Abstract
Recent evidence indicates that salt-sensitive hypertension can result from a subclinical injury that impairs the kidneys’ capacity to properly respond to a high salt diet. However, how this occurs is not well understood. Here, we showed that while previously salt resistant wild-type mice became salt-sensitive after the induction of renal injury with the nitric oxide synthase inhibitor Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME); mice lacking renal angiotensin-converting enzyme, exposed to the same insult, did not become hypertensive when faced with a sodium load. This is because the activity of renal angiotensin-converting enzyme plays a critical role in: 1) augmenting the local pool of angiotensin II and, 2) the establishment of the anti-natriuretic state via modulation of glomerular filtration rate and sodium tubular transport. Thus, this study demonstrates that the presence of renal angiotensin-converting enzyme plays a pivotal role in the development of salt sensitivity in response to renal injury.
Keywords: Angiotensin-converting enzyme, salt-sensitive hypertension, inflammation, renal sodium transporters, glomerular filtration rate
INTRODUCTION
The blood pressure of one in every two hypertensive patients increases in response to high salt intake, a condition known as salt-sensitive hypertension. Yet, despite its high prevalence, salt sensitivity continues to be poorly understood. There is no consensus definition, it is not easy to detect in the clinic and above all, there are fundamental gaps in the understanding of its origin. While it is recognized that salt sensitivity has a strong genetic component,1,2 it has also become apparent that acquired factors increase the pressor response to dietary sodium. Most notably, recent evidence suggests that salt-sensitive hypertension can result from a chronic renal inflammatory state that reduces the kidneys’ capacity to excrete a sodium load without elevating blood pressure.3–5
Over the last two decades a plethora of studies suggest that inflammation is central to the etiology of hypertension. Investigators have shown that T lymphocytes and other components of the inflammatory response, including macrophages, dendritic cells, pro-inflammatory cytokines, and reactive oxygen species (ROS) actively modulate blood pressure.6–11 These studies add to the well-established observation that renal inflammation and injury are common in experimental and human hypertension and other associated conditions like obesity and diabetes.12–14 However, even accepting the kidney injury hypothesis, a key question remains how inflammation affects the renal sodium excretory pathway to cause sodium retention and hypertension.
We hypothesize that the activity of the renal angiotensin-converting enzyme (ACE)/Angiotensin (Ang) II pathway is crucial in blunting natriuresis and establishing salt sensitivity in the setting of renal inflammation. This novel concept builds on recent analyses showing that the renal renin-angiotensin system (RAS) becomes activated in conditions of renal injury. It also builds upon previous work emphasizing the importance of Ang II actions locally within the kidney.15 Specifically, our group found that local Ang II synthesis by renal ACE is indispensable for the development of experimental hypertension: in mice lacking renal ACE, neither Ang II infusion nor nitric oxide depletion can induce hypertensive disease.16 This is because renal ACE activity is required to increase local Ang II, which in turn, induces sodium and fluid retention via activation of key sodium transporters in the proximal tubule [Na+/H+ exchanger 3 (NHE3)], the thick ascending limb [Na+-K+-2Cl− co-transporter 2 (NKCC2)], and the distal nephron [NaCl co-transporter (NCC), epithelial Na+ channel (ENaC) and pendrin].16,17 Thus, our data link renal Ang II and local regulation of sodium transport as obligatory for the development of hypertension.
We now present evidence that two mouse models lacking ACE in renal tissues, the ACE 3/3 and the ACE 10/10 mice, do not develop salt sensitivity in response to renal injury. Specifically, in the absence of renal ACE these mice maintain a normal renal natriuretic response to high salt despite substantial levels of renal inflammation induced by the protocol. In other words, our data suggest that without renal ACE, there is no salt-sensitive hypertension despite the presence of renal inflammation.
METHODS
For detailed description see Methods in the online-only Data Supplement.
RESULTS
Mouse models
The ACE 10/10 mouse is an inbred line (C57Bl/6J) that expresses ACE only in myelomonocytic cells.18 The ACE 3/3 mouse has a mixed background (C57Bl/6-129) and expresses ACE mostly in hepatocytes.19 Both strains share features that were important for this study: normal levels of circulating ACE, normal basal blood pressure and normal basal renal morphology and physiology. Even more important, in ACE 10/10 mice, renal ACE activity is reduced by 98% (Figure S1A), while in the ACE 3/3 mice it is reduced by 86%. Finally, both wild-type (WT) and ACE 10/10 mice are salt resistant at baseline, as they can be exposed to a high salt diet without discernable blood pressure changes (Figure S1B).
Renal ACE absence blunts salt sensitivity
We used the post L-NAME model to test our hypothesis. In this protocol, a transient exposure (4 weeks) to Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME) was followed by a washout phase (1 week) and then, exposure to a high salt diet (3 weeks). We selected this model based on previous publications showing that the transient exposure to L-NAME elicited renal injury that converted rodents from salt-resistant to salt-sensitive.20 Indeed, WT mice, previously salt-resistant, developed hypertension when fed a high salt diet, regardless of their background strain (Figures 1). Because ACE 10/10 and ACE 3/3 mice are more resistant to L-NAME than WT mice,17 we increased their L-NAME dosing three-fold to raise blood pressure to the level observed in the WT mice. Remarkably, even after equivalent levels of hypertension to WT (Figure 1), mice lacking renal ACE remained completely resistant to the development of salt sensitivity. After 3 weeks of high salt diet, ACE 10/10 mice had a blood pressure that was 109 ± 2 mmHg vs. 107 ± 5 mmHg at baseline (Figure 1A; not significant, NS). The blood pressure of ACE 3/3 mice was 102 ± 4 mmHg vs. 102 ± 7 mmHg at baseline (Figure 1B; NS).
