Abstract
Background
There is a significantly higher incidence of cardiovascular disease (CVD) among type 1 diabetic (T1D) patients than among non-diabetic subjects. T1D is associated with hyperketonemia, a condition with elevated blood levels of ketones, in addition to hyperglycemia. The biochemical mechanism by which vitamin D (VD) may reduce the risk of CVD is not known. This study examines whether VD can be beneficial in reducing hyperketonemia (acetoacetate, AA) induced oxidative stress in endothelial cells.
Methods
HUVEC were pretreated with 1,25(OH)2D3, and later exposed to the ketone body acetoacetate.
Results
The increases in ROS production, ICAM-1 expression, MCP-1 secretion, and monocyte adhesion in HUVEC treated with AA were significantly reduced following treatment with 1,25(OH)2D3. Interestingly, an increase in glutathione (GSH) levels was also observed with 1,25(OH)2D3 in ketone treated cells. The effects of 1,25(OH)2D3 on GSH, ROS, and monocyte-endothelial adhesion were prevented in GCLC knockdown HUVEC. This suggests that 1,25(OH)2D3 inhibits ROS, MCP-1, ICAM-1, and adherence of monocytes mediated by the upregulation of GCLC and GSH.
Conclusion
This study provides evidence for the biochemical mechanism through which VD supplementation may reduce the excess monocyte adhesion to endothelium and inflammation associated with T1D.
Keywords: Vitamin D; 1,25(OH)2D3; oxidative stress; endothelium; ketones; type 1 diabetes
Introduction
Epidemiological studies have demonstrated that there is a prevalence of 25(OH)-vitamin D (VD) deficiency in the population worldwide [1–4]. Diabetic patients show a higher incidence of 25(OH)VD deficiency [5–7]. 25(OH)VD deficiency has been associated with an increased risk of all-cause and cardiovascular mortality [8–11]. Studies show that low levels of 25(OH)VD may detrimentally affect vascular function [12, 13], and that supplementation with VD can be beneficial in improving endothelial function [14–16]. Several in vitro and in vivo studies provide evidence to the beneficial role of VD in the vasculature. VD supplementation in VD-deficient diabetic patients was shown to improve endothelial function [17]. Similar improvement with VD was shown in stroke patients [18]. VD significantly downregulated platelet activating factor-induced intercellular adhesion molecule-1 (ICAM-1) expression in pulmonary microvascular endothelial cells isolated from Sprague Dawley rats; in addition, the adhesion rate of polymorphonuclear leukocytes to these cells was decreased [14]. LPS induced ICAM-1 expression was downregulated by VD in peripheral blood mononuclear cells isolated from patients as well as in HUVEC [19]. Tumor necrosis factor alpha (TNF-α) induced vascular cell adhesion molecule-1 (VCAM-1) and interleukin-8 (IL-8) upregulation was reported to be inhibited in coronary artery endothelial cells [20]. Additionally, the effects of VD in downregulating the pro-inflammatory profile that includes the reduced expression of cytokines (IL-6, TNF-α) and chemokines (IL-8), cholesterol uptake, and endoplasmic reticular stress in monocytes from type 2 and type 1 diabetic patients were also shown [9, 21, 22]. The potential role of VD in reducing the level of oxidative stress and monocyte adhesion is not known.
Development of atherosclerosis is more frequently observed in children and adolescents with type 1 diabetes (T1D) than in healthy control subjects despite insulin treatment and controlled glycemia [23]. Diabetic patients have elevated levels of oxidative stress and inflammation that can increase their risk for atherosclerosis and CVD [24–28]. Oxidative stress and monocyte adhesion to the endothelium are considered important in the progression of atherosclerosis [29]. T1D is most commonly associated with hyperketonemia along with hyperglycemia. Previous studies have shown that hyperketonemia can bring about an increase in the lipid peroxidation in T1D patients [30]. In vitro data have provided evidence that ketones can upregulate oxygen radical generation [31] along with the expression of adhesion molecules such as ICAM-1 and the secretion of IL-8 and MCP-1 cytokines [32, 33]. It has also been shown that ketone treatment increases monocyte-endothelial adhesion, considered as an important and critical step in the initiation of atherosclerosis [32].
This study investigates the hypothesis that supplementation with 1,25(OH)2D3 can inhibit ketone induced ROS production due to the VDR mediated upregulation of GCLC (and GSH), thereby reducing ICAM-1 upregulation, and adhesion of monocytes in HUVEC.
Materials and Methods
Human umbilical vein endothelial cells (HUVEC)
HUVEC were purchased from Lonza (Walkersville Inc., Walkersville, MD) and cultured in Endothelial Growth Medium-2 BulletKit (Lonza) as described previously [34]. Cells were pretreated with vitamin D (1,25(OH)2D3; 25 nM) for 24 h and then exposed to acetoacetate (AA; 4 mM) for 24 h.
Human THP-1 Monocytes
Human THP-1 monocytes were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in RPMI 1640 medium as described previously [34]. Cells were pretreated with vitamin D (1,25(OH)2D3; 25 nM) and then with acetoacetate (AA; 4 mM). HUVEC and THP-1 cells were cultured in glucose conditions similar to that used in previous publications [35]. The concentration of 25 nM 1,25(OH)2D3 was selected based on the consistency of our initial results and previous studies [36].
