 18 (12.8%) | 0.86c | 0.62 |
| Total STAI | 67.3 (19.2) | 63.7 (18.1) | 65.0 (17.8) | 0.30b | 0.02 |
| SCOPA-Autonomic | 11.4 (6.7) | 8.5 (5.4) | 8.8 (6.0) | 0.001b | 0.024 |
Abbreviations: UPSIT = University of Pennsylvania Smell Identification Test; UPDRS = Unified Parkinson's Disease Rating Scale; RBD = REM sleep Behavior Disorder; STAI = State Trait Anxiety Inventory; SCOPA-Autonomic = Scales for Outcomes In Parkinson's disease – Autonomic.
Data are shown as mean (SD), median (IQR) or N (%). The unadjusted p-value is the p-value for linear trend in one-way ANOVA, or from chi square or Kruskal-Wallis tests.
The adjusted p-value is from linear or logistic regression controlling for age and sex.
P-value for linear trend in one-way ANOVA
p-value for χ2
p-value for Kruskal-Wallis
Lowest vs Middle <0.05
Middle vs Highest <0.05
Lowest vs Highest <0.05
Olfaction
The overall mean UPSIT score was 22.4 (8.2). Some degree of olfactory impairment was found in 90.8% (384/423) of the participants. Using published normative data controlling for age and sex, 34.8% (147/423) were classified as anosmic while 28.6% (121/423) had severe microsmia and 27.4% (116/423) displayed mild to moderate microsmia.
Association between olfaction and baseline motor, non-motor, radiologic and CSF measures
At baseline, participants in the lowest olfactory tertile had significantly higher scores on the UPDRS part IA (p<0.001) compared to the middle and higher olfactory tertiles, but there was no difference in UPDRS part IB or part II among the tertiles. Differences in motor severity (UPDRS part III) did not remain significant (p=0.07) after adjusting for age and sex (Table 1). As shown in Table 1, total STAI (p=0.02) and SCOPA-AUT (p=0.024) demonstrated a linear trend with higher scores on both tests (more impaired) in the lowest olfaction tertile and lower (better) scores in the highest olfactory tertile. Those in the lowest olfactory tertile were more likely to report cognitive impairment (UPDRS part 1 question 1) compared to participants in the middle and highest tertiles (p<0.001). After adjusting for age and sex, there was no difference in MoCA score among the tertiles. Individual neuropsychological tests were similar at baseline after adjusting for age and sex, except for the Benton Judgement of Line orientation, in which the baseline score was slightly lower in the lowest olfactory tertile compared to the middle and the highest tertiles (p=0.04) (Supplementary Table 1). Baseline performance on the neuropsychological test battery in this cohort as a whole has been published elsewhere [26]. Baseline CSF values are shown in table 2. Total Aβ1-42 was associated with olfactory tertile, with lower values present in those with worse olfaction.
Table 2.
Baseline CSF biomarkers by olfactory tertile.
| Olfactory Tertile | |||||
|---|---|---|---|---|---|
| CSF Biomarkers | Lowest (N=142) | Middle (N=130) | Highest (N=136) | Unadjusted P-value for trenda | Adjusted p-valueb |
| Aβ1-42 | 352.9 (103.1) | 367.4 (91.2) | 392.4 (102.6) | 0.001 | 0.01 |
| T-Tau | 46.7 (20.3) | 44.4 (18.5) | 42.9 (15.5) | 0.09 | 0.65 |
| T-Tau/Aβ1-42 | 0.140 (0.08) | 0.125 (0.06) | 0.113 (0.04) | <0.001 | 0.046 |
| P-Tau | 14.7 (7.9) | 16.2 (12.0) | 16.1 (10.0) | 0.24 | 0.17 |
Abbreviations: Aβ1-42 = amyloid beta 1-42; T-Tau = total tau; P-Tau = phosphorylated tau
Data are shown as mean (SD).
P-value for linear trend in one-way ANOVA
The adjusted p-value is from linear regression controlling for age and sex.