Figure 1. The absence of renal ACE prevents the development of salt-sensitive hypertension.
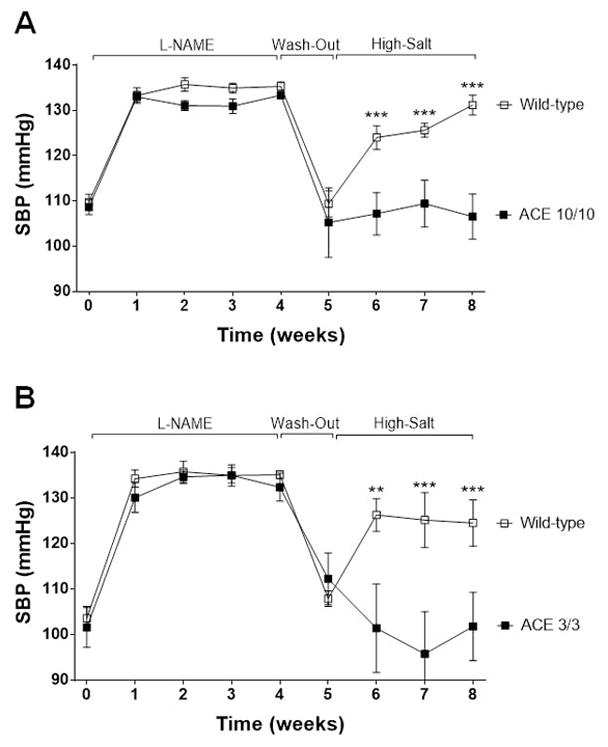
ACE 10/10 (A), ACE 3/3 (B) and wild-type (WT) mice were exposed to 4 weeks of L-NAME, a 1-week washout period, and then, 3 weeks of high salt diet (4%). L-NAME was given to WT (0.5 mg/mL) and mutant mice (1.5 mg/mL) in the drinking water. SBP: Systolic blood pressure. Values represent mean ± SEM. n = 6–17 per group, **p<0.01, ***p<0.001.
We focused on the ACE 10/10 mice because of their nearly total lack of renal ACE activity. Also, the renal function of the C57Bl strain is well characterized.16,21 First, we investigated if the dissimilar responses to the sodium load were due to differences in nitric oxide (NO) availability and/or residual effects of L-NAME by measuring urinary nitrite/nitrate (NOx) excretion. However, WT and mutant mice exhibited similar reductions in NOx excretion during L-NAME treatment that recovered to pre-existing levels during the washout and the high salt phase (Figure S2).
Renal inflammation and fibrosis
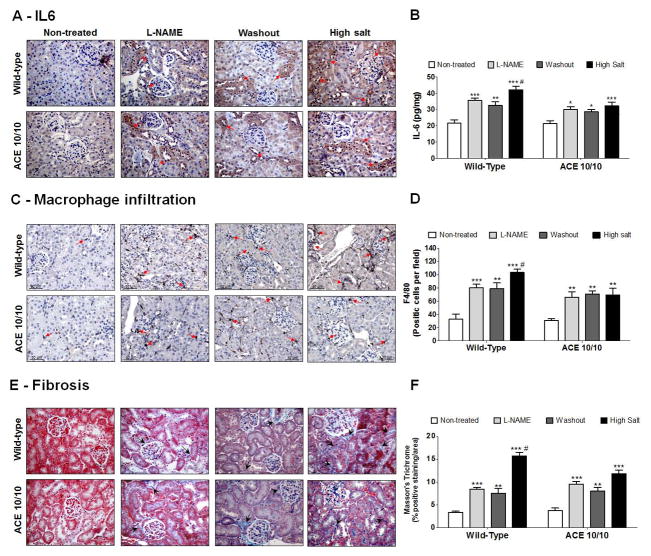
We determined whether differences in renal injury induced by L-NAME accounted for the dissimilar blood pressure response to high salt diet by assessing the renal content of interleukin 6 (IL-6), Tumor necrosis factor α (TNF-α), IL-17, transforming growth factor β (TGF-β), macrophage infiltration, fibrosis, and proteinuria (Figures 2, S3 and S4). Baseline level of these parameters was the same among genotypes. After L-NAME, IL-6 (Figure 2A, 2B and S3C), TNF-α content (Figure S3A, S3B and S3D), IL-17 (Figure S3E), TGF-β (Figure S3F), macrophage number (Figure 2C and 2D), fibrosis (Figure 2E and 2F), and proteinuria (Figure S4) increased to the same extent in WT mice and ACE 10/10 mice. Further, all these parameters remained elevated throughout the washout in WT and mutant mice. Hence, the blunted blood pressure of mutant mice in response to the sodium load cannot be attributed to a lesser degree of renal injury. Finally, the high salt diet exacerbated the degree of inflammation and injury in WT kidneys, but not in the kidneys from mutant mice (Figure 2, S3 and S4).
Figure 2. Wild-type (WT) and ACE 10/10 mice display similar levels of renal inflammation and injury during post L-NAME hypertension.