ROS assay
Cells grown to subconfluence were first pretreated with 1,25(OH)2D3 (25 nM) for 24 h and then exposed to AA for 24 h. The oxidant-sensitive probe dicholorodihydrofluorescein diacetate (H2DCFDA, Sigma Chemical Co., St. Louis, MO, USA) at a concentration of 20μM was added, and the cells were incubated at 37°C for 30 minutes. Fluorescence was measured using the plate reader with the filter settings of excitation 485 nm and emission 528 nm.
Western blotting
After treatment HUVEC were lysed in RIPA lysis buffer with protease and inhibitors purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Lysates were then centrifuged for 15 min at 13,000 rpm at 4°C. Supernatants were collected and the protein concentrations were determined using a BCA assay kit (Pierce/Thermo Scientific, Rockford, IL). An aliquot of 20 μg of protein from each sample lysate was prepared in sample buffer and run on 8% Tris-SDS acrylamide gels as described previously [34, 37]. ICAM-1 primary antibody was purchased from Santa Cruz Biotechnology, Inc. GCLC, p-NF-κB, NF-κB, β-Actin, α-Tubulin, and VDR primary antibodies used in this study were purchased from Abcam, Inc. (Cambridge, MA).
Monocyte-HUVEC adhesion assay
HUVEC were plated and allowed to grow to confluent monolayers. They were pretreated with 1,25(OH)2D3 (25 nM) for 24 h and then exposed to AA (4 mM) for 24 h. Monocytes were labeled with 8 μM CellTracker Green (CMFDA; Invitrogen, Eugene, OR) and then treated with 1,25(OH)2D3 and AA at the same time as were the HUVEC. After 24 h monocytes were added to the endothelial monolayers and incubated. The fluorescent intensity of the monocytes added to the monolayer (input) and the nonadherent cells was measured at excitation 485 nm and emission 528 nm as previously described [32, 34, 37].
Cytokine assays
MCP-1 levels were determined using ELISA method with commercially available ELISA kit purchased from R&D Systems, Inc. (Minneapolis, MN). The chemokine concentrations were determined by evaluating the supernatant from endothelial cells. All appropriate controls and standards as specified by the manufacturer’s kit were used. The data were expressed as picograms per milliliter of cell supernatant.
siRNA knockdown in HUVEC
GCLC and VDR siRNA were purchased from Santa Cruz Biotechnology. siRNA was diluted in serum free transfection medium (Santa Cruz) and mixed with lipofectamine (Invitrogen) and then plated into wells as described previously [34]. Confluent cells were used to perform ROS, Western blotting, or adhesion assays.
QPCR analysis
TRIzol reagent (Invitrogen) was used to extract RNA from HUVEC. The concentration of the extracted RNA was determined on a NanoDrop spectrophotometer (Thermo Scientific). High Capacity RNA-To-cDNA kit (Invitrogen) was used to synthesize cDNA. QPCR was performed on a 7900HT Real Time PCR system and software (Applied Biosystems) using the primer/probe set Hs00155249_m1 for GCLC, Hs00172113_m1 for VDR, and Hs02758991_g1 for GAPDH (Invitrogen) respectively. The relative amount of mRNA was calculated using the relative quantification (ΔΔCT) method.
GSH analysis
GSH analysis was carried out following the modified method of Pfeiffer et al. [38]. Cell lysates were used to determine GSH levels using high-performance liquid chromatography (Waters, Milford, MA) complexed to a 2475 fluorescent detector at settings 385 nm emission and 515 nm excitation as described earlier [39].
All chemicals, unless specified, were purchased from Sigma. Analysis of data was carried out with Sigma Plot statistical software using one way analysis of variance (ANOVA, SPSS, Chicago, IL, USA). A p value of 0.05 or less was considered significant.
Results
Effect of 1,25(OH)2D3 inhibition of monocyte adherence to the endothelial cells
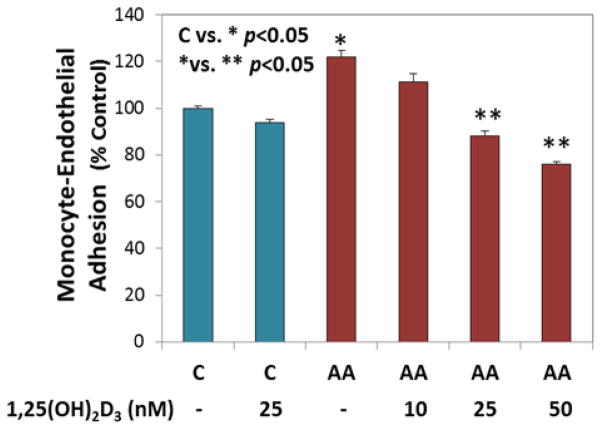
Hyperketonemia is the elevation of the ketone bodies acetoacetate (AA) and 3-β-hydroxybutyrate (BHB) in the blood of diabetic patients [40]. Studies from our lab and others have shown that AA, but not BHB, increases both oxidative stress [31, 34], and adhesion molecule and cytokine expression [32, 33, 41, 42], promotes fatty acid peroxidation [43], and activates ERK1/2 and MAPK signaling mediated by oxidative stress [44]. Hence, we focused our studies only on AA. Monocyte-endothelial cell adhesion plays a critical role in the progression of inflammation and vascular disease. Treatment of cells with AA increased the adherence of monocytes to HUVEC when compared to that of non-treated cells. THP-1 monocyte adhesion to HUVEC with AA was significantly blocked by 1,25(OH)2D3 supplementation (Figure 1). There was a dose dependent response with different concentrations of 1,25(OH)2D3. The 25 nM treatment showed a significant downregulation in the percentage of monocytes that adhered to HUVEC as compared to that of 10nM, so we used the concentration of 25 nM in the following 1,25(OH)2D3 supplementation experiments. VD concentrations of 25 nM have been used in our previous studies as well [36].
