Abstract
Purpose of review
Regulatory T cells (Treg) are now well established as vital participants in maintaining self tolerance and preventing autoimmunity. Tregs have already been shown to be effective in preventing graft-versus-host disease in clinical bone marrow transplantation, and numerous animal studies have suggested a therapeutic role for Treg in solid organ transplantation. Recent advances in Treg isolation and expansion have the field poised to perform trials of therapeutic Treg infusion in solid organ transplantation around the world. An important component of these trials will be the detection of infused cells and the assessment Treg activity after infusion.
Recent findings
Several animal studies have demonstrated that infused Treg migrate to transplanted tissue in the early period after transplantation. This finding has important implications for the interpretation of biopsy results in human trials. Recent refinements in Treg identification, quantification, and functional assays will be discussed in the context of immune monitoring.
Summary
Understanding the migration/localization and persistence of infused Treg into transplanted tissues as well as how they impact the peripheral immune response will be critical to the interpretation of early Treg trials.
Keywords: Regulatory T cells, organ transplant, clinical trials, immune monitoring
Introduction
Regulatory T cells (Treg) are now well established as critical modulators of the immune system and are essential for preventing autoimmune diseases(1). The therapeutic potential of Treg has now been extensively explored in animal models, establishing a strong rationale for testing their potential efficacy in preventing autoimmunity as well as alloimmunity in humans(2). Treg have already shown promise in preventing graft-versus-host disease in the setting of human bone marrow transplantation(3)(4, 5). Recent advances in ex-vivo expansion and manufacturing of polyclonally expanded Treg as well as donor-reactive Treg has made the infusion of clinically meaningful doses of Tregs feasible(6). Currently there are multiple groups worldwide preparing to test Treg in the setting of solid organ transplantation in phase I/II trials with most studies planning dose escalation(7).
Because these trials have been primarily designed to test safety, it is unlikely that they will yield efficacy data. Thus, much of the focus of the trials will be on mechanistic outcomes such as detection of infused Treg, longevity of infused Treg, and their impact on the overall immune responses of the recipients.
In this review, we will discuss recent data on infused Treg migration to allografts and how these may inform our interpretation of biopsy specimens from clinical trialsin humans. In addition, we will discuss recent advances in Treg identification, quantification of alloreactivity in the Treg pool, as well as functional assays that may help elucidate how the infusion of Treg impacts the immune system. These data will be particularly important to estimate the cell numbers required to significantly impact immune responses for subsequent efficacy trials.
Interpretation of Transplant biopsies following Treg cell therapy
A key question in Treg therapy is whether the administered Treg will migrate to the allograft, and how this will impact the histology of allograft biopsies. Treg appear to home similarly to Teff, including to sites of inflammation(8, 9). Due to the injury associated with surgery, as well as ischemia/reperfusion injury, allografts are known to recruit inflammatory cells as well as T lymphocytes. Another consideration is that, even in instances of spontaneous(9) or induced transplant tolerance(10), lymphocytes (including Treg) can be found within allografts. Foxp3 positive T cells have also been demonstrated in numerous human allograft biopsy studies(11, 12).
In disparate rodent transplant models, infused Treg have been shown to migrate to allografts and co-localize with Teff cells (13) (14, 15). Treg/Teff ratios of greater than 1:3 have been shown to be associated with graft survival, while lower ratios tend to be associated with rejection(6). Antigen specificity is not required for localization, though graft-infiltrating cells appear to be enriched for allospecific Treg(16). The preponderance of pre-clinical studies would therefore suggest that infused Treg should localize to the allograft. However, in preclinical models, Treg have been generally infused before or at the time of transplant, and in the absence of generalized immunosuppression. For safety reasons, immunosuppression will clearly need to be administered in Phase I/II trials, with an unknown impact on Treg migration and survival. Varied immunosuppressive regimens as well as timing of Treg administration are additional variables that may impact Treg migration.