Kidneys from WT and ACE 10/10 mice were collected at the end of each phase of the post L-NAME protocol. Renal sections were stained for IL-6 (A) and results were confirmed by ELISA in kidney homogenates. Data were expressed as pg of IL-6 per mg of total kidney protein (B). Macrophage infiltration was performed by F4/80 staining (C) and data were expressed as F4/80 positive cells per area (D). Fibrosis was assessed by Masson’s Trichrome staining (E) and expressed as percentage of positive staining per area (F). L-NAME was given to WT (0.5 mg/mL) and ACE 10/10 (1.5 mg/mL) in the drinking water. Values represent the mean ± SEM. n = 5–11 per group, *p<0.05; **p<0.01; ***p<0.001 vs. non-treated mice; #p<0.05 vs. all groups.
The absence of renal ACE prevents local Ang II accumulation
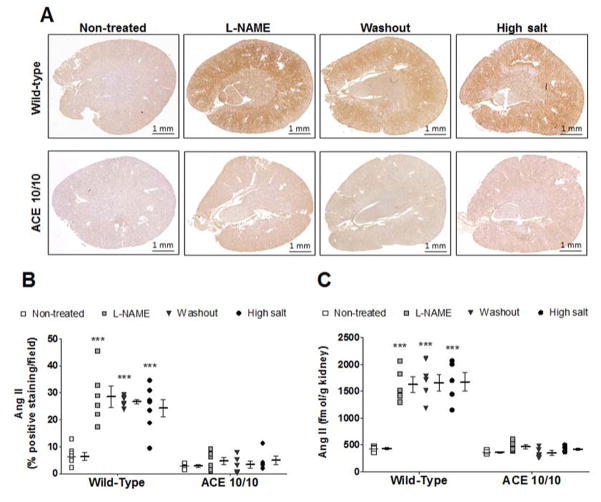
We used a validated immunohistochemical method to measure tissue Ang II content (Figure 3A, 3B and S5A).17 Baseline renal Ang II levels were similar among genotypes. In WT mice, L-NAME treatment increased renal Ang II, and this increase persisted throughout the washout and the sodium load (Figure 3A, 3B and S5A). In contrast, renal Ang II content in ACE 10/10 mice did not change significantly throughout the experiment. Renal Ang II levels were confirmed by an enzyme immunoassay in total kidney homogenate (Figure 3C).
Figure 3. Renal angiotensin (Ang) II immunostaining during post L-NAME hypertension.
Kidneys from wild-type and ACE 10/10 mice were collected after each phase of the post L-NAME protocol. Renal sections were stained for Ang II (A) and values were expressed as percentage of positive staining per area (B). Renal Ang II content was confirmed by an enzyme immunoassay and data were expressed as fmol of Ang II per gram of kidney (C). L-NAME was given to WT (0.5 mg/mL) and ACE 10/10 (1.5 mg/mL) in the drinking water. n = 5–10 per group, ***p<0.001 vs. non-treated mice.
In view of these findings, we analyzed the expression of two key renal RAS components: angiotensinogen and ACE (Figure S5). As previously reported,16,17 baseline angiotensinogen was significantly higher in WT mice than in the mutant mice. In WT mice, renal angiotensinogen abundance increased in response to L-NAME, and stayed elevated during the washout and the sodium load. In the ACE 10/10 mice, angiotensinogen did not change (Figure S5B). As expected, baseline ACE expression was also significantly higher in WT mice than in ACE 10/10 mice. Further, while renal ACE expression remained unchanged in the ACE 10/10, in WT mice ACE appeared to progressively increase during L-NAME treatment, washout and salt load (Figure S5C).
Renal ACE is obligatory for sodium retention during renal injury
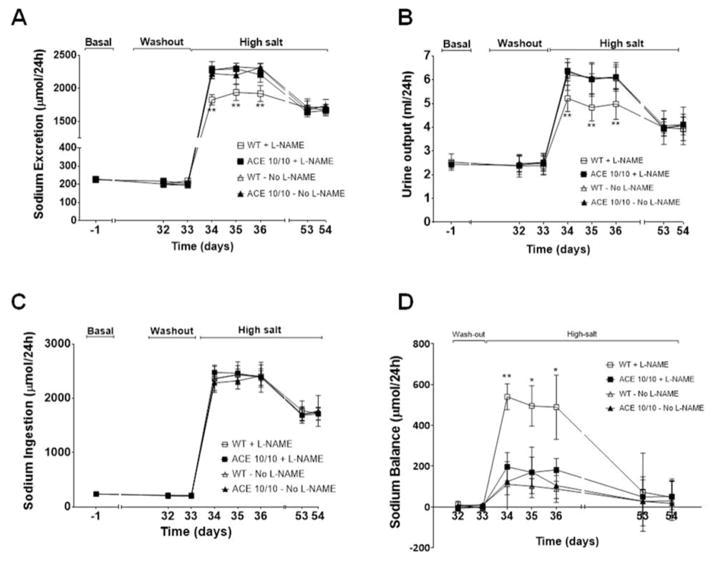
We previously showed that baseline sodium and urine excretion were similar in WT mice and mice lacking renal ACE. We also demonstrated that in response to L-NAME, WT mice exhibited transient reductions in sodium excretion and a positive sodium balance that were corrected at the expense of hypertension. In contrast, these anti-natriuretic responses were blunted in the ACE 10/10 mice.17 In view of these findings, we focused on renal sodium handling during the washout and high salt phases (Figure 4, S6 and S7). During washout, sodium and urine excretion were similar in both strains. While sodium and urine excretion increased in both WT and mutant mice in response to a high salt diet, ACE 10/10 mice displayed a markedly enhanced natriuretic and diuretic response (4A and 4B). Importantly, the greater natriuresis of the mutant mice is not a reflection of higher sodium intake, as this was indistinguishable from WT mice (Figure 4C and S6). Importantly, sodium balance studies showed that WT mice developed a positive daily sodium balance during the first 72 h of the sodium load (508 ± 62 μmol/24h, Figure 4D) as well as sodium accumulation (Figure S6E) that were later corrected at the expense of the increased blood pressure. In sharp contrast, the greater natriuresis observed in mice lacking renal ACE was associated with lesser sodium retention (108 ± 43 μmol/24h; p<0.01 vs. WT; Figures 4D and S6E), and retained normal blood pressure. Plotting sodium excretion against blood pressure revealed a rightward shift in the pressure-natriuresis relationship of WT mice (Figures S7A), but not in the mutant mice (Figures S7B), after L-NAME treatment. Finally, none of these differences between WT and mutant mice in response to the salt challenge were observed in mice not pre-treated with L-NAME (Figure 4, S6 and S7).