Figure 1. Effect of 1,25(OH)2D3 supplementation on inhibition of monocyte adhesion to endothelial cells.
Adherence of monocytes to HUVEC was determined after both cell lines were pretreated with 1,25(OH)2VD followed by treatment with AA (4 mM). The difference between C (untreated control cells) vs. * (AA treated cells) is significant at a p value <0.05. The difference between * (AA treated cells) vs. ** (AA+1,25(OH)2VD treated cells) is significant at a p value <0.05. Values are mean±SE (n=3).
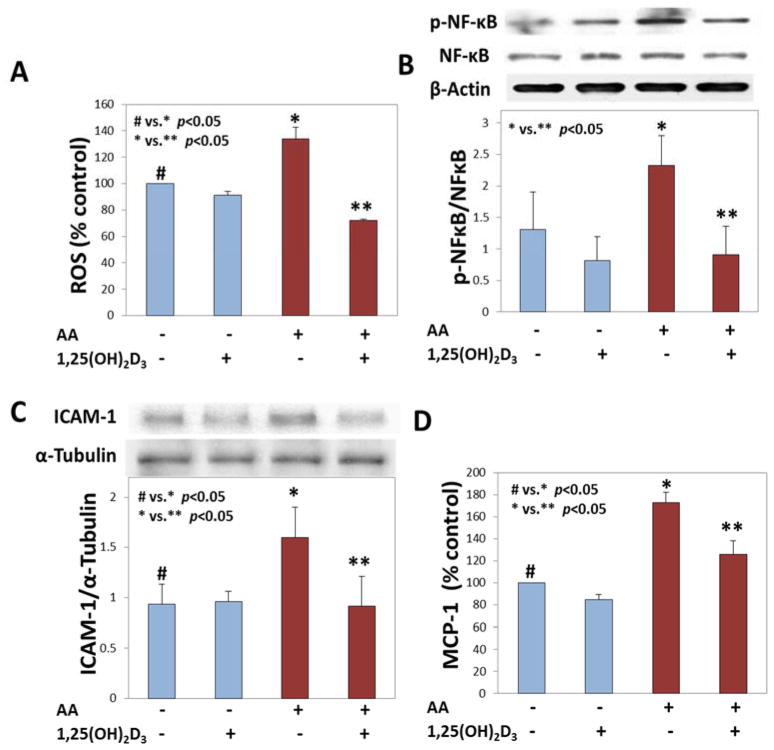
Effect of 1,25(OH)2D3 supplementation on ROS levels in HUVEC treated with acetoacetate
There is evidence from previous studies that 1,25(OH)2D3 supplementation has a beneficial effect on high glucose induced ROS production [39, 45]. This study investigated the effect of 1,25(OH)2D3 supplementation on AA induced ROS production (2A). 1,25(OH)2D3 supplementation significantly (p <0.05) reduced ROS production in AA treated cells. Activation of NF-κB by its phosphorylation is important for the transcription of various genes that are regulated by it. Treatment of HUVEC with AA increased the expression levels of p-NFκB compared to untreated cells. The inhibition of p-NFκB by 1,25(OH)2D3 is shown in Figure 2B. Each experiment was repeated at least 3 times and we believe that an n value greater than 3 might bring out the significant differences much clearly between groups.
Figure 2. Effect of 1,25(OH)2D3 supplementation on oxidative stress inhibition in ketone treated endothelial cells.
Panel A shows the ROS levels in HUVEC pretreated with 1,25(OH)2VD for 24 h and then treated with AA (4 mM) for another 24 h. Panel B shows a phospho-NF-κB blot and its quantification. Panel C shows ICAM-1 protein expression in cells pretreated with 1,25(OH)2VD (25 nM) for 24 h and then exposed to AA (4 mM) for a period of 24 h. MCP-1 secretion is shown in panel D. The difference between # (untreated control cells) vs. * (AA treated cells) is significant at a p value <0.05. The difference between * (AA treated cells) vs. ** (AA+1,25(OH)2VD treated cells) is significant at a p value <0.05. Values are mean±SE (n=3).
Effect of 1,25(OH)2D3 supplementation on downregulation of Adhesion molecule expression and cytokine secretion
ICAM-1 is an adhesion molecule that is characterized as a marker of atherosclerosis and CVD. Previously it was shown that treatment with AA increases the expression of ICAM-1. Here (Figure 2C) it is shown that supplementation with 1,25(OH)2D3 prior to AA treatment prevents this increase in its expression. There was a significant downregulation of ICAM-1 expression with 1,25(OH)2D3. The cytokine MCP-1 is important in mediating activation of monocytes, and functions along with ICAM-1 in mediating the adhesion of monocytes to endothelial cells. Figure 2D shows that cytokine MCP-1 secretion increases in cells that were exposed to AA. This secretion was significantly (p <0.05) reduced in the presence of 1,25(OH)2D3.
