Abstract
Reorganization of the actin cytoskeleton during mitosis is crucial for regulating cell division. A functional role for γ-actin in mitotic arrest induced by the microtubule-targeted agent, paclitaxel, has recently been demonstrated. We hypothesized that γ-actin plays a role in mitosis. Herein, we investigated the effect of γ-actin in mitosis and demonstrated that γ-actin is important in the distribution of β-actin and formation of actin-rich retraction fibers during mitosis. The reduced ability of paclitaxel to induce mitotic arrest as a result of γ-actin depletion was replicated with a range of mitotic inhibitors, suggesting that γ-actin loss reduces the ability of broad classes of anti-mitotic agents to induce mitotic arrest. In addition, partial depletion of γ-actin enhanced centrosome amplification in cancer cells and caused a significant delay in prometaphase/metaphase. This prolonged prometaphase/metaphase arrest was due to mitotic defects such as uncongressed and missegregated chromosomes, and correlated with an increased presence of mitotic spindle abnormalities in the γ-actin depleted cells. Collectively, these results demonstrate a previously unknown role for γ-actin in regulating centrosome function, chromosome alignment and maintenance of mitotic spindle integrity.
Keywords: γ-actin, abnormal mitotic spindles, centrosome amplification, mitosis, mitotic abnormalities, mitotic progression
Introduction
During mitosis the actin cytoskeleton rapidly rearranges and localizes to the cortical plasma membrane in early mitotic phases and to the contractile ring during cytokinesis.1 This recruitment of actin and actin regulatory proteins to the cell cortex during mitosis is essential for the interaction between astral microtubules and cortical actin, which is believed to be important in regulating mitotic spindle orientation.2-4 The actin filaments together with several actin regulatory proteins such as myosin-10,5,6 myosin II,7-11 moesin12-14 and formin 215-17 have been implicated to be critically important in regulating mitosis and/or meiosis in various systems. The role of actin and its regulatory proteins in these processes ranges from regulating centrosome separation18,19 to proper spindle assembly and orientation6-8,12,13,15-17 to elongation of kinetochore microtubules.11,20 Importantly, disruption of the actin cytoskeleton affects mitotic and/or meiotic spindle structure and function, resulting in mitotic defects6-8,11-13,20 and/or meiotic defects.15-17 These studies provide evidence of how critically important actin and its regulatory proteins are in regulating cell division. However, the relative contribution of the 2 major non-muscle β-actin and γ-actin isoforms to mitotic progression is poorly understood.
Previous studies from our group have demonstrated a role for γ-actin in cell polarity and motility21 and angiogenesis,22 as well as resistance to microtubule-targeted agents.23 Microtubule-targeted agents such as the anti-cancer drug paclitaxel, induce mitotic arrest and subsequent cell death. In this regard, we have recently demonstrated that partial depletion of γ-actin suppresses microtubule dynamics and also inhibits paclitaxel-induced mitotic arrest,24 suggesting that γ-actin may be playing an important role that impacts microtubule function during mitosis. To functionally dissect the role of γ-actin in mitosis, we have used confocal microscopy and live cell imaging, coupled with γ-actin siRNA knockdown. Our findings identify γ-actin to be involved in regulating centrosome function, chromosome alignment, mitotic spindle integrity and mitotic progression.