Figure 4. Mice lacking renal ACE display improved sodium (Na+) handling.
Wild-type (WT) and ACE 10/10 mice were housed individually in metabolic cages with free access to food and water before and during the high-salt diet. Na+ excretion (A) and urine output (B) as well as Na+ ingestion (C) were used to calculate Na+ balance (D). Values were expressed as μmol Na+ per day. L-NAME was previously given to WT (0.5 mg/mL) and ACE 10/10 (1.5 mg/mL) in the drinking water. WT and ACE 10/10 mice not pre-treated with L-NAME were used as controls. Values represent mean ± SEM. n = 8 per group, *p<0.05; **p<0.01.
Renal ACE blunts the GFR responses of the injured kidney to a sodium load
The GFR of conscious unrestrained mice was assessed by a transcutaneous method.17,22 At baseline, the GFR of WT and ACE 10/10 mice was indistinguishable (1461 ± 32 vs. 1481 ± 27 μL/min/100 g b.w., NS, Figure 5). In response to high salt diet without L-NAME pre-treatment, both strains showed acute GFR increases that were similar in magnitude, approximately 20% (Figure 5A). Importantly, L-NAME pre-treatment abolished this rise in GFR in WT but not in ACE 10/10 mice (Figure 5B), even though the mutant strain was exposed to three times more L-NAME; ACE 10/10 still exhibit an acute GFR increase when switched to high salt diet (from 1406 ± 37 to 1596 ± 49 μL/min/100 g b.w, p<0.01, Figure 5B). Thus, these data indicate a very important role of renal ACE in counteracting the adaptive GFR increase in response to high salt diet in the context of renal inflammation.
Figure 5. Renal ACE impairs the acute increase of glomerular filtration rate (GFR) elicited by a high salt diet.
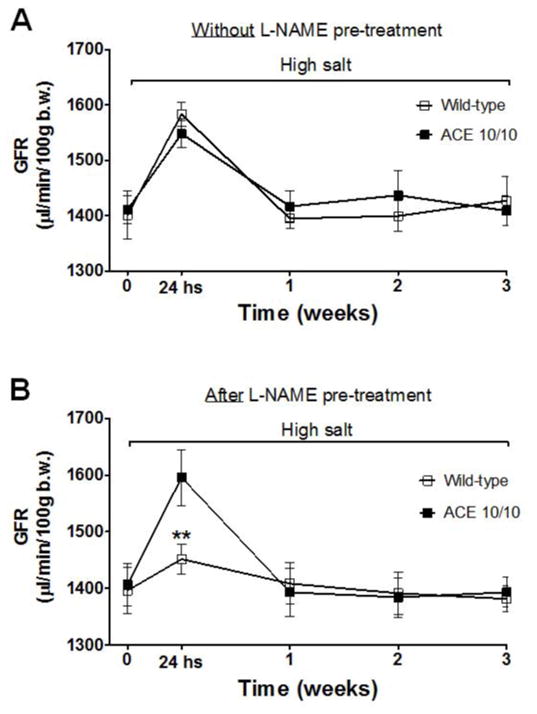
GFR was estimated by the transcutaneous method at the indicated times during the 3-week course of the high salt diet in: naïve, not pre-treated with L-NAME, wild-type and ACE 10/10 mice (A), and in animals previously exposed to the L-NAME (B). Values represent the mean ± SEM. n = 5–7 per group, **p<0.01.