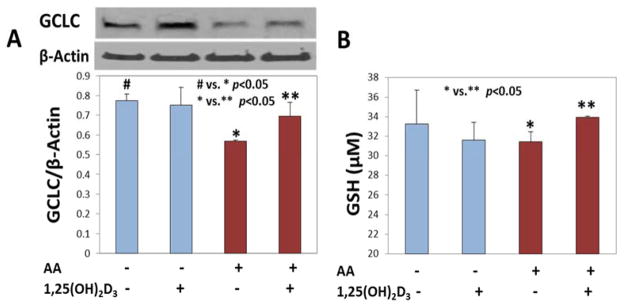
1,25(OH)2D3 supplementation increases glutathione levels
Glutamate cysteine ligase (GCL) is a key enzyme in glutathione (GSH) synthesis. It is a rate limiting enzyme composed of a catalytic (GCLC) subunit and a modifier (GCLM) subunit [46]. It was observed that in ketone treated HUVEC, the levels of GSH were reduced, which correlated with low expression of GCLC, but in the cells that received 1,25(OH)2D3, GCLC and GSH levels increased and were near to control levels. The expression of GCLC and the corresponding GSH levels in cells supplemented with 1,25(OH)2D3 and treated with AA are shown in Figure 3A and 3B respectively. The differences seen here are small, but significant. If the experiments were repeated more number of times the differences between groups would have been much sharper.
Figure 3. Effect of 1,25(OH)2D3 supplementation on increases in glutathione levels.
Panel A shows the expression of GCLC in AA treated and 1,25(OH)2VD supplemented HUVEC. The bar graph in panel B shows the cellular levels of GSH. The difference between # (untreated control cells) vs. * (AA treated cells) is significant at a p value <0.05 in panel A. The difference between * (AA treated cells) vs. ** (AA+1,25(OH)2VD treated cells) is significant at a p value <0.05. Values are mean±SE (n=3).
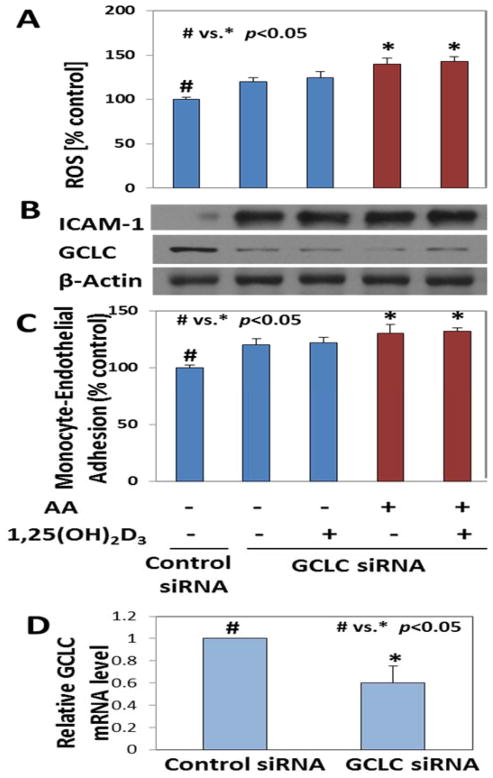
1,25(OH)2D3 supplementation has no beneficial effects in GSH deficient HUVEC
To investigate the importance of GCLC in mediating the beneficial effects of 1,25(OH)2D3, GCLC specific siRNA was used to knock it down. The GSH levels were significantly reduced (p <0.05) in these cells (39.13±0.8 μM) compared to those in control cells (53.07±1.4 μM). The ability of these cells to reduce basal levels of ROS is diminished, as seen by higher levels of ROS and ICAM-1 with GCLC knockdown, not only in the absence of ketone treatment, but also with 1,25(OH)2D3 supplementation. Results show that knocking down GCLC completely diminished the ability of 1,25(OH)2D3 to reduce ICAM-1. This suggests that 1,25(OH)2D3 increases GCLC expression, which in turn makes GSH and thereby enables a reduction in the levels of ketone-induced ROS. When GCLC is lacking, the beneficial effects of 1,25(OH)2D3 are not observed. ROS levels in GCLC knockdown cells are shown in Figure 4A. GCLC and ICAM-1 protein expression is shown in 4B and monocyte-endothelial adhesion in shown in panel 4C. GCLC mRNA levels in control and GCLC knockdown cells are shown in panel D of Figure 4.
Figure 4. Effect of 1,25(OH)2D3 supplementation on ROS and monocyte adhesion to endothelial cells in glutathione-deficient HUVEC.
The ROS levels in GCLC knockdown HUVEC treated with 1,25(OH)2VD and AA are shown in panel A. The knockdown efficiency of GCLC siRNA along with ICAM-1 protein expression is shown in panel B. The percentage of monocyte adhesion to the GCLC knockdown HUVEC is shown in panel C. Panel D shows the mRNA levels of GCLC in HUVEC. p<0.05 when untreated control cells (#) were compared with GCLC knockdown AA treated or AA+1,25(OH)2VD treated cells (*). 1,25(OH)2D3 supplementation has no beneficial effects in glutathione deficient HUVEC. Values are mean±SE (n=3).
