Abstract
Long-term renal function is compromised in patients receiving deceased donor kidneys which require cold storage exposure prior to transplantation. It is well established that extended cold storage induces renal damage and several labs, including our own, have demonstrated renal mitochondrial damage after cold storage alone. However, to our knowledge, few studies have assessed renal and mitochondrial function after transplantation of rat kidneys exposed to short-term (4 hr) cold storage compared to transplant without cold storage (autotransplantation). Our data reveal that cold storage plus transplantation exacerbated renal and mitochondrial dysfunction when compared to autotransplantation alone.
Keywords: Kidney, Mitochondria, Cold storage, Transplantation
Introduction
Kidney transplantation is the preferred modality of treatment for end stage kidney disease. While advances in tissue-type matching and immunosuppressive protocols have greatly reduced short-term graft dysfunction after renal transplantation, long-term graft function continues to be problematic, especially in patients receiving deceased donor kidneys [1–3]. The kidneys of deceased donors are routinely flushed and stored in cold storage (CS) solutions [4]. Short-term CS lowers the metabolic demand, yet, prolonged CS causes extensive tissue damage and is associated with a higher incidence of delayed graft function and poor long-term outcome after transplant [1,5,6]. Despite this, the mechanism of how CS negatively impacts overall graft function and survival is not well understood. Many laboratories, including our own, have published that renal CS (in vitro and ex vivo) induces mitochondrial injury, reactive oxygen species (ROS) generation, and cell death [7–12]. However, characterization of mitochondrial function after renal CS plus transplantation remains unknown. The respiratory enzyme complexes (complexes I-V) comprise the electron transfer chain, and through mitochondrial respiration generate ATP as the terminal product with ROS as a byproduct. When mitochondrial dysfunction occurs, cells are deprived of ATP and ROS are increased, consequently impacting numerous cellular pathways responsible for tissue regeneration/repair or damage. Therefore, our goal was to evaluate the contribution of short-term CS to early renal dysfunction and altered mitochondrial respiratory function following CS plus transplantation (CS/Tx) by comparing to autotransplant (ATx) (without CS exposure), and sham surgery.
Methods
Animals
Male Lewis rats weighing 200 – 250 g were used as transplant donors and recipients in this study. All of the animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Arkansas for Medical Sciences (UAMS), and all animal experiments described below were performed in compliance with the IACUC at UAMS using NIH guidelines.
Syngeneic rat renal transplant model with cold storage
Orthotopic renal transplant surgery was performed in male Lewis rats. For the donor rat surgery, rats were anesthetized using isoflourane, and the left kidney was flushed with cold saline solution containing heparin (100 units/ml) via the aorta. The left kidney was removed en bloc (with the renal artery, vein and ureter attached) and stored in University of Wisconsin (UW) solution at 4°C for 4 hr. For the recipient rat surgery, rats were anesthetized using isoflourane, the native left kidney was removed, and the donor left kidney was transplanted using end-to-end anastomosis as described previously. The surgical ischemia time was ~ 45 min. The right native kidney was immediately removed, making renal function dependent on the transplanted left kidney. The ureter was anastomosed end-to-end over a 5 mm PE-50 polyethylene stent. Postoperatively, the animals were given 0.9% (w/v) NaCl in the abdominal cavity and placed under a heating lamp to recover from the anesthesia. Rats were given buprenorphine (0.06 mg/kg, SC) for pain. After 24hr of reperfusion, the transplanted left kidney and blood were collected under anesthesia and saved as 4hr cold storage plus transplantation (4h CS/Tx) group (n = 4).
Control groups
Autotransplant surgery
The autotransplant (ATx) surgery was performed as described in the recipient surgery method, except that the left kidney was removed and immediately transplanted in the same rat without CS exposure, followed by right nephrectomy. The surgical ischemia time was ~ 45 min. After 24 hr, the transplanted kidney was harvested under anesthesia, and the organs were referred to as Autotransplantation (ATx) group (n=4).
Sham surgery
Rats underwent identical surgery (right nephrectomy), but without the renal transplantation (Sham operation). The right kidney from a donor rat was removed and the left kidney remained functioning for 24 hr, and then the sham kidney and blood were harvested and saved as the Sham group (n = 4).
Blood chemistry
Blood chemistry was determined in heparinized blood (arterial) using a hand-held clinical chemistry analyzer, iSTAT™ [13], and cartridges (CHEM8+) as described by the manufacturer (Vetscan®, Abaxis, USA).
High resolution respirometry (HRR)
Mitochondrial respiratory complex activity was measured in the saponin permeabilized renal biopsies by high resolution respirometry (HRR) (Oroboros instruments - Oxygraph-2k, Innsbruck, Austria), according to substrate-inhibitor-titration (SIT) protocol as described earlier [11,14]. DATLAB 4.2 software (Oroboros) was used to analyze data, and tissue respiration was shown as oxygen flux (pmol/mg/s).