Results
Partial depletion of γ-actin affects β-actin distribution and reduces formation of retraction fibers during mitosis
Changes in γ-actin expression have been shown to affect the activity of anti-microtubule agents.23,24 To gain an understanding of how partial depletion in γ-actin can inhibit microtubule-targeted agent induced mitotic arrest,24 we initially examined the localization and reorganization of β-actin and γ-actin during mitosis following siRNA-mediated knockdown of γ-actin in SH-EP transfected neuroblastoma cells. SH-EP cells were used in this study as they have good (~100%) transfection efficiency with siRNA24 making them suitable for single cell imaging. In addition, successful siRNA-mediated knockdown of γ-actin protein expression (~50%) in the SH-EP cell line has been previously demonstrated.23
β-Actin and γ-actin display distinct localization in interphase SH-EP cells which is consistent with our previous finding.21 β-Actin was predominantly localized to the actin stress fibers (Fig. 1A) whereas γ-actin was observed as a fine meshwork of actin filaments throughout the cell body and also found at the cell periphery, near lamella and lamellipodial-like structures (Fig. 1A). However, when γ-actin was partially depleted in SH-EP cells, formation of prominent stress fibers that lack lamella and lamellipodial-like structures was observed, with γ-actin being more prominent in the stress fibers (Fig. 1B). In dividing SH-EP cells, β-actin was observed throughout the cell body, cell membrane and retraction fibers during prometaphase (Fig. 1C), metaphase (Fig. 1E) and anaphase (Fig. 1G). As cells moved to telophase, β-actin was enriched to the contractile ring (Fig. 1I) which is consistent with a previous observation in human keratinocyte cells.25 Similar to β-actin, localization of γ-actin changes throughout mitosis. γ-Actin was observed throughout the cell body and also found localized to the retraction fibers in prometaphase (Fig. 1C) and metaphase (Fig. 1E). When cells proceeded to anaphase (Fig. 1G) and telophase (Fig. 1I), γ-actin was found at the cell membrane. Predominant localization of γ-actin at the cell membrane during telophase in SH-EP cells is consistent with a previous observation that γ-actin is predominantly at the cell membrane during telophase in human keratinocyte cells.25 Interestingly, the distinct localization of β-actin and γ-actin observed in the control siRNA transfected cells was altered in the γ-actin knockdown cells. β-Actin and γ-actin was observed throughout the cell body during prometaphase to telophase (Fig. 1D, 1F, 1H and 1J) and at the cell membrane during prometaphase to anaphase (Fig. 1D, 1F and 1H). The most notable alteration due to γ-actin depletion was the reduction in actin-rich retraction fibers, which was evident in prometaphase (Fig. 1D) and metaphase (Fig. 1F), as well as the disappearance of β-actin enrichment from the contractile ring in some of the mitotic cells during telophase (Fig. 1J). Redistribution of β-actin and γ-actin following knockdown of γ-actin (Fig. S1A) was also observed in the breast cancer cells, MCF-7 (Fig. S2). Partial depletion of β-actin significantly impaired cell proliferation and caused cell death, therefore we were unable to examine the effect of β-actin knockdown on mitotic progression. These findings show that partial depletion of γ-actin perturbs the localization of β-actin and inhibits the formation of actin-rich retraction fibers in SH-EP cells during mitosis.
Figure 1.

Spatial distribution of β-actin and γ-actin in different mitotic phases. Confocal images of SH-EP cells transfected with either the control or γ-actin siRNA in interphase (A and B), prometaphase (C and D), metaphase (E and F), anaphase (G and H) and telophase (I and J). The cells were dual stained for β-actin (green) or γ-actin (red). Confocal images show maximum projections and images were acquired using the same settings and process exactly the same using the ZEN software. Scale bar, 5 µm.