Renal sodium transporter profiling
To investigate the blunted natriuresis in the post L-NAME model, we measured the abundance, phosphorylation and processing of several key sodium transporters: NHE3, NKCC2, NCC, and ENaC. We showed in the past that the baseline transporter profile of WT and ACE 10/10 mice is indistinguishable.16 We also showed that WT mice respond to L-NAME with adaptations that facilitate natriuresis including decreases in total abundance and phosphorylation (P) of NKCC2 as well as decreased full length and the active cleaved product of αENaC (αENaC-CL). In L-NAME treated ACE 10/10 mice, more extensive and substantial natriuretic adaptations occurred. These include: decreases in NHE3, Na+-P(i) cotransporter 2 (NaPi2), further decrease in NKCC2-P, decrease in NCC-P and αENaC modulation.17 We now show that after the washout week, WT mice transporter profiling returned to baseline levels (Figure 6A, left). In contrast, most changes induced by L-NAME persisted after the washout in ACE 10/10 mice. Specifically, ACE 10/10 mice displayed reductions in either the abundance or the phosphorylation levels of NKCC2, NCC, and cleaved αENaC (αENaC-CL) as compared to baseline (Figure 6A, right). Most of these changes were significantly greater when compared to WT mice (Figure 6B). Thus, the more extensive transporter expression reduction of the ACE 10/10 mice suggests less potential for active sodium reabsorption by the time these mice are exposed to the high salt diet.
Figure 6. Lower abundance and/or phosphorylation of NHE3, NKCC2, NCC and the cleaved (CL) subunit of ENaC were observed in animals lacking renal ACE after the washout phase.
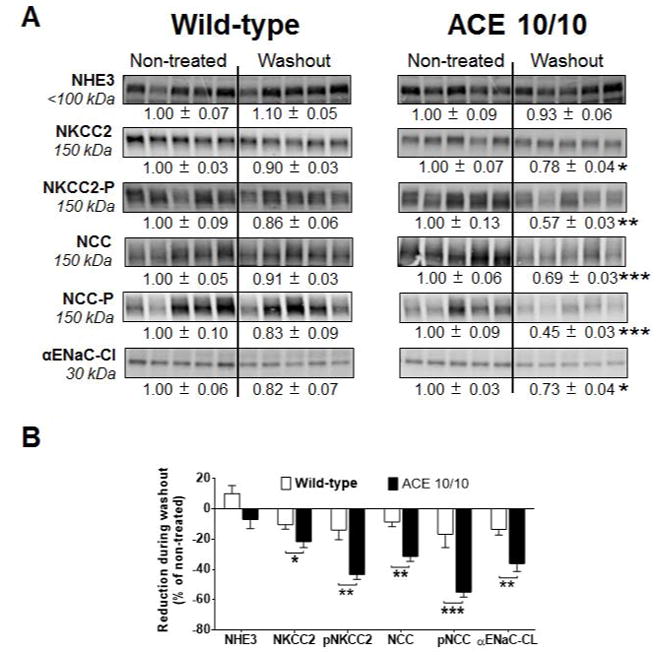
(A) Transporter expression was analyzed in kidney homogenates from non-treated mice and after the washout phase. L-NAME was given to wild-type (0.5 mg/mL) and mutant mice (1.5 mg/mL) in the drinking water. Immunoblots were performed with a constant amount of protein per lane. Relative abundance from each group is displayed below the corresponding blot as mean ± SEM. n = 12 per group, *p<0.05; **p<0.01; ***p<0.001 vs. corresponding non-treated mice. (B) Bars represent the relative reduction vs. non-treated mice (set as 0), *p<0.05; **p<0.01; ***p<0.001.
WT and ACE 10/10 mice never treated with L-NAME displayed equivalent reductions in sodium transporters when exposed to a 3-week high salt diet (Figure S8). However, different responses were observed in L-NAME pre-treated mice. WT mice responded with reduced abundance or phosphorylation of NKCC2, NCC, and αENaC-CL when compared to baseline levels (Figure 7A, left). Nonetheless, these mice still developed hypertension. L-NAME treated ACE 10/10 mice also displayed reductions in sodium transporters (Figure 7A, right) but maintained normal blood pressure. This was associated with significantly greater reductions of NCC abundance and phosphorylation, as well as αENaC-CL abundance, than observed in WT mice (Figure 7B). Thus, these findings indicate that the presence of renal ACE in WT mice was associated with higher levels of transporter expression and impaired natriuresis during the high salt challenge.
Figure 7. The absence of renal ACE amplifies reductions in abundance and/or phosphorylation of NCC and the cleaved (CL) portion of αENaC after 3 weeks of a high salt diet.
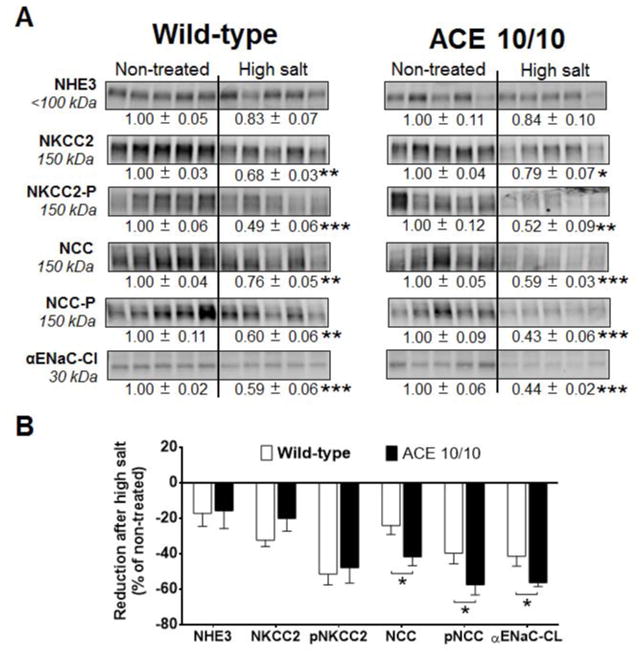
(A) Transporter expression was analyzed in total kidney homogenates from non-treated mice and after a 3-week high salt phase. L-NAME was given to wild-type (0.5 mg/mL) and mutant mice (1.5 mg/mL) in the drinking water. Immunoblots were performed with a constant amount of protein per lane. Relative abundance from each group is displayed below the corresponding blot as mean ± SEM. n = 12 per group, *p<0.05; **p<0.01; ***p<0.001 vs. corresponding non-treated mice. (B) Bars represent the relative reduction vs. non-treated mice (set as 0), *p<0.05; **p<0.01; ***p<0.001.