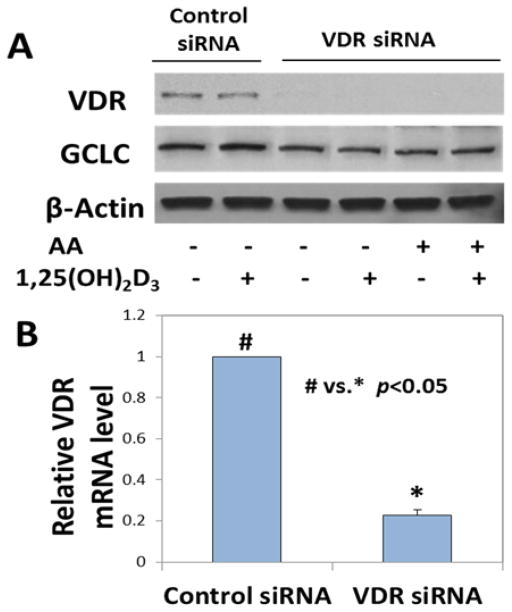
Effect of 1,25(OH)2D3 supplementation on GCLC in VDR-deficient HUVEC
VDR was knocked down with siRNA to investigate the role of VDR in mediating 1,25(OH)2D3 effects on increasing GCLC expression. It was observed that 1,25(OH)2D3 was unable to induce an upregulation in GCLC expression in the absence of VDR. Figure 5A shows that the GCLC upregulation that occurs with 1,25(OH)2D3 treatment is lost when cells do not have VDR. Knockdown efficiency is shown in Figure 5B.
Figure 5. Effect of 1,25(OH)2D3 supplementation on GCLC levels in vitamin D receptor-deficient HUVEC.
Panel A shows the expression levels of VDR and GCLC in VDR knockdown cells treated with 1,25(OH)2VD and ketones. The mRNA levels of VDR after knockdown are shown in panel B. p<0.05 when control siRNA cells (#) were compared with VDR knockdown cells (*). Values are mean±SE (n=3).
Discussion
Diabetes is known to increase the incidence of atherosclerosis in patients [47]. Risk factors such as oxidative stress [48], adhesion molecule expression [49], cytokine secretion [50], oxidized LDL formation [51], and monocyte-endothelial cell adhesion increase the progression and development of atherosclerosis. Hyperglycemia and hyperketonemia are hallmarks of T1D [52]. It has been suggested that T1D is associated with higher incidence of vascular inflammation and atherosclerosis [53]. T1D patients more frequently show elevated ketone levels in associated with an elevation in glucose levels in their blood. Previously we have studied the effects of the combination of ketones and high glucose together and found that in HUVEC the combination treatment showed an additive effect, while in adipocytes this was not the case [34, 54]. It is possible that ketones and high glucose might turn on different signaling pathways in these cells. The role of high glucose in endothelial dysfunction and vascular inflammation is already well documented [55, 56] and several studies have also looked at the beneficial effects of VD in diminishing high glucose mediated damage in the endothelium [45]. Various studies have stated the beneficial effect of VD supplementation, especially 1,25(OH)2D3, in the prevention of atherosclerosis and CVD [9, 11, 21, 57]. However, no previous study has examined the effect of 1,25(OH)2D3 supplementation on hyperketonemia induced ROS and monocyte-endothelial adhesion. There is evidence that ketones might play a role in increasing the risk of T1D patients to vascular dysfunction [32, 58]. The aim of this study is to look at alternative treatment options in reducing the endothelial cell dysfunction arising due to elevation in ketone levels. Ketones when elevated in the body can elicit responses that mimic the pro-inflammatory state [58–60]. Given the beneficial effects of VD in reducing the inflammatory markers we wanted to investigate the protective effects provided by VD in inhibiting ketone mediated oxidative stress and damage that could potentially inhibit the adherence of monocytes to the endothelial cells. This study suggests that 1,25(OH)2D3 supplementation reduces oxidative stress, NF-κB activation, ICAM-1 and MCP-1 levels leading to the inhibition of monocyte adhesion to HUVEC in AA treated cells.
The observation that 1,25(OH)2D3 increases GCLC expression and improves cellular GSH levels is novel. The beneficial effects of 1,25(OH)2D3 on ROS production and adhesion of monocytes to HUVEC were abolished in GCLC knockdown cells. This suggests that the upregulation of GCLC and GSH mediate the beneficial effect of 1,25(OH)2D3 on monocyte adhesion on endothelial cells. Knocking down VDR also eliminated the beneficial effect of 1,25(OH)2D3 as expected, thereby suggesting that the actions of 1,25(OH)2D3 are mediated via its receptor VDR in upregulating GCLC expression. Increase in GSH synthesis could be of tremendous importance in diabetic patients as they most often show GSH deficiency [61] along with deficiencies in VD [7]; therefore, VD supplementation in these patients could be vital in restoring normal cellular functions by increasing their GSH pools to combat with excess oxidative stress load.