γ-Actin is required to activate the G2/M cell cycle checkpoint induced by broad categories of anti-mitotic agents
We have previously shown that the microtubule-targeted antimitotic agent, paclitaxel, failed to induce mitotic arrest in γ-actin depleted SH-EP cells to the same level as that of the control siRNA cells.24 Consistent with this result, cell cycle analysis shows that γ-actin knockdown reduces the ability of paclitaxel to induce G2/M cell cycle checkpoint compared to the control siRNA cells (Fig 2A). In order to investigate the process by which this occurred, the expression of several cell cycle markers in γ-actin-depleted cells were examined, to determine whether these may be associated with the lack of mitotic arrest in paclitaxel-treated γ-actin siRNA cells. We initially focused on the basal expression of cyclins in non-drug treated cells (22 and 70 h timepoints). Cyclin D1 and cyclin E regulate G1 phase exit and both these cyclins were highly expressed in the γ-actin-depleted cells by comparison with control siRNA cells (Fig. 2B-D). Analysis of other G1 phase cell cycle markers revealed a moderate increase in p21 and p27 and a decrease in polo-like kinase 1 (Plk1) basal expression in the γ-actin depleted cells compared to the control siRNA cells, h post-transfection (Fig. S3A). These alterations were also confirmed with γ-actin siRNA duplex 2 (Fig. S3B). Conversely, the cell cycle markers that regulate S (cyclin A), G2 (cyclin B1) and M (securin) phase exit, were expressed at low levels at the 22 h timepoint in γ-actin knockdown cells compared to the control cells (Fig. 2B and Fig. S4A, B and C). Thus, the aberrant expression of G1 phase cell cycle markers accompanied by low levels of S, G2 and M phase markers, would impede cell proliferation24 and cause accumulation of cells in the G0/G1 phase of the cell cycle as observed in the γ-actin-depleted cells.
Figure 2.
Aberrant expression of cell cycle markers in the γ-actin-depleted cells. The SH-EP GFP-β-Itubulin cells were transfected with either the control or γ-actin siRNA and treated with 0–10 nM paclitaxel for 22 or 70 h to investigate the cell cycle. Aliquots of the cells were taken for flow cytometry to confirm that paclitaxel induces G2/M cell cycle checkpoint and the rest of the cells were lysed for western blot analysis. (A) Cell cycle analysis of the control and γ-actin siRNA transfectants treated with 0–10 nM paclitaxel for either 22 or 70 h. (B) Representative western blot analyses showing the expression of several cell cycle markers, cyclin A, cyclin B1, cylin D1, cyclin E and securin, in control and γ-actin siRNA transfected cells. γ-Actin was included to confirm γ-actin knockdown and GAPDH was included as a control for equal loading. Graphs showing the relative expression of cyclin D1 (C) and cyclin E (D) in control and γ-actin siRNA transfected cells. Data are mean ± SEM of at least 3 independent experiments. *P < 0 .05, #P < 0 .005, statistically significant between the drug free γ-actin siRNA cells and drug free control siRNA cells or between the drug treated cells and its corresponding drug free cells.
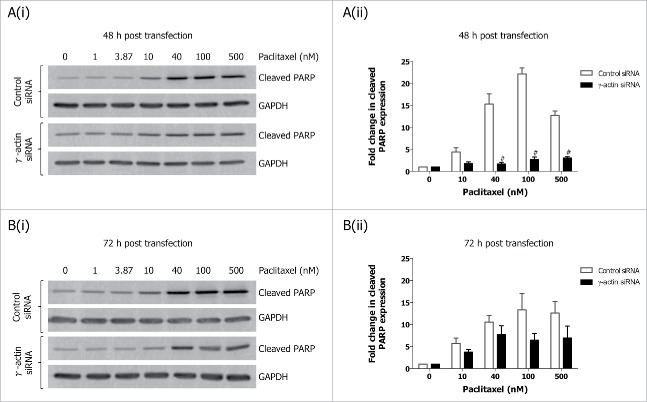