ATP assay
An ATP-luciferase-based bioluminescence assay kit (Sigma, MO, USA) and TD 20/20 luminometer (Turner Designs Sunnyvale, CA, USA) were used to measure ATP levels in the sham and transplant kidney tissue lysates as described earlier [14].
Statistical analysis
Results are presented as mean ± standard error of the mean (S.E.M.) using Graph Pad Prism software (version 4.0). The Student’s t-test was used to compare differences between the mean of two groups at a 95% level of confidence. Differences with a P value <0.05 were considered statistically significant. Comparisons were made between the groups: sham vs. ATx, sham vs. 4hrCS/Tx, and AutoTx vs. 4hrCS/Tx.
Results and Discussion
We previously reported in isolated rat and pig kidneys that CS alone induced significant renal and mitochondrial injury [10,11]. In this study, we evaluated renal function after 4 hr CS combined with transplantation using a syngeneic transplantation model to minimize confounding effects of the host immune system. Similarly, the autotransplant model without CS was chosen to evaluate the effects of warm ischemia combined with surgical trauma on kidney and mitochondrial function following transplantation. Sham rats showed no significant changes in plasma creatinine (Cr; ~0.5 mg/dl) and blood urea nitrogen (BUN; ~20 mg/dl), but these parameters were increased after autotransplant (ATx; Figure 1A and 1B). However, rat kidneys exposed to 4 hr CS plus transplantation showed markedly increased levels of Cr and BUN (4hrCS/Tx, Figure 1A and 1B).
Figure 1.
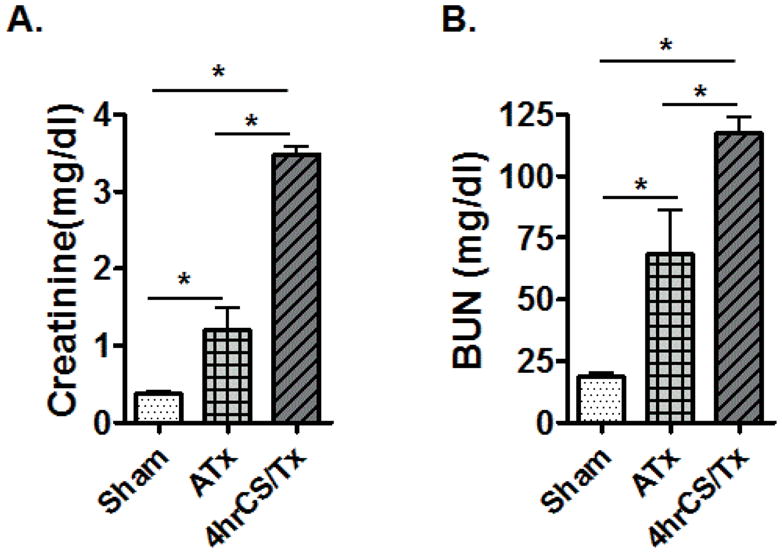
Cold storage worsens renal function after transplantation. Whole blood (arterial) creatinine and blood urea nitrogen (BUN) from the sham, autotransplant (ATx), and 4hCS/Tx rats were analyzed using hand-held clinical chemistry analyzer (iSTAT™) and Chem8+ cassettes as described in materials and methods. Values were expressed as Mean ± S.E.M. (n = 3–4); * indicates means are significantly different (P < 0.05).
Our laboratory previously showed that CS (4 hr) alone induces altered renal mitochondrial function (reduced respiratory complex I-IV activity, using a spectrophotometric assay and isolated renal mitochondria), leading to renal tubular injury [10]. However, it was not clear if mitochondrial damage extended to post-transplant. In the current study, High Resolution Respirometry (HRR) was used, which, in contrast to the spectrophotometric assay, monitors real-time mitochondrial respiration in fresh renal biopsies, thereby excluding the possibility of introducing assay artifacts on functional analysis via mitochondrial isolation.
A significant decline in renal mitochondrial complex I respiratory function (~50%) was detected by reduced oxygen flux following CS/Tx when compared to sham or ATx groups (Figure 2A). Similarly, complex III activities was significantly declined after CS/Tx when compared to ATx (Figure 2A). There was no change in complex II or IV activity following CS/Tx when compared to sham or ATx. To our knowledge, this is the first report demonstrating a reduction of renal mitochondrial respiratory complex (I and III) activity following renal CS/Tx. Since compromised respiratory complex activity can lead to reduced ATP levels, we also evaluated ATP levels. CS plus transplantation showed a dramatic loss of renal ATP when compared to the sham or ATx ATP levels (Figure 2B). These data suggest that short-term (4 hr) CS negatively impacts mitochondrial function following transplantation.