An open question then is how allograft biopsies will appear and be interpreted in the upcoming clinical trials, especially in the early days to weeks following transplantation/Treg infusion. It is likely from preclinical data that infused Treg will migrate to the allograft and co-localize with potentially pathogenic T cells. With standard H&E staining, it will be impossible to distinguish between Treg and Teff cells within the graft. Thus, protocol biopsies in the absence of clinical signals will need to be interpreted with caution, as a lymphocytic infiltrate may not necessarily indicate rejection. It is even possible that Treg will localize to areas such as subendothelial areas, renal tubules or bile ducts, which would conventionally contribute to a diagnosis of rejection. Foxp3 staining of biopsy specimens may help distinguish Treg from Teff; however, activated human Teff cells are known to transiently express Foxp3. Stable expression of Foxp3 is dependent on stable epigenetic modification of an area within the Foxp3 gene termed the Treg-specific demethylated region, or TSDR. A pcr based method for quantitating frequency of non-methylated TSDR in peripheral blood samples has been recently devised(17). This methodology correlates well with multiparameter flow cytometry in the quantitation of Treg/Teff ratios(18). This assay can also be used for tissue samples and may be of particular value in this setting. However, this assay does not give information at the single cell level.
The interpretation of protocol biopsies will therefore be difficult in the absence of clinical indicators of rejection, especially in the early period after transplantation. The use of adjunctive modalities such as Foxp3 staining, TSDR analysis, and potentially gene expression/proteomic analysis, and clinical correlation should be extremely informative, along with eventual clinical data.
Identification and enumeration of Treg
Seminal studies by Sakaguchi and others demonstrated that CD4+ CD25hi T cells contained suppressive activity(19). Treg were subsequently shown to express the intracellular protein Foxp3, providing a molecular marker for Treg. From a technical standpoint, however, CD25 staining as well as Foxp3 staining can be problematic and often do not generate distinct cell populations, making accurate quantitation difficult. Importantly, a subsequent study showed that Treg activity in peripheral blood was shown to primarily reside in the CD127lo population, providing another maker to help separate Treg from Teff(20). More recently, the transcription factor Helios was correlated with Foxp3 expression, potentially discriminating natural Treg from induced Treg(21). Like Foxp3, however, Helios also can be expressed transiently by proliferating Teff cells and its expression should be interpreted in context(22). A more recent study demonstrated that approximately 10% of natural Treg clones do not express Helios, and therefore gating on Helios may miss a subpopulation of Treg (23). Nevertheless, it should serve as another marker to help separate most Treg from Teff, to improve quantitation. These additional markers may make identification more accurate. Overall the accurate identification of Treg is not straightforward and any quantitation should be planned/reviewed carefully. TSDR analysis can help quantify Treg/Teff ratios but cannot be used at the single cell level(18).
The number of Teff and Treg in humans is not known but is a critical factor to consider for Treg therapy and subsequent detection of infused cells. A recent paper has estimated on the order of 166 × 109 CD4+ Tcells in an average human, with approximately 13 × 109 Treg(24). Thus, in a patient that has not had T cell depletion as part of their immunosuppression, the infusion of even large numbers of Treg may not result in an appreciable rise in the overall numbers of Treg in the periphery. However, the pharmacokinetics and volume of distribution of exogenously manipulated/expanded Treg are not known.
In a trial of polyclonal Treg administered in new onset diabetics, the infusion of 10-30 × 106 Treg/kg resulted in readily detectable increases in overall Treg number for as long as 2 to 4 months after infusion. These patients were lymphoreplete and did not receive any other treatment. It is likely therefore, that increases in overall numbers of Tregs will be detectable in the peripheral blood of subjects after infusion, if given similar numbers of Treg. Lymphodepletion prior to infusion with agents such as anti-thymocyte globulin will likely make lower doses detectable, as exemplified by the results in the extremely lymphodepleted context of bone marrow transplantation.
Notably, it will not be possible to distinguish between endogenous Treg and infused Treg in humans using standard flow techniques. Additional techniques for labeling and/or tracking infused Treg have been developed but are beyond the scope of this review.
Quantification of Alloreactive Treg/Teff ratios
Preclinial studies of therapeutic Treg administration have shown that donorreactive Treg are more potent at suppressing donor-specific proliferation in vitro and in preventing allograft rejection in vivo(6). Additional lines of evidence suggest that achieving a ratio of Treg/Teff of approximately 1:3 or greater is important in conferring protection(19). Approximately 5-10% of Treg are reactive to a full MHC mismatch(24), similar to Teff. Thus, simply quantifying overall Teff and Treg numbers will not be sufficient to profile immunologic changes after Treg administration since most of the quantified cells are likely to be less relevant.