Olfactory function as a predictor of disease progression
In longitudinal analysis, there was no association between baseline UPSIT score and change in UPDRS motor score, anxiety assessment (STAI), or autonomic symptoms (SCOPA-AUT). As shown in Table 3, worse baseline olfaction was associated with higher rates of decline in general cognition: MoCA scores decreased by 0.02 points for every 1 point decrease in UPSIT score (95% CI: 0.01, 0.03; p=0.001). For example, a subject with an UPSIT score of 13 (mean of lowest olfactory tertile) might be expected to experience an additional 1 point decline in MoCA score over 3 years compared to a subject with an UPSIT score of 32 (mean of highest olfactory tertile). CSF Aβ1-42 and t-tau/ Aβ1-42 ratio were each associated with decline in MoCA (p<0.001) alone, and there was a significant interaction between UPSIT score and t-tau/ Aβ1-42 ratio (−0.23, p=0.001) and decline in MoCA score. Because of this interaction, a composite measure of olfaction and CSF biomarkers was developed to create a more clinically meaningful classification of risk for decline in cognition. The composite measure of olfaction and tau/ Aβ1-42 ratio was also associated with an addition 0.5 point decline in MoCA score per year (β= −0.48, p<0.001). Those in Group 1 (worse olfaction and higher tau/ Aβ1-42 ratio) had an additional 1.5 point decline in MoCA score over 3 years compared to those in Group 3 (better olfaction and lower tau/ Aβ1-42 ratio). Baseline UPSIT score was significantly associated with the annual rate of change of individual neuropsychological tests except for the Benton Judgement of Line and Semantic Fluency (Supplementary Table 3).
Conversion to MCI
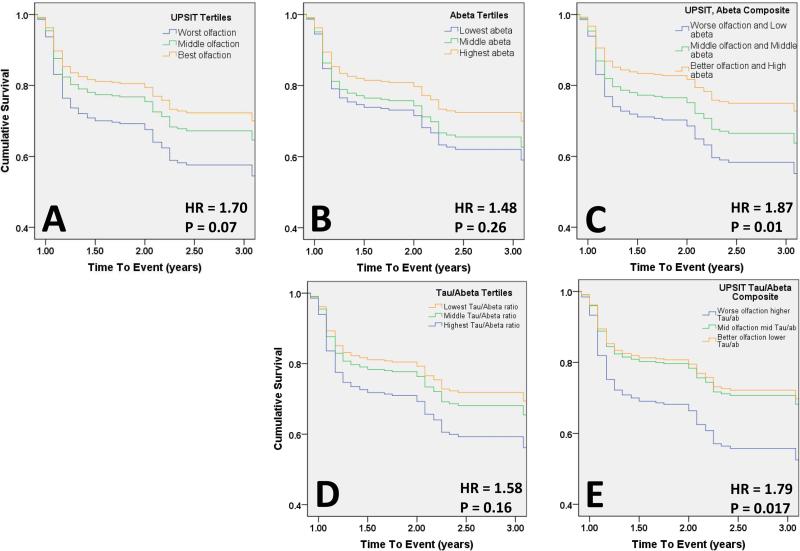
The proportion of participants who met criteria for MCI at baseline was 28.5% (42/147) in the lowest olfactory tertile, 18.5% (25/135) in the middle tertile and 17.0% (24/141) in the highest olfactory tertile. Rates of MCI among the Aβ1-42 tertiles (p=0.39) and composite measure tertiles (p=0.96) were similar at baseline. Participants with baseline MCI (91/423; 21.5%) were excluded from the Cox regression analyses. The number of cases of MCI identified at each visit out of the total number of participants that were included in the cox regression at each time point are as follows: year 1, 83/306; year 2, 21/201; year 3, 8/97. In Cox proportional hazards models with time to conversion to MCI as the dependent variable, there was a suggestion of an effect of olfaction alone on the risk of developing MCI (HR=1.7; p=0.07). CSF biomarker Aβ1-42 and tau/ Aβ1-42 tertiles were similarly not significant predictors of conversion to MCI alone. However, when UPSIT scores and Aβ1-42 were combined, the participants with worse olfaction and lower Aβ1-42 ratio were 87% more likely to develop MCI over the study period compared to those with better olfaction and higher Aβ1-42 (HR=1.87; p=0.01) (Figure 1). In a separate analysis, we defined MCI by neuropsychological testing criteria and found that the event rate decreased by half. The direction of effects were similar to that seen with the MoCA- defined MCI in longitudinal analyses, however, they did not reach significance (data not shown).
Figure 1. Olfaction and CSF biomarkers predict conversion to MCI.
Survival curves for conversion to MCI from cox proportional hazards regression. Panel A: Conversion to MCI by UPSIT Tertiles; Panel B: Conversion to MCI by Aβ1-42 Tertiles; Panel C: Conversion to MCI by composite measure of UPSIT and Aβ1-42 . Panel D: Conversion to MCI by tau/Aβ1-42 Tertiles; Panel E: Conversion to MCI by composite measure of UPSIT and tau/Aβ1-42 ratio.