DISCUSSION
Using the post L-NAME hypertension model, there was a major difference in salt sensitivity between WT and ACE 10/10 mice. Namely, while the transient exposure to L-NAME sensitized WT mice to salt-sensitive hypertension, ACE 10/10 mice could excrete a salt load without a rise in blood pressure when challenged with a high salt diet; in other words, they remained salt-resistant. The ACE 3/3 mice, a second genetic model of reduced renal ACE expression, also remained normotensive following a high salt diet. ACE 10/10 and ACE 3/3 mice have different mutations, different genetic backgrounds and a different pattern of ACE expression. However, both strains maintain normal basal renal structure and function, and have normal systemic Ang II levels. What both strains lack is the ability to produce normal amounts of renal ACE.
Notwithstanding genetic predilection, a theory put forward to explain salt sensitivity is that it arises from a subtle acquired renal injury.4,23,24 Salt-sensitive hypertension is thought the result of three renal insults. The first is a transient elevation in blood pressure caused by an environmental stress. This establishes the second insult, a chronic renal inflammatory state that, although significant, is not overwhelming. The final insult is high salt intake itself, which surpasses the compromised compensatory ability of the kidney and leads to overt hypertension. We intended to replicate these three insults with the post L-NAME model. While we used L-NAME to induce the initial renal injury, others have reported the induction of salt sensitivity by other experimental protocols of renal damage, including Ang II infusion25 and chronic ischemia.26 Our previous study established that both the ACE 10/10 and ACE 3/3 mice are resistant to the development of hypertension induced by L-NAME.17 Thus, in order to apply the post-L-NAME protocol, we increased the dose of L-NAME to induce an equivalent level of hypertension in the ACE 10/10 and ACE 3/3 mice as in WT mice. One obvious question was whether we did, in fact, achieve equivalent levels of injury in both WT and mutant mice. Since analysis showed comparable levels of inflammatory and injury markers, we believe there was equivalent injury in both groups of mice throughout the experimental protocol.
What then explains the difference in response to a salt load? Our hypothesis is that the inflammation triggered by L-NAME activates the renal RAS, resulting in renal Ang II accumulation and, ultimately, impaired renal natriuretic responses. In the ACE 10/10 mice lacking renal ACE, this does not occur. A strong body of evidence supports the concept that the renal RAS is regulated independently from the systemic RAS.15 Specifically, inflammation, ROS, and other pathological agents can stimulate several renal RAS components, including angiotensinogen, tubular renin, ACE and the AT1 receptor.15,27–29 Here, we show that in WT mice, the initial L-NAME insult stimulates renal expression of angiotensinogen and ACE resulting in increased local Ang II synthesis. Thus, WT mice accumulate increasing amounts of renal Ang II during post-L NAME hypertension while the intrarenal Ang II is never increased in the ACE 10/10 mice. Because ACE cleaves many substrates, it is possible that at least some of our observations are due to other peptide(s) besides Ang II.
Both ACE 10/10 and ACE 3/3 mice are unique animals with very little renal ACE. In addition, the genetic mutations used to create these mice result in endothelium (both intra- and extra-renal endothelium) that make no ACE.18,19 In terms of systemic effects, studies have shown that under basal conditions, plasma Ang II levels are normal in these mice. Further, basal GFR and renal concentrating function is also equivalent to WT mice.16,17 Both L-NAME administration and high salt result in plasma renin suppression and low systemic Ang I levels. All this suggests that the lack of systemic endothelial ACE has little effect on the physiology of these mice. Indeed, both previously published genetic manipulations of endothelial ACE levels and computer simulations of the effects of systemic ACE on blood pressure control strongly suggest that the lack of systemic endothelial ACE has minimal effects in ACE 10/10 and ACE 3/3 mice.30,31 However, the absence of ACE from endothelium and other tissues such as the central nervous system could also contribute to our results and cannot be discounted.