Classically, mixed lymphocyte reaction assays have been used to measure proliferation or cytokine production in bulk cultures. Some assessment of donor reactive Treg activity has been made by removing CD4+CD25hi T cells, but this gives no indication as to the frequency of either Teff or Treg. ELISPOT assays can also be used to quantitate the frequency of donor-reactive Teff but is not useful for determining the frequency of donor reactive Treg.
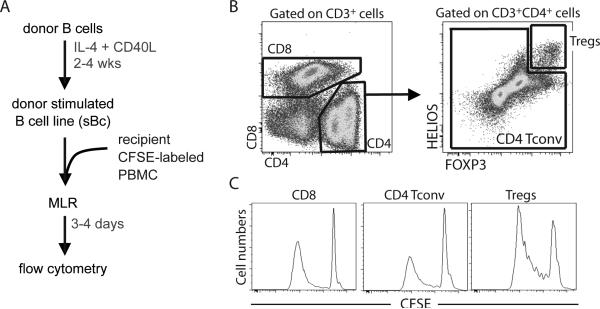
Recently, it has been shown that CD40L-stimulated B cells (sBc) can effectively drive proliferation of alloreactive Tregs (25). The use of CD40L-sBc as APC in MLR together with CFSE labeled responder cells allows simultaneous assessment of the proliferation of CD8+ and CD4+ Teff cells along with the proliferation of CD4+ Treg(Figure 1). Proliferated cells that have diluted CFSE can be identified after 4 days of culture and their precursor frequency can be readily back calculated. The presence of Treg in the samples does not appear to affect the proliferation of the effector cells, as depletion of Treg from the sample did not increase Teff proliferation (unpublished data). This is likely due to the high expression of CD80, CD86, and HLA on the CD40L-sBc, which like activated dendritic cells, can negate the suppressive effects of Treg on proliferation in vitro. Thus, this enhanced MLR can be used to quantify frequencies of donor-reactive Tregs and effector T cells in Treg therapy trials and other trials aimed at boosting Tregs.
Figure 1.
In Vitro Alloreactive T cell proliferation assay to simultaneously estimate the frequency of Teff and Treg. A) Schematic representation of assay. Donor B cells are expanded and stimulated with CD40L to generate highly stimulatory “sBc” with high levels of MHC, CD80 and CD86. Recipient CFSE-labeled PBMC are then mixed with sBc for 3-4 days and analysed by flow cytometry. B) Gating strategy to differentiate CD8 Teff, CD4 Teff, and CD4 Treg. C) Representative CFSE dilution profiles. (Tconv= Teff)
Functional Assays for Treg activity
A defining characteristic of Treg is that they suppress the proliferation of Teff in response to activation. In general Treg suppressive activity has been measured by progressive addition of Treg to responder Teff in vitro. Although Treg require stimulation through the TCR to gain suppressive activity, once they are activated, they suppress in a non-specific fashion, at least in vitro. It is important to distinguish assays that use non-specific activation from those that use antigen as non-specific activation. Assays that utilize non-specific TCR activation, for example with anti-CD3/CD28 antibody coated beads, will stimulate nearly all T cells and thus be a general measure of Teff/Treg activity. This type of assay will give no indication as to whether Treg activity for a specific antigen (e.g. alloantigen) has changed during the course of treatment. The administration of donor reactive Treg, for example, could theoretically make only a small impact on the overall Treg activity of a patient but have a significant impact on the pool of donor reactive Treg.
Assessment of Treg activity against a specific donor requires the use of donor APC as stimulators. The addition of Treg at increasing ratios to Teff then can be utilized to determine the potency of suppression against a donor. Teff proliferation can be measured using a variety of methods such as CFSE dilution or incorporation of labeled nucleotides. It is important to note that donor-reactive Teff activity could also be changing over time in a given recipient due to the presence of the graft as well as immunosuppression/modulation; thus, we believe it is important to use responder Teff from a single time point, ideally pre-transplant. These assays tend to require relatively high cell numbers, are technically demanding, and can demonstrate significant variability.