We next examined how paclitaxel-induced mitotic arrest would affect the expression of these cell cycle markers. Treatment with 10 nM paclitaxel for 22 h, led to decreased cyclin A expression (Fig. 2B and Fig. S4A), and increased cyclin B1 (Fig. 2B and Fig. S4B), cyclin D1 (Fig. 2B-C) and the upper band of securin (Fig. 2B) in the control siRNA cells, indicative of activation of the G2/M cell cycle checkpoint (Fig. 2A) or mitotic arrest.24 In contrast, the expression of cyclin A (Fig. 2B and Fig. S4A), cyclin B1 (Fig. 2B and Fig. S4B), cyclin D1 (Fig. 2B-C) and total securin (Fig. 2B and Fig. S4C) did not significantly change in paclitaxel-treated γ-actin-depleted cells, which is consistent with the reduced ability of paclitaxel to activate a G2/M cell cycle checkpoint (Fig. 2A) or mitotic arrest24 in the these cells. In contrast, there was no significant differences in cyclin E expression in the 22 h paclitaxel-treated control and γ-actin siRNA transfected cells when compared to their respective non-drug treated counterparts (Fig. 2D). Interestingly, overexpression of cyclin E was maintained in both the non-drug treated and 22 h paclitaxel-treated γ-actin-depleted cells (Fig. 2B and D). In addition, prolonged exposure to paclitaxel (70 h) also affects the expression of these cell cycle markers. Increased cyclin D1 and cyclin E expression was observed in the 70 h paclitaxel-treated control siRNA cells compared to the non-drug treated control siRNA cells (Fig. 2B-D). In contrast, there was no significant alterations in cyclin D and cyclin E expression detected in the 70 h paclitaxel-treated γ-actin knockdown cells compared to the non-drug treated γ-actin knockdown cells (Fig. 2B-D). Decreased expression in cyclin A, cyclin B1 and securin following 70 h paclitaxel treatment was detected in both the control and γ-actin knockdown cells when compared to their respective non-drug treated cells (Fig. 2B and Fig. S4A, B and C). These changes in the expression of cyclin A, cyclin B1, cyclin D and securin, were associated with the cell cycle profiles displayed by the control and γ-actin siRNA transfected cells (Fig. 2A). The lack of mitotic arrest24 or G2/M cell cycle checkpoint (Fig. 2A) in the γ-actin knockdown cells was not due to increased cell death, since there was less cleaved PARP detected, a measure of apoptosis, in the paclitaxel-treated γ-actin-depleted cells compared to the control siRNA cells (Fig. 3A-B).
Figure 3.
Decreased expression of cleaved PARP in paclitaxel-treated γ-actin-depleted cells. Western blotting analysis of cleaved PARP expression performed on lysates from SH-EP GFP-β-Itubulin siRNA transfected cells, treated with 0–500 nM paclitaxel for 48 h to induce cell death, following either 48 (A) or 72 h (B) siRNA transfection. Both floating and adherence cells were collected for western blot analysis. GAPDH was included as a control for equal loading. Graphs represent the fold change in cleaved PARP expression. Data are mean ± SEM of 3 independent experiments. #P < 0 .005, statistically significant when comparing the γ-actin siRNA cells to the control siRNA cells.
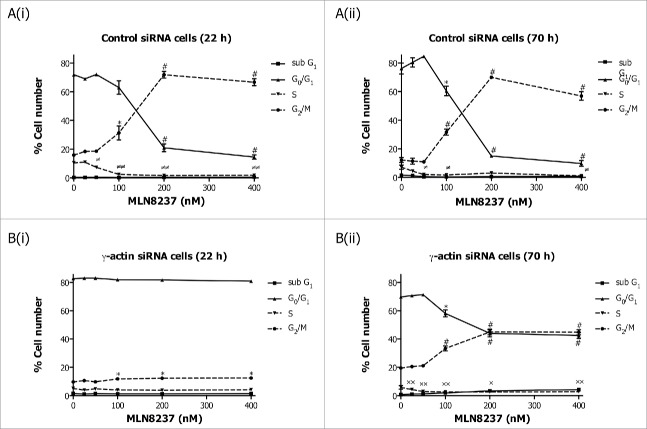
Our data clearly shows that loss of γ-actin inhibits the ability of paclitaxel to exert an antimitotic effect,24 possibly due to slower cell proliferation24 and accumulation of cells in G0/G1 cell cycle phase. This raised the question as to the requirement for γ-actin in the activity of non-microtubule targeted antimitotic agents. To address this, γ-actin siRNA and control siRNA transfected cells were treated with Eg5 or Aurora kinase A inhibitors prior to cell cycle analysis being performed. The Eg5 inhibitor III, dimethylenastron (DIMEN), arrests cells at prometaphase which results in accumulation of cells in mitosis. Treatment of control siRNA transfected SH-EP cells with increasing concentrations of DIMEN for 22 or 70 h induced a dose-dependent decrease in G0/G1 cell populations with a concomitant increase in G2/M accumulation (Fig. 4A(i) and (ii)). Interestingly, a small but significant induction in G2/M with no significant decrease in G0/G1 was observed in the γ-actin-depleted SH-EP cells following DIMEN treatment for 22 h (Fig. 4B(i)). Prolonged treatment (70 h) of γ-actin knockdown cells with DIMEN caused a significant decrease in G0/G1 cell populations with a concomitant increase in G2/M (Fig. 4B (ii)). However, this dose-dependent increase in G2/M cell populations observed in the γ-actin knockdown cells was less than that of the control siRNA cells (Fig. 4). Similar to SH-EP cells, DIMEN induced a dose-dependent increase in G2/M cell populations in the γ-actin siRNA transfected MCF-7 cells but to a lesser extend compared to the control siRNA cells (Fig. S5). These results were supported by treatment with another antimitotic agent, MLN8237, an Aurora kinase A inhibitor, that induced mitotic block and accumulation of polyploid cells.26 A dose-dependent increase in G2/M (Fig. 5A(i) and (ii)) and polyploid (Fig. S6 A) cell populations was observed in the control siRNA cells when treated with MLN8237 for 22 and 70 h. In γ-actin siRNA cells there was a dose-dependent increase in G2/M (Fig. 5B(i) and (ii)) and polyploid (Fig. S6B) cell populations but to a lesser extend than control siRNA cells. These findings provide strong evidence that γ-actin is required for G2/M checkpoint block induced by a broad range of anti-mitotic agents, suggesting that γ-actin is regulating mitotic progression.
Figure 4.
Inhibition of Eg5 inhibitor III, dimethylenastron, induced G2/M cell cycle checkpoint in the γ-actin knockdown cells. Cell cycle analysis of SH-EP GFP-β-Itubulin cells transfected with either control (A) or γ-actin siRNA (B) and treated with 0–2 µM dimethylenastron for either 22 h (A(i) and B(i)) or 70 h (A(ii) and B(ii)). Data are mean ± SEM of 3 independent experiments.*P < 0 .05, #P < 0 .005, statistically significant between the drug treated cells and its corresponding drug free cells in G0/G1 and G2/M cell cycle phases. ≠P < 0 .05, ≠≠P < 0 .005, statistically significant between the drug treated cells and its corresponding drug free cells in S phase. xP < 0 .05, xxP < 0 .005, statistically significant between the drug treated cells and its corresponding drug free cells in sub-G1.
Figure 5.
Partial depletion of γ-actin inhibits Aurora kinase A inhibitor, MLN8273, induced G2/M cell cycle checkpoint in SH-EP cells. Cell cycle distribution in SH-EP GFP-β-Itubulin cells transfected with either the control (A) or γ-actin siRNA (B) then treated with increasing concentrations of MLN8237, for either 22 (A(i) and B(i)) or 70 h (A(ii) and B(ii)). Data are mean ± SEM of 3 independent experiments.*P < 0 .05, #P < 0 .005, statistically significant between the drug treated cells and its corresponding drug free cells in G0/G1 and G2/M cell cycle phases. ≠P < 0 .05, ≠≠P < 0 .005, statistically significant between the drug treated cells and its corresponding drug free cells in S phase. *P < 0 .05, **P < 0 .005, statistically significant between the drug treated cells and its corresponding drug free cells in sub-G1.
Depletion of γ-actin leads to enhanced centrosome amplification
The results showing that γ-actin is required for drug-induced mitotic arrest, raised the possibility that γ-actin may be involved in regulating mitotic progression. To address this, we initially investigated whether depletion of γ-actin affects centrosome amplification since a correct number of centrosomes are essential for normal mitosis to occur. Extra centrosomes were frequently observed in SH-EP cells treated with γ-actin siRNA (7.3%, a 2.1-fold increase) compared to the control siRNA (3.4%) (Fig. 6A). Furthermore, a trend toward increased number of extra centrosomes was also obtained in MCF-7 cells transfected with γ-actin siRNA, where a 2.1-fold increase in centrosome amplification was observed, compared to the control siRNA (Fig. 6B). These results were further confirmed in SH-EP cells transfected with either of 2 additional γ-actin siRNAs, duplex 1 and duplex 2 (a 3.0-fold and 2.5-fold increase in centrosome amplification was observed, respectively) (Fig. S7A).
Figure 6.
Enhanced centrosome amplification in cancer cells due to γ-actin depletion. Quantification of centrosome numbers in the control and γ-actin siRNA transfected SH-EP (A), MCF-7 (B) and MRC-5 cells (C) were analyzed by co-staining the cells with γ-tubulin and α-tubulin. Confocal images were acquired and then centrosome number was scored in interphase cells as 1, 2 or more than 2 centrosomes. n = the total number of interphase cells analyzed from 4 independent experiments. *P < 0 .05, #P < 0 .005, statistically significant when comparing the γ-actin siRNA cells to the control siRNA cells.
In contrast to cancer cells, normal human fetal lung fibroblast cells (MRC-5) centrosome amplification occurred at similar frequencies in the control (3.0%) and γ-actin-depleted cells (2.8%) (Fig. 6C), indicating that abnormal centrosome amplification due to depletion of γ-actin was detected only in the cancer cell lines but not in normal human fibroblast cells examined in this study. We did not detect γ-actin in the centrosome of interphase and mitotic SH-EP cells when staining with the γ-actin monoclonal antibody (data not shown), and γ-actin depletion in SH-EP cells did not influence the localization of various centrosomal proteins such as γ-tubulin, pericentrin, Aurora kinase A and p150Glued (data not shown). These findings suggest that γ-actin plays a role in maintaining centrosome integrity.
Partial depletion of γ-actin induces mitotic defects and delays mitotic progression
To assess how the presence of multiple centrosomes would affect mitotic progression in γ-actin depleted cells, fluorescence time-lapse microscopy was performed in SH-EP cells stably expressing a mCherry-histone-H2B plasmid construct to visualize the chromosomes. The majority of the control cells underwent multiple mitoses (Supplemental movie 1). In contrast, in the γ-actin siRNA cells, firstly, there were fewer cells undergoing mitosis and secondly, there were fewer multiple mitoses (Supplemental movie 2). In the few γ-actin siRNA transfected cells that underwent mitosis, 18.72% (Fig. 7B) of them exhibited some form of mitotic defect such as uncongressed chromosomes (Fig. 7A, bottom panels) compared to just 8.37% of such defects seen in the control cells (Fig. 7B). Mitotic abnormalities observed included uncongressed chromosomes in metaphase (see arrows in Fig. 7A, bottom panels) and incorrect chromosome segregation in anaphase (Fig. 7A, bottom panels). Persistence of mitotic abnormalities caused a prolonged prometaphase/metaphase arrest in the γ-actin depleted cells. It took the γ-actin depleted cells longer to complete metaphase (mean 97.2±10.6 min) compared to the control siRNA cells (mean 37.0±1.62 min) (Fig. 7B). Among the mitotic cells that displayed defects due to γ-actin depletion, these cells, either die during mitosis, exit mitosis and die in cytokinesis or the following interphase, or exit mitosis and divide into 2 daughter cells (Supplemental movie 2).
Figure 7.
Partial depletion of γ-actin induces mitotic defects. (A) Still images from movies in Supplemental Movie