The most remarkable aspect of our study is the significant impact of renal ACE on the pressure-natriuresis relationship. In the present study, the renal responses of naïve WT and ACE 10/10 mice to a high salt diet are undistinguishable. Yet, after injury, the presence of renal ACE in WT mice obliges a rightward shift of the pressure-natriuresis relationship and the establishment of hypertension. Remarkably, in the ACE 10/10 mice, animals lacking renal ACE, this major change is not required to maintain salt balance despite equivalent levels of renal injury. Although we observed a strong correlation between sodium balance and blood pressure, we cannot establish with absolute certainty that greater sodium retention is the sole mechanism explaining the hypertension.32
In theory, renal ACE could dysregulate sodium handling during a high salt diet by causing abnormalities of renal hemodynamics and GFR, or by blunting the expected decrease in sodium reabsorption along the nephron. We observed that both naïve WT and ACE 10/10 mice, never exposed to L-NAME, displayed an acute and transient GFR increase in response to a salt load. This has been described by others as a first line of defense against sodium-induced extracellular volume expansion.33,34 After L-NAME treatment, this adaptive response to a salt load was lost in WT, but not in the ACE 10/10 mice. The lack of increase in GFR after high salt is likely the consequence of the vasoconstrictive effects of Ang II on pre-glomerular and post-glomerular vasculature.35 While it is thought that Ang II preferentially constricts efferent arterioles, enough Ang II can constrict pre-glomerular arterioles via direct activation of AT1 receptors and through increasing the sensitivity of the tubule-glomerular feedback mechanism. Although we did not assess this mechanism directly, we find that activated (phosphorylated) NKCC2, the tubuloglomerular feedback sensor, is reduced by 40% in the ACE 10/10 mice after L-NAME treatment and washout. Thus, it is logical to predict that the activity of tubuloglomerular feedback depends on renal ACE
Although we observed reductions in sodium transporters in WT mice, the establishment of hypertension in this group indicates that such compensation was insufficient to prevent the blood pressure increase. We present here evidence to support renal Ang II accumulation as the underlying factor for the reduced compensatory capacity. However, it is also possible that downregulation of counterbalancing mechanisms, like the dopamine system,36,37 play a role in our observations. In contrast to what was observed in WT mice, ACE 10/10 mice have greater reductions of abundance, phosphorylation and processing of key sodium transporters. Thus, the absence of ACE in the mutant mice reduced nephron sodium avidity and preserved the capacity to balance sodium output to the elevated sodium intake without increasing blood pressure. Left here unexplored, it is also the possibility that other mechanisms, including alterations in the vascular responses, participate in our observations.
In conclusion, our data support a hypothesis in which L-NAME induced hypertension injures the kidney, thereby triggering local renal ACE derived Ang II production. The increase of local Ang II blunts the appropriate natriuretic responses to a sodium load and elevates the blood pressure. The absence of renal ACE prevents changes in renal salt handling and the shifting of the pressure-natriuresis relationship, keeping blood pressure values in the normal range. Thus, our study strongly suggests that renal ACE exerts a critical causative role in the induction of salt sensitivity in response to renal parenchymal injury.
Supplementary Material
PERSPECTIVES.
Salt-sensitive hypertension affects half of hypertensive patients. Yet, it continues to be a poorly understood aspect of hypertension. Here, study of mice genetically lacking renal ACE shows that renal ACE plays a pivotal role in the development of salt sensitivity following renal injury. We propose a new paradigm in which renal sodium handling, including the regulation of the glomerular filtration rate and key sodium transporter activity, is ultimately controlled by renal, instead of systemic, Ang II levels. Specifically, renal ACE/Ang II pathway facilitates the development of salt-sensitive hypertension. Thus, our data offers a very novel mechanistic explanation for the development of salt sensitivity following renal parenchymal injury.
NOVELTY AND SIGNIFICANCE.
What Is New?
Renal injury is associated with renal ACE overexpression, Ang II accumulation in the kidney and sodium retention.
During renal parenchymal injury, renal ACE and Ang II modulate GFR and sodium transporter activity to induce a sodium-retentive state that establishes salt-sensitive hypertension.
What Is Relevant?
Renal ACE, and not the systemic RAS, plays a pivotal role in establishing a sodium-retentive state that precedes hypertension.
This study offers a very novel mechanistic explanation as to how renal injury causes sodium retention.
Summary
Renal ACE is fundamental for the establishment of an anti-natriuretic state via modulation of the glomerular filtration rate and multiple sodium tubular transporters. This, in turn, impairs the capacity of the kidney to respond to a sodium load and results in hypertension.
Acknowledgments
SOURCES OF FUNDING
This study was supported by NIH grants R00DK083455 and R03DK101592 (to RAGV), R01HL110353 (to KEB), and R01DK083785 (To AMcD), a grant from the University Kidney Research Organization (to AMcD), and a grant from the AHA 13BGIA14680069 (to X.Z.S.). JFG has a postdoctoral fellowship from AHA (15POST22520015).
Footnotes
DISCLOSURES
RAGV is an employee and a stockholder of Pfizer, Inc. All other authors have no disclosures.