Overall, recent advances in determining Treg and Teff frequencies as well as functional activity will hopefully provide some indication as to the doses of Treg that need to be administered to have a significant impact on overall Treg as well as donor-reactive Treg populations. These data, in combination with specific tracking of the administered Treg, will help establish pharmacokinetic and pharmacodynamic data that will and provide important insights that will help guide the design of subsequent efficacy trials.
Conclusions
Trials evaluating the safety of Treg therapy in solid organ transplantation are poised to begin but will likely be underpowered or not designed to test efficacy. Thus, mechanistic data will play a large role in determining the impact of various doses of Treg and for the design of subsequent efficacy trials. Standard histological analysis of allograft biopsies may not be accurate due anticipated infiltration of the graft by the infused Treg; therefore, additional analyses such as immunohistochemistry to identify Treg vs. non-Treg will be required to characterize lymphocytic infiltrates. Additional information such as cellular damage, recruitment of other inflammatory cells, and perhaps gene expression profiling and/or proteomics will be useful to distinguish a “benign” infiltrate from a pathogenic infiltrate. Regardless, caution should be exercised before treating a histologic finding in the absence of clinical correlates of rejection.
Recent improvements in the identification of Treg, as well as assays to determine specificity of both Treg and Teff cells, will allow us to develop a pharmacokinetic profile of infused Treg and to understand the impact of dosing on overall Treg/Teff ratios as well as on the donor specific Treg/Teff ratios. Finally, new tracking technology fwill allow us to understand the relationship of the duration of survival of infused Treg with overall Treg/Teff ratios, and allow the identification of “infectious tolerance” in humans.
Acknowledgements
The authors received funding from NIAID 1U01AI110658-01 and the UCSF Transplant Innovative Fund,
Footnotes
S-M.K has no conflicts of interest to declare. QT is a co-inventor on two patents on regulatory T cell therapy and has received support from BD Biosciences and Neostem for the development of regulatory T cell therapy.
Contributor Information
Qizhi Tang, Department of Surgery, University of California, San Francisco 513 Parnassus Avenue, Box 0780, HSE520, San Francisco, CA 94143-0780, USA. Tel: +1 415 476 1739, Fax: +1 415 502 8326, Qizhi.Tang@ucsfmedctr.org
Sang-Mo Kang, Department of Surgery, University of California, San Francisco 513 Parnassus Avenue, Box 0780, HSE520, San Francisco, CA 94143-0780, USA. Tel: +1 415 502 5337, Fax: +1 415 502 8326, Sang-Mo.Kang@ucsfmedctr.org
References
- 1*.Waldmann H, Hilbrands R, Howie D, Cobbold S. Harnessing FOXP3+ regulatory T cells for transplantation tolerance. The Journal of clinical investigation. 2014;124(4):1439–45. doi: 10.1172/JCI67226. [excellent, concise overview of Treg in the context of transplanatation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. Journal of molecular cell biology. 2012;4(1):11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 5*.Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, et al. HLAhaploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124(4):638–44. doi: 10.1182/blood-2014-03-564401. [Phase II trial of donor Treg administration to prevent GVHD in patients with leukemia who were also given Teff along with donor bone marrow. Low GVH rates and cancer relapse were observed compared to historical controls.] [DOI] [PubMed] [Google Scholar]
- 6*.Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harbor perspectives in medicine. 2013;3(11) doi: 10.1101/cshperspect.a015552. [review of important considerations in applying Tregs to human disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissler EK, Hutchinson JA. Cell therapy as a strategy to minimize maintenance immunosuppression in solid organ transplant recipients. Current opinion in organ transplantation. 2013;18(4):408–15. doi: 10.1097/MOT.0b013e328363319d. [DOI] [PubMed] [Google Scholar]
- 8*.Issa F, Robb RJ, Wood KJ. The where and when of T cell regulation in transplantation. Trends in immunology. 2013;34(3):107–13. doi: 10.1016/j.it.2012.11.003. [excellent review of the role of Treg in transplantation and therapeutic prospects.] [DOI] [PubMed] [Google Scholar]