DISCUSSION
Olfactory dysfunction is one of the most prevalent non-motor manifestations of PD and often one of the first signs of Parkinson's disease [3], which, along with the low cost and ease of assessment, make it an attractive biomarker for other clinical features that may present later in the disease course. In this study, we examined the association of olfaction with baseline motor and non-motor symptoms in a cohort of early PD patients, as well as the ability of baseline olfactory testing to predict progression of these features. Similar to prior studies [1-2], 91% of the patients in this cohort demonstrated some degree of olfactory impairment at the time of PD diagnosis [1-2]. Worse baseline olfaction was associated with more severe NMS, worse longitudinal scores on assessments of verbal memory and executive function, and in combination with lower Aβ1-42, predicted incident MCI over up to three years of follow up.
Our findings suggest that olfactory impairment may be a predictor of NMS, particularly cognitive decline. Worse olfaction was associated with a faster rate of decline in both global cognition and several cognitive domains, specifically verbal memory, executive function and attention, which corroborates findings from prior studies [9-12]. According to Braak staging, the olfactory system is one of the first areas affected by Lewy body pathology according [4], so worse olfaction may reflect more severe extranigral disease and therefore be associated with earlier cognitive impairment through cortical involvement.
In addition to cortical Lewy body pathology, Alzheimer's disease (AD) pathology has been linked to cognitive decline in PD and PDD. Up to one third or more of patients who develop PDD will also meet pathologic criteria for AD, involving beta amyloid plaques and tau-containing neurofibrillary tangles, which may have an additive effect with alpha-synuclein pathology to worsen prognosis [30-32]. Autopsy studies in PD-MCI are more limited, but in a report of eight cases, neuropathology revealed limbic or neocortical Lewy body pathology in five of the cases and the majority had diffuse amyloid plaques, suggesting that PDD and PD-MCI may be on a spectrum [33]. Prior studies have demonstrated associations between CSF tau and Aβ1-42 and cognitive impairment in PD and DLB in cross sectional studies, and in longitudinal studies, reduced CSF Aβ1-42 predicted future cognitive decline [15-18]. We found that Aβ1-42 and tau/ Aβ1-42 ratio were associated with decline in MoCA, and the combination of these CSF biomarkers with olfaction was predictive of long-term decline in global cognition and conversion to MCI, while olfaction and CSF biomarkers alone each showed trends toward predicting MCI. Those with worse olfaction and lower Aβ1-42 were 87% more likely to develop MCI than participants with better olfaction and higher Aβ1-42. The additive effect of olfaction and tau/ Aβ1-42 in predicting cognitive decline in PD may reflect each marker as an independent proxy of distinct molecular pathologies that combine to determine the clinical phenotype.
There are several study limitations to note. First, while olfaction can be measured in multiple ways (odor identification, discrimination and threshold), the UPSIT only measures odor identification. Odor identification requires more cognitive and memory processing than discrimination or threshold testing, so it is possible that baseline cognitive impairment at the time of olfaction assessment may influence olfactory identification scores [34]. A prior study addressed this issue by assessing picture recognition in those who had undergone odor identification testing with UPSIT and found that picture identification did not vary by cognitive or olfactory status, suggesting that baseline MCI in this early cohort did not affect UPSIT scores [13]. Second, use of the MoCA alone is a level I category for determining PD-MCI by MDS guidelines and a diagnosis by these criteria are considered less certain than a diagnosis of PD-MCI by level II category assessment. However, since comprehensive testing may not always be available or feasible, an MCI definition based on an abbreviated assessment is of interest. Analysis using MCI categorization based on neuropsychological testing (PD-MCI level II) did not reach significance, likely due to low event rate. Third, some of the observed effect sizes were relatively small, such an additional 1.5 point decline in MoCA score over 3 years in those with worse olfaction and higher T-Tau/ Aβ1-42 ratio. However, given that the mean MoCA score of the lowest olfactory tertile was 26.7, a subject with a decline of 1.5 points would make the transition between normal cognition and MCI. Given that the PPMI cohort has a very short disease duration, it is also possible that these small changes may herald larger ones in the future. Finally, while the longitudinal design starting with de novo PD subjects is a major strength of the PPMI cohort, the follow up period is relatively short with limited cases of MCI and no dementia converters. While olfaction and CSF biomarkers were significant predictors of decline in MoCA over time in linear mixed effects models, they did not independently predict conversion to MCI, likely because power was decreased by dichotomizing a continuous variable into a categorical variable and the event rate of MCI was relatively low. As the cohort is followed for a longer period of time and more events are accrued, olfaction and the CSF markers alone may prove to predict conversion to MCI.