Figure 2.
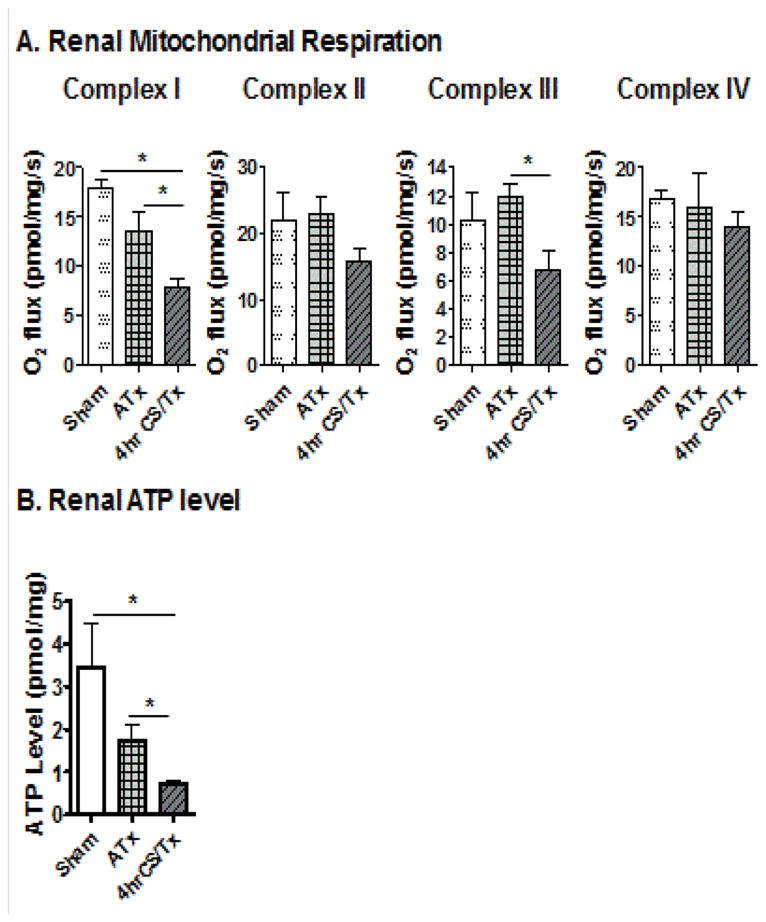
Cold storage worsens mitochondrial function after transplantation. (A) Graph showing respiratory complex I, II, III, and IV activity of the electron transport chain using high resolution respirometry in sham kidneys, or after transplantation with 4 hr cold storage (CS/Tx) or without CS (autotransplant, ATx). (B) Graph showing ATP levels in renal homogenates in sham, CS plus transplantation (CS/Tx), or autotransplant (ATx) groups. Error bar indicates Mean ± S.E.M. (n = 3–4); *indicates means are significantly different (P < 0.05).
Several other studies have documented renal dysfunction following various times of CS, although many are much longer than 4 hr [4,15,16]. Our study revealed, for the first time, that CS (4 hr) leads to a selective respiratory complex inhibition (I and III, but not II and IV) and ATP depletion following transplantation. Therefore, we anticipate that loss of mitochondrial function is a critical event that leads to energy depletion and exacerbated renal dysfunction during CS/Tx.
The profound decline in complex I activity occurring after CS/Tx is significant because complex I has been shown to be a major source of ROS in many diseases [17–19]. In addition, complex III, which was also inactivated after CS/Tx, also participates in ROS generation [20–22]. Future studies will evaluate CS-induced oxidant generation and its involvement in the mechanism of respiratory complex inactivation following CS/Tx. Similarly, additional studies employing longer CS and reperfusion times are warranted to evaluate how CS negatively impacts mitochondrial function that causes sustained loss of ATP following CS/Tx. One limitation of the current study is that rat kidneys were harvested from living animals, whereas clinically the majority of kidney donors are harvested from deceased patients. Future studies could be performed using a non-heart beating donor rat prior to CS, but the rationale for the current study was to dissect the effect that CS alone has on mitochondrial damage. Another limitation is certainly the time of CS as well as reperfusion, and new studies are underway to determine the extent of mitochondrial/renal damage with longer CS times (up to 24 hr). Finally, new studies will need to look at 3–7 days post-transplant to determine if function is improved or worsened.
Acknowledgments
We thank National Institutes of Health (NIH) and for financial support. We also would like to thank Pragya Singh for technical help.
Grant
National Institutes of Health (NIH) grants: RO1DK078936 (LAMC), T32DK061921 (NP), and T32 GM106999 (SS).