- 9**.Hu M, Wang C, Zhang GY, Saito M, Wang YM, Fernandez MA, et al. Infiltrating Foxp3(+) regulatory T cells from spontaneously tolerant kidney allografts demonstrate donor-specific tolerance. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(11):2819–30. doi: 10.1111/ajt.12445. [This study demonstrates that , in a spontaneously tolerant mouse model of renal transplantation, that Treg can be found in the allograft and can mediate tolerance upon adoptive transfer.] [DOI] [PubMed] [Google Scholar]
- 10.Farris AB, Taheri D, Kawai T, Fazlollahi L, Wong W, Tolkoff-Rubin N, et al. Acute renal endothelial injury during marrow recovery in a cohort of combined kidney and bone marrow allografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(7):1464–77. doi: 10.1111/j.1600-6143.2011.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bestard O, Cunetti L, Cruzado JM, Lucia M, Valdez R, Olek S, et al. Intragraft regulatory T cells in protocol biopsies retain foxp3 demethylation and are protective biomarkers for kidney graft outcome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(10):2162–72. doi: 10.1111/j.1600-6143.2011.03633.x. [DOI] [PubMed] [Google Scholar]
- 12.Chung BH, Oh HJ, Piao SG, Hwang HS, Sun IO, Choi SR, et al. Clinical significance of the ratio between FOXP3 positive regulatory T cell and interleukin-17 secreting cell in renal allograft biopsies with acute T-cell-mediated rejection. Immunology. 2012;136(3):344–51. doi: 10.1111/j.1365-2567.2012.03588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan TV, Tang Q, Liu FC, Hoang V, Bi M, Bluestone JA, et al. Requirements for prolongation of allograft survival with regulatory T cell infusion in lymphosufficient hosts. The Journal of surgical research. 2011;169(1):e69–75. doi: 10.1016/j.jss.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Lee K, Nguyen V, Lee KM, Kang SM, Tang Q. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(1):27–38. doi: 10.1111/ajt.12509. [this study demonstrates the need for “debulking” alloreactive Teff to prevent rejection in the absence of adjunct immunosuppression, and shows the infused donor reactive Treg migrate to iselt allografts.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho-Gaspar M, Jones ND, Luo S, Martin L, Brook MO, Wood KJ. Location and time-dependent control of rejection by regulatory T cells culminates in a failure to generate memory T cells. Journal of immunology. 2008;180(10):6640–8. doi: 10.4049/jimmunol.180.10.6640. [DOI] [PubMed] [Google Scholar]
- 16.Waldmann H, Adams E, Fairchild P, Cobbold S. Regulation and privilege in transplantation tolerance. Journal of clinical immunology. 2008;28(6):716–25. doi: 10.1007/s10875-008-9249-5. [DOI] [PubMed] [Google Scholar]
- 17.Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer research. 2009;69(2):599–608. doi: 10.1158/0008-5472.CAN-08-2361. [DOI] [PubMed] [Google Scholar]
- 18**.Nettenstrom L, Alderson K, Raschke EE, Evans MD, Sondel PM, Olek S, et al. An optimized multi-parameter flow cytometry protocol for human T regulatory cell analysis on fresh and viably frozen cells, correlation with epigenetic analysis, and comparison of cord and adult blood. Journal of immunological methods. 2013;387(1-2):81–8. doi: 10.1016/j.jim.2012.09.014. [excellent comparison between multi-parameter flow cytomety and TSDR analysis for identifying and quantifying Treg in clinical samples.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nature reviews Immunology. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. The Journal of experimental medicine. 2006;203(7):1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. Journal of immunology. 2010;184(7):3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PloS one. 2011;6(8):e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios-cells coexist within the natural FOXP3+ T regulatory cell subset in humans. Journal of immunology. 2013;190(5):2001–8. doi: 10.4049/jimmunol.1201379. [this study demonstrates that approximately 10% of natural Treg do not express Helios.] [DOI] [PubMed] [Google Scholar]
- 24.Tang Q, Lee K. Regulatory T-cell therapy for transplantation: how many cells do we need? Current opinion in organ transplantation. 2012;17(4):349–54. doi: 10.1097/MOT.0b013e328355a992. [DOI] [PubMed] [Google Scholar]
- 25*.Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(11):3010–20. doi: 10.1111/ajt.12433. [This study demonstrates the feasibility of GMP grade manufacturing of donor reactive Treg and includes details of a novel CFSE based proliferation assay using sBc to simultaneously estimate the frequency of donor reactive Teff and Treg.] [DOI] [PMC free article] [PubMed] [Google Scholar]