References
- 1.Sanada H, Jones JE, Jose PA. Genetics of salt-sensitive hypertension. Current hypertension reports. 2011;13:55–66. doi: 10.1007/s11906-010-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lifton RP. Molecular genetics of human blood pressure variation. Science. 1996;272:676–680. doi: 10.1126/science.272.5262.676. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2012;39:96–103. doi: 10.1111/j.1440-1681.2011.05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco M, Tapia E, Bautista R, Pacheco U, Santamaria J, Quiroz Y, Johnson RJ, Rodriguez-Iturbe B. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. American journal of physiology. Renal physiology. 2013;304:F982–990. doi: 10.1152/ajprenal.00463.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-ii hypertension is blunted in interferon-gamma−/− and interleukin-17a−/− mice. Hypertension. 2015;65:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. The Journal of experimental medicine. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-c-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 8.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin ii-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin ii hypertension is attenuated in interleukin-6 knockout mice. American journal of physiology. 2006;290:H935–940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 10.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating t lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol. 2011;300:F734–742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirabo A, Fontana V, de Faria AP, et al. Dc isoketal-modified proteins activate t cells and promote hypertension. The Journal of clinical investigation. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden MR, Whaley-Connell A, Sowers JR. Renal redox stress and remodeling in metabolic syndrome, type 2 diabetes mellitus, and diabetic nephropathy: Paying homage to the podocyte. Am J Nephrol. 2005;25:553–569. doi: 10.1159/000088810. [DOI] [PubMed] [Google Scholar]
- 13.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–376. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ. The immunological basis of hypertension. American journal of hypertension. 2014;27:1327–1337. doi: 10.1093/ajh/hpu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, et al. The absence of intrarenal ace protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giani JF, Janjulia T, Kamat N, Seth DM, Blackwell WL, Shah KH, Shen XZ, Fuchs S, Delpire E, Toblli JE, Bernstein KE, McDonough AA, Gonzalez-Villalobos RA. Renal angiotensin-converting enzyme is essential for the hypertension induced by nitric oxide synthesis inhibition. Journal of the American Society of Nephrology : JASN. 2014;25:2752–2763. doi: 10.1681/ASN.2013091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JA, Williams IR, Capecchi MR, Taylor WR, Bernstein KE. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am J Pathol. 2007;170:2122–2134. doi: 10.2353/ajpath.2007.061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole J, Quach DL, Sundaram K, Corvol P, Capecchi MR, Bernstein KE. Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure. Circulation research. 2002;90:87–92. doi: 10.1161/hh0102.102360. [DOI] [PubMed] [Google Scholar]
- 20.Quiroz Y, Pons H, Gordon KL, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ, Rodriguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. American journal of physiology. Renal physiology. 2001;281:F38–47. doi: 10.1152/ajprenal.2001.281.1.F38. [DOI] [PubMed] [Google Scholar]
- 21.Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin ii-infused mice. Hypertension. 2009;54:120–126. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, Koenig S, Heinrich R, Hoecklin F, Pill J, Friedemann J, Schweda F, Gretz N, Schock-Kusch D. Transcutaneous measurement of renal function in conscious mice. American journal of physiology. Renal physiology. 2012;303:F783–788. doi: 10.1152/ajprenal.00279.2012. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Iturbe B, Johnson RJ. The role of renal microvascular disease and interstitial inflammation in salt-sensitive hypertension. Hypertens Res. 2010;33:975–980. doi: 10.1038/hr.2010.148. [DOI] [PubMed] [Google Scholar]
- 25.Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to angiotensin ii. Hypertension. 1999;33:1013–1019. doi: 10.1161/01.hyp.33.4.1013. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa T, Kang DH, Ohashi R, Suga S, Herrera-Acosta J, Rodriguez-Iturbe B, Johnson RJ. Tubulointerstitial disease: Role of ischemia and microvascular disease. Curr Opin Nephrol Hypertens. 2003;12:233–241. doi: 10.1097/00041552-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Urushihara M, Acres OW, Navar LG, Kobori H. Il-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Molecular and cellular endocrinology. 2009;311:24–31. doi: 10.1016/j.mce.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prieto MC, Gonzalez AA, Navar LG. Evolving concepts on regulation and function of renin in distal nephron. Pflugers Archiv : European journal of physiology. 2013;465:121–132. doi: 10.1007/s00424-012-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin ii in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krege JH, Kim HS, Moyer JS, Jennette JC, Peng L, Hiller SK, Smithies O. Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension. 1997;29:150–157. doi: 10.1161/01.hyp.29.1.150. [DOI] [PubMed] [Google Scholar]
- 31.Smithies O, Kim HS, Takahashi N, Edgell MH. Importance of quantitative genetic variations in the etiology of hypertension. Kidney international. 2000;58:2265–2280. doi: 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- 32.Kanagy NL, Fink GD. Losartan prevents salt-induced hypertension in reduced renal mass rats. The Journal of pharmacology and experimental therapeutics. 1993;265:1131–1136. [PubMed] [Google Scholar]
- 33.Buranakarl C, Mathur S, Brown SA. Effects of dietary sodium chloride intake on renal function and blood pressure in cats with normal and reduced renal function. American journal of veterinary research. 2004;65:620–627. doi: 10.2460/ajvr.2004.65.620. [DOI] [PubMed] [Google Scholar]
- 34.Wiggins WS, Manry CH, Lyons RH, Pitts RF. The effect of salt loading and salt depletion on renal function and electrolyte excretion in man. Circulation. 1951;3:275–281. doi: 10.1161/01.cir.3.2.275. [DOI] [PubMed] [Google Scholar]
- 35.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiological reviews. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 36.Khan F, Spicarova Z, Zelenin S, Holtback U, Scott L, Aperia A. Negative reciprocity between angiotensin ii type 1 and dopamine d1 receptors in rat renal proximal tubule cells. American journal of physiology. Renal physiology. 2008;295:F1110–1116. doi: 10.1152/ajprenal.90336.2008. [DOI] [PubMed] [Google Scholar]
- 37.Li D, Scott L, Crambert S, Zelenin S, Eklof AC, Di Ciano L, Ibarra F, Aperia A. Binding of losartan to angiotensin at1 receptors increases dopamine d1 receptor activation. Journal of the American Society of Nephrology : JASN. 2012;23:421–428. doi: 10.1681/ASN.2011040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.