Conclusions
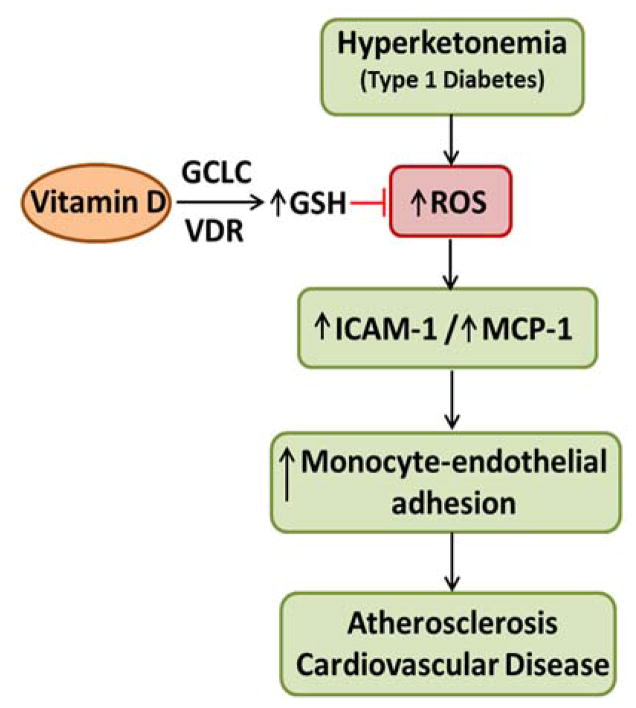
The role of VD in restoring the normal function of the endothelium is important not only in preventing monocyte adhesion to the endothelium, but also in preventing monocyte/macrophage transmigration into the intima or the underlying tissue. This study shows that 1,25(OH)2D3 plays a prominent role in suppressing ROS, regenerating GSH, and reversing the pro-atherogenic phenotype in HUVEC caused by hyperketonemia (Figure 6), emphasizing that intake of VD can prove beneficial in reducing the burden of oxidative stress in patients with T1D. Further studies in T1D animal models need to be carried out to demonstrate the beneficial effect of VD supplementation in the prevention of TID induced risk for vascular disease mediated by hyperketonemia.
Figure 6. Proposed mechanism by which vitamin D supplementation can reduce hyperketonemia induced endothelial cell dysfunction.
This schematic diagram represents the events that follow diabetic hyperketonemia, eventually leading to monocyte adherence to the endothelium that may progress into atherosclerosis. The various steps at which VD supplementation can have an effect in reducing ROS and the development of cardiovascular disease in T1D are indicated.
Highlights.
1,25(OH)2vitamin D inhibits ketone induced oxidative stress and monocyte adhesion in HUVEC.
Beneficial effects of 1,25 (OH)2vitamin D are impaired in cells that are deficient in glutathione.
1,25 (OH)2vitamin D upregulates glutathione in a vitamin D receptor-dependent manner.
Acknowledgments
The authors are supported by grants from NIDDK, the Office of Dietary Supplements of the National Institutes of Health RO1 DK072433 and RO1 AT007442, the Malcolm Feist Endowed Chair in Diabetes, and funded by a fellowship from the Malcolm Feist Cardiovascular Research Endowment from LSUHSC, Shreveport. The authors thank Georgia Morgan for excellent editing of this manuscript.
Abbreviations
- CVD
cardiovascular disease
- T1D
type 1 diabetes
- VD
vitamin D
- AA
acetoacetate
- ROS
reactive oxygen species
- ICAM-1
intercellular adhesion molecule-1
- MCP-1
monocyte chemotactic protein-1
- GSH
glutathione
- GCLC
glutamate cysteine ligase catalytic subunit
- TNF-α
Tumor necrosis factor alpha
- VCAM-1
vascular cell adhesion molecule-1
- and IL-8
interleukin-8
Footnotes
Conflict of interest
Authors declare they have no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daly RM, Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Sikaris KA, Zimmet PZ, Ebeling PR, Shaw JE. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: a national, population-based study. Clin Endocrinol (Oxf) 2012;77:26–35. doi: 10.1111/j.1365-2265.2011.04320.x. [DOI] [PubMed] [Google Scholar]
- 2.Farrokhyar F, Tabasinejad R, Dao D, Peterson D, Ayeni OR, Hadioonzadeh R, Bhandari M. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45:365–378. doi: 10.1007/s40279-014-0267-6. [DOI] [PubMed] [Google Scholar]
- 3.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez MM, Reiff e Vieira AC, Luiz RR, da Veiga GV. Validation of capillary glycemia as a strategy for the screening of diabetes mellitus in adolescents. Pediatr Diabetes. 2009;10:449–454. doi: 10.1111/j.1399-5448.2009.00508.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261–266. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Anagnostis P, Athyros VG, Adamidou F, Florentin M, Karagiannis A. Vitamin D and cardiovascular disease: a novel agent for reducing cardiovascular risk? Curr Vasc Pharmacol. 2010;8:720–730. doi: 10.2174/157016110792006978. [DOI] [PubMed] [Google Scholar]
- 9.Riek AE, Oh J, Sprague JE, Timpson A, de las Fuentes L, Bernal-Mizrachi L, Schechtman KB, Bernal-Mizrachi C. Vitamin D Suppression of Endoplasmic Reticulum Stress Promotes an Anti-Atherogenic Monocyte/Macrophage Phenotype in Type 2 Diabetic Patients. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M112.386912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Targher G, Bertolini L, Padovani R, Zenari L, Scala L, Cigolini M, Arcaro G. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–597. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 11.Weng S, Sprague JE, Oh J, Riek AE, Chin K, Garcia M, Bernal-Mizrachi C. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS One. 2013;8:e54625. doi: 10.1371/journal.pone.0054625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozos I, Marginean O. Links between Vitamin D Deficiency and Cardiovascular Diseases. BioMed research international. 2015;2015:109275. doi: 10.1155/2015/109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SF. 1 alpha, 25-Dihydroxyvitamin D3 decreased ICAM-1 and ELAM-1 expressions on pulmonary microvascular endothelial cells and neutrophil motivation. J Steroid Biochem Mol Biol. 1995;52:67–70. doi: 10.1016/0960-0760(94)00153-d. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Ichiyama T, Ohsaki A, Hasegawa S, Shiraishi M, Furukawa S. Anti-inflammatory effect of 1alpha,25-dihydroxyvitamin D(3) in human coronary arterial endothelial cells: Implication for the treatment of Kawasaki disease. J Steroid Biochem Mol Biol. 2009;113:134–138. doi: 10.1016/j.jsbmb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Sokol SI, Srinivas V, Crandall JP, Kim M, Tellides G, Lebastchi AH, Yu Y, Gupta AK, Alderman MH. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease, Vascular medicine (London. England) 2012;17:394–404. doi: 10.1177/1358863X12466709. [DOI] [PubMed] [Google Scholar]