References
- 1.Debout A, Foucher Y, Trébern-Launay K, Legendre C, Kreis H, et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015;87:343–349. doi: 10.1038/ki.2014.304. [DOI] [PubMed] [Google Scholar]
- 2.Floerchinger B, Oberhuber R, Tullius SG. Effects of brain death on organ quality and transplant outcome. Transplant Rev (Orlando) 2012;26:54–59. doi: 10.1016/j.trre.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Salahudeen AK. Consequences of cold ischemic injury of kidneys in clinical transplantation. J Investig Med. 2004;52:296–298. doi: 10.1136/jim-52-05-28. [DOI] [PubMed] [Google Scholar]
- 4.Hoeger S, Lueg G, Tsagogiorgas C, Schneider M, Theisinger S, et al. UW is superior compared with HTK after prolonged preservation of renal grafts. J Surg Res. 2011;170:e149–157. doi: 10.1016/j.jss.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Hoeger S, Petrov K, Reisenbuechler A, Fontana J, Selhorst J, et al. The additional detrimental effects of cold preservation on transplantation-associated injury in kidneys from living and brain-dead donor rats. Transplantation. 2009;87:52–58. doi: 10.1097/TP.0b013e318191b2ca. [DOI] [PubMed] [Google Scholar]
- 6.van der Vliet JA, Warlé MC. The need to reduce cold ischemia time in kidney transplantation. Curr Opin Organ Transplant. 2013;18:174–178. doi: 10.1097/MOT.0b013e32835e2a08. [DOI] [PubMed] [Google Scholar]
- 7.Salahudeen AK, Huang H, Joshi M, Moore NA, Jenkins JK. Involvement of the mitochondrial pathway in cold storage and rewarming-associated apoptosis of human renal proximal tubular cells. Am J Transplant. 2003;3:273–280. doi: 10.1034/j.1600-6143.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 8.Green CJ, Healing G, Lunec J, Fuller BJ, Simpkin S. Evidence of free-radical-induced damage in rabbit kidneys after simple hypothermic preservation and autotransplantation. Transplantation. 1986;41:161–165. doi: 10.1097/00007890-198602000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell T, Saba H, Laakman J, Parajuli N, Macmillan-Crow LA. Role of mitochondrial-derived oxidants in renal tubular cell cold-storage injury. Free Radic Biol Med. 2010;49:1273–1282. doi: 10.1016/j.freeradbiomed.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell T, Rotaru D, Saba H, Smith RA, Murphy MP, et al. The mitochondria-targeted antioxidant mitoquinone protects against cold storage injury of renal tubular cells and rat kidneys. J Pharmacol Exp Ther. 2011;336:682–692. doi: 10.1124/jpet.110.176743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parajuli N, Campbell LH, Marine A, Brockbank KG, Macmillan-Crow LA. MitoQ blunts mitochondrial and renal damage during cold preservation of porcine kidneys. PLoS One. 2012;7:e48590. doi: 10.1371/journal.pone.0048590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cekauskas A, Bruns H, Manikas M, Herr I, Gross ML, et al. Sulforaphane decreases kidney injury after transplantation in rats: role of mitochondrial damage. Ann Transplant. 2013;18:488–496. doi: 10.12659/AOT.884013. [DOI] [PubMed] [Google Scholar]
- 13.Mock T, Morrison D, Yatscoff R. Evaluation of the i-STAT system: a portable chemistry analyzer for the measurement of sodium, potassium, chloride, urea, glucose, and hematocrit. Clin Biochem. 1995;28:187–192. doi: 10.1016/0009-9120(95)90708-6. [DOI] [PubMed] [Google Scholar]
- 14.Patil NK, Parajuli N, Macmillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306:F734–F743. doi: 10.1152/ajprenal.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goujon JM, Vandewalle A, Baumert H, Carretier M, Hauet T. Influence of cold-storage conditions on renal function of autotransplanted large pig kidneys. Kidney Int. 2000;58:838–850. doi: 10.1046/j.1523-1755.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- 16.Sener A, Tran KC, Deng JP, Garcia B, Lan Z, et al. Carbon monoxide releasing molecules inhibit cell death resulting from renal transplantation related stress. J Urol. 2013;190:772–778. doi: 10.1016/j.juro.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Ruiz I, Fernandez-Moreira D, Solis-Munoz P, Rodriguez-Juan C, Diaz-Sanjuan T, et al. Mitochondrial complex I subunits are decreased in murine nonalcoholic fatty liver disease: implication of peroxynitrite. J Proteome Res. 2010;9:2450–2459. doi: 10.1021/pr9011427. [DOI] [PubMed] [Google Scholar]
- 18.He Y, Leung KW, Zhang YH, Duan S, Zhong XF, et al. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Invest Ophthalmol Vis Sci. 2008;49:1447–1458. doi: 10.1167/iovs.07-1361. [DOI] [PubMed] [Google Scholar]
- 19.Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 22.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]