In summary, olfactory dysfunction in early PD is associated with multiple measures of cognitive decline, suggesting that olfaction may be a sensitive biomarker of cognitive abilities in early PD. The combination of an AD CSF biomarker with olfactory performance at disease onset, compared with each biomarker individually, may substantially increase our ability to identify those at highest long-term risk of developing clinically significant cognitive impairment. Future prospective studies, including analysis of the PPMI cohort as more patients are followed for a longer time period, will clarify the usefulness of olfaction as a biomarker for cognition in PD, including how this simple and inexpensive assessment may be combined in multi-modal biomarker approaches.
Supplementary Material
HIGHLIGHTS.
Olfactory impairment is common in Parkinson's disease.
Lower UPSIT score was associated with more severe non-motor symptoms.
Worse olfaction was associated with a greater decline in MoCA score.
Addition of AD CSF biomarkers to olfaction may improve ability to predict MCI.
ACKNOWLEDGMENTS
PPMI is supported by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including Abott, Avid Radiopharmaceuticals, Biogen Idec, Covance, Bristol-Myers Squibb, Meso Scale Discovery, Priamal, Eli Lilly and co, F. Hoffman-La Roche Ltd, GE Healthcare, Genetech, GlaxoSmithKline, Merck and Co, Pfizer Inc, and UCB PharmaSA.
Dr. Fullard is funded by NIH training grant# 5T32NS061779-07. B. Tran reports no disclosures. Dr. Xie is currently funded by NIH grants P30-AG010124, P50-NS053488, P01-AG032953, and P01-AG017586. Dr. Toledo is supported by P01 AG032953, PO1 AG017586, P30 AG010124 and P50 NS053488 Dr. Weintraub has received research funding from the Michael J. Fox foundation for Parkinson's Research, NIH, Novartis Pharmaceuticals, Department of Veterans Affairs, and Alzheimer's’ disease Cooperative Study; honoraria from Biotie, Teva Pharmaceuticals, Lundbeck Inc., Pfizer, Avanir Pharmaceuticals, Acadia Pharmaceuticals, Merck & Co., UCB, Bristol-Myers Squibb Company, Novartis Pharmaceuticals, Clintrex LLC, Theravance, Medivation, CHDI foundation, and the Weston Foundation; license fee payments from the University of Pennsylvania for the QUIP and QUIP-RS; royalties from Wolters Kluwer; and fees for testifying in 2 court cases related to impulse disorders in Parkinson's disease (March 2013 to April 2014, payments by Eversheds and Roach, Brown, McCarthy & Gruber, P.C.).
Dr. Duda receives research support from the Department of Veterans Affairs, the NIH, and the Michael J. Fox Foundation for Parkinson's Research. Dr. Chahine receives support from the NIH (P50 NS053488), receives support as site Principal Investigator of the Parkinson's Progression Marker's Initiative and receives royalties from Wolters Kluwel for book authorship. Dr. Morley receives research support from the Department of Veterans Affairs and GE Healthcare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
M. Fullard: data analysis and interpretation, drafting and revision of manuscript
B. Tran: data analysis and interpretation, drafting and revision of manuscript
S. Xie: data analysis and interpretation, revision of manuscript
J. Toledo: data analysis and revision of manuscript
C. Scoridia: data analysis
C. Linder: data acquisition and analysis
R. Purri: data acquisition and analysis
D. Weintraub: data interpretation, revision of manuscript
J. Duda: revision of manuscript
L. Chahine: study concept/design, data interpretation, revision of manuscript
J. Morley: study concept/design, data interpretation, revision of manuscript
All authors edited the manuscript for accuracy and content and approved the final version for submission.
Disclosures:
C. Scordia reports no disclosures. C. Linder reports no disclosures. R. Purri reports no disclosures.
REFERENCES
- 1.Boesveldt S, Verbaan D, Knol DL, Visser M, van Rooden SM, van Hilten JJ, et al. A comparative study of odor identification and odor discrimination deficits in Parkinson's disease. Mov Disord. 2008;23(14):1984–1990. doi: 10.1002/mds.22155. [DOI] [PubMed] [Google Scholar]
- 2.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38(8):1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 3.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters EC, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol. 2004;56(2):173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 5.Berendse HW, Roos DS, Raijmakers P, Doty RL. Motor and non-motor correlates of olfactory dysfunction in Parkinson's disease. J Neurol Sci. 2011;310(1-2):21–24. doi: 10.1016/j.jns.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Kang WY, Chen S, Wang Y, Xiao Q, Wang G, et al. Hyposmia correlates with SNCA variant and non-motor symptoms in Chinese patients with Parkinson's disease. Parkinsonism Relat Disord. 2015;21(6):610–614. doi: 10.1016/j.parkreldis.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Cramer CK, Friedman JH, Amick MM. Olfaction and apathy in Parkinson's disease. Parkinsonism Relat Disord. 2010;16(2):124–126. doi: 10.1016/j.parkreldis.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Verbaan D, Boesveldt S, van Rooden SM, Visser M, Marinus J, Macedo MG, et al. Is olfactory impairment in Parkinson disease related to phenotypic or genotypic characteristics? Neurology. Dec 2. 2008;71(23):1877–82. doi: 10.1212/01.wnl.0000336651.48596.c7. [DOI] [PubMed] [Google Scholar]
- 9.Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, et al. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson's disease. Brain. 2010;133(6):1747–1754. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postuma R, Gagnon JF. Cognition and olfaction in Parkinson's disease. Brain. 2010;133(12):e160. doi: 10.1093/brain/awq228. author reply e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morley JF, Duda JE. Neuropsychological correlates of olfactory dysfunction in Parkinson's disease. J Neurol Sci. 2011;310(1-2):228–230. doi: 10.1016/j.jns.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Damholdt MF, Borghammer P, Larsen L, Ostergaard K. Odor identification deficits identify Parkinson's disease patients with poor cognitive performance. Mov Disord. 2011;26(11):2045–2050. doi: 10.1002/mds.23782. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson R, Houghton D, Sundarararjan S, Doty RL, Stern M, Xie SX, et al. Odor identification deficits are associated with increased risk of neuropsychiatric complications in patients with Parkinson's disease. Mov Disord. 2010;25(13):2099–2104. doi: 10.1002/mds.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baba T, Kikuchi A, Hirayama K, Nishio Y, Hosokai Y, Kanno S, et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson's disease: a 3 year longitudinal study. Brain. 2012;135(1):161–169. doi: 10.1093/brain/awr321. [DOI] [PubMed] [Google Scholar]
- 15.Bibl M, Mollenhauer B, Esselmann H, Lewczuk P, Klafki HW, Sparbier K, et al. CSF amyloid-beta-peptides in Alzheimer's disease, dementia with Lewy bodies and Parkinson's disease dementia. Brain. 2006;129(5):1177–1187. doi: 10.1093/brain/awl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compta Y, Marti MJ, Ibarretxe-Bilbao N, Junque C, Valldeoriola F, Munoz E, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Mov Disord. 2009;24(15):2203–2210. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 17.Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-Plotkin A, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75(12):1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parnetti L, Farotti L, Eusebi P, Chiasserini D, De Carlo C, Giannandrea D, et al. Differential role of CSF alpha-synuclein species, tau, and Abeta42 in Parkinson's Disease. Front Aging Neurosci. Mar 31. 2014;6:53. doi: 10.3389/fnagi.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkinson Progression Marker Initiative The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 21.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 22.Nomura T, Inoue Y, Kagimura T, Uemura Y, Nakashima K. Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson's disease patients. Sleep Med. 2011;12(7):711–713. doi: 10.1016/j.sleep.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson's disease: the SCOPA-AUT. Mov Disord. 2004;19(11):1306–1312. doi: 10.1002/mds.20153. [DOI] [PubMed] [Google Scholar]
- 24.Weintraub D, Oehlberg KA, Katz IR, Stern MB. Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry. 2006;14(2):169–175. doi: 10.1097/01.JGP.0000192488.66049.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. Mar. 2012;27(3):349–56. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Siderowf A, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Mov Disord. Jun. 2015;30(7):919–27. doi: 10.1002/mds.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, et al. Association of cerebrospinal fluid beta-amyloid 1-42, T-tau, P-tau181, and alpha-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013;70(10):1277–1287. doi: 10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54(4):343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 29.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 30.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson's disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci. 2013;14(9):626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 32.Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, Collins C, et al. Lewyand Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain. 2011;134(5):1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adler CH, Caviness JN, Sabbagh MN, Shill HA, Connor DJ, Sue L, et al. Heterogeneous neuropathological findings in Parkinson's disease with mild cognitive impairment. Acta Neuropathol. Dec. 2010;120(6):827–8. doi: 10.1007/s00401-010-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy C, Cain WS, Gilmore MM, Skinner RB. Sensory and semantic factors in recognition memory for odors and graphic stimuli: elderly versus young persons. Am J Psychol. 1991;104(2):161–192. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.