- 17.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabetic medicine: a journal of the British Diabetic Association. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 18.Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients - a randomised controlled trial. Nutrition, metabolism and cardiovascular diseases: NMCD. 2012;22:864–870. doi: 10.1016/j.numecd.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Martinesi M, Treves C, d’Albasio G, Bagnoli S, Bonanomi AG, Stio M. Vitamin D derivatives induce apoptosis and downregulate ICAM-1 levels in peripheral blood mononuclear cells of inflammatory bowel disease patients. Inflamm Bowel Dis. 2008;14:597–604. doi: 10.1002/ibd.20354. [DOI] [PubMed] [Google Scholar]
- 20.Kudo K, Hasegawa S, Suzuki Y, Hirano R, Wakiguchi H, Kittaka S, Ichiyama T. 1alpha,25-Dihydroxyvitamin D(3) inhibits vascular cellular adhesion molecule-1 expression and interleukin-8 production in human coronary arterial endothelial cells. J Steroid Biochem Mol Biol. 2012;132:290–294. doi: 10.1016/j.jsbmb.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Riek AE, Oh J, Bernal-Mizrachi C. 1,25(OH)2 vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol. 2013;136:309–312. doi: 10.1016/j.jsbmb.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margeirsdottir HD, Stensaeth KH, Larsen JR, Brunborg C, Dahl-Jorgensen K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care. 2010;33:2043–2048. doi: 10.2337/dc10-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capellini VK, Celotto AC, Baldo CF, Olivon VC, Viaro F, Rodrigues AJ, Evora PR. Diabetes and vascular disease: basic concepts of nitric oxide physiology, endothelial dysfunction, oxidative stress and therapeutic possibilities. Curr Vasc Pharmacol. 2009;8:526–544. doi: 10.2174/157016110791330834. [DOI] [PubMed] [Google Scholar]
- 25.Fatehi-Hassanabad Z, Chan CB, Furman BL. Reactive oxygen species and endothelial function in diabetes. Eur J Pharmacol. 2010;636:8–17. doi: 10.1016/j.ejphar.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy Y, Blum S, Levy AP. Antioxidants in the prevention of atherosclerosis: the importance of proper patient selection. Clin Nutr. 2009;28:581–582. doi: 10.1016/j.clnu.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 28.van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YW, Kim PH, Lee WH, Hirani AA. Interleukin-4, Oxidative Stress, Vascular Inflammation and Atherosclerosis. Biomol Ther (Seoul) 2010;18:135–144. doi: 10.4062/biomolther.2010.18.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain SK, Kannan K, McVie R. Effect of hyperketonemia on blood monocytes in type-I diabetic patients and apoptosis in cultured U937 monocytes. Antioxid Redox Signal. 1999;1:211–220. doi: 10.1089/ars.1999.1.2-211. [DOI] [PubMed] [Google Scholar]
- 31.Jain SK, Kannan K, Lim G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic Biol Med. 1998;25:1083–1088. doi: 10.1016/s0891-5849(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 32.Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:E298–306. doi: 10.1152/ajpendo.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman WH, Cheng C, Passmore GG, Carroll JE, Hess D. Acetoacetate increases expression of intercellular adhesion molecule-1 (ICAM-1) in human brain microvascular endothelial cells. Neurosci Lett. 2002;334:71–74. doi: 10.1016/s0304-3940(02)00816-9. [DOI] [PubMed] [Google Scholar]
- 34.Kanikarla-Marie P, Jain SK. Hyperketonemia (acetoacetate) upregulates NADPH oxidase 4 and elevates oxidative stress, icam-1, and monocyte adhesivity in endothelial cells. Cell Physiol Biochem. 2015;35:364–373. doi: 10.1159/000369702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manna P, Jain SK. Effect of PIP3 on adhesion molecules and adhesion of THP-1 monocytes to HUVEC treated with high glucose. Cell Physiol Biochem. 2014;33:1197–1204. doi: 10.1159/000358688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manna P, Jain SK. Vitamin D Up-regulates Glucose Transporter 4 (GLUT4) Translocation and Glucose Utilization Mediated by Cystathionine-{gamma}-lyase (CSE) Activation and H2S Formation in 3T3L1 Adipocytes. J Biol Chem. 2012;287:42324–42332. doi: 10.1074/jbc.M112.407833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanikarla-Marie P, Jain SK. L-Cysteine supplementation reduces high-glucose and ketone-induced adhesion of monocytes to endothelial cells by inhibiting ROS. Mol Cell Biochem. 2014;391:251–256. doi: 10.1007/s11010-014-2009-3. [DOI] [PubMed] [Google Scholar]
- 38.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–292. [PubMed] [Google Scholar]
- 39.Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun. 2013;437:7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15:412–426. doi: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Rains JL, Jain SK. Effect of hyperketonemia (Acetoacetate) on nuclear factor-kappaB and p38 mitogen-activated protein kinase activation mediated intercellular adhesion molecule 1 upregulation in endothelial cells. Metab Syndr Relat Disord. 2015;13:71–77. doi: 10.1089/met.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rains JL, Kanikarla-Marie P, Jain SK. Hyperketonemia induces upregulation of LFA-1 in monocytes, which is mediated by ROS and P38 MAPK activation. Can J Physiol Pharmacol. 2012;90:1642–1646. doi: 10.1139/y2012-131. [DOI] [PubMed] [Google Scholar]
- 43.Harrison JE, Saeed FA. Acetoacetate is an electron donor to myeloperoxidase and a promoter of myeloperoxidase-catalyzed fatty acid peroxidation. Biochem Med. 1981;26:339–355. doi: 10.1016/0006-2944(81)90010-7. [DOI] [PubMed] [Google Scholar]
- 44.Abdelmegeed MA, Kim SK, Woodcroft KJ, Novak RF. Acetoacetate activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in primary cultured rat hepatocytes: role of oxidative stress. J Pharmacol Exp Ther. 2004;310:728–736. doi: 10.1124/jpet.104.066522. [DOI] [PubMed] [Google Scholar]
- 45.Zitman-Gal T, Golan E, Green J, Bernheim J, Benchetrit S. Vitamin D receptor activation in a diabetic-like environment: potential role in the activity of the endothelial pro-inflammatory and thioredoxin pathways. J Steroid Biochem Mol Biol. 2012;132:1–7. doi: 10.1016/j.jsbmb.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colwell JA, Lopes-Virella M, Halushka PV. Pathogenesis of Atherosclerosis in Diabetes Mellitus. Diabetes Care. 1981;4:121–133. doi: 10.2337/diacare.4.1.121. [DOI] [PubMed] [Google Scholar]
- 48.Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler Thromb Vasc Biol. 2007;27:2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 49.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 50.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 51.Steinberg D. The LDL modification hypothesis of atherogenesis: an update. J Lipid Res. 2009;50(Suppl):S376–381. doi: 10.1194/jlr.R800087-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain SK, McVie R, Bocchini JA., Jr Hyperketonemia (ketosis), oxidative stress and type 1 diabetes. Pathophysiology. 2006;13:163–170. doi: 10.1016/j.pathophys.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Marcovecchio ML, Chiarelli F. Microvascular disease in children and adolescents with type 1 diabetes and obesity. Pediatr Nephrol. 2010;26:365–375. doi: 10.1007/s00467-010-1624-9. [DOI] [PubMed] [Google Scholar]
- 54.Manna P, Gungor N, McVie R, Jain SK. Decreased cystathionine-gamma-lyase (CSE) activity in livers of type 1 diabetic rats and peripheral blood mononuclear cells (PBMC) of type 1 diabetic patients. The Journal of biological chemistry. 2014;289:11767–11778. doi: 10.1074/jbc.M113.524645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee CH, Shieh YS, Hsiao FC, Kuo FC, Lin CY, Hsieh CH, Hung YJ. High glucose induces human endothelial dysfunction through an Axl-dependent mechanism. Cardiovascular diabetology. 2014;13:53. doi: 10.1186/1475-2840-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis. 2007;193:328–334. doi: 10.1016/j.atherosclerosis.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 57.Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 58.Close TE, Cepinskas G, Omatsu T, Rose KL, Summers K, Patterson EK, Fraser DD. Diabetic ketoacidosis elicits systemic inflammation associated with cerebrovascular endothelial cell dysfunction. Microcirculation. 2013;20:534–543. doi: 10.1111/micc.12053. [DOI] [PubMed] [Google Scholar]
- 59.Jain SK, Kannan K, Lim G, Matthews-Greer J, McVie R, Bocchini JA., Jr Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003;26:2139–2143. doi: 10.2337/diacare.26.7.2139. [DOI] [PubMed] [Google Scholar]
- 60.Jain SK, Kannan K, Lim G, McVie R, Bocchini JA., Jr Hyperketonemia increases tumor necrosis factor-alpha secretion in cultured U937 monocytes and Type 1 diabetic patients and is apparently mediated by oxidative stress and cAMP deficiency. Diabetes. 2002;51:2287–2293. doi: 10.2337/diabetes.51.7.2287. [DOI] [PubMed] [Google Scholar]
- 61.Sekhar RV, McKay SV, Patel SG, Guthikonda AP, Reddy VT, Balasubramanyam A, Jahoor F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2010;34:162–167. doi: 10.2337/dc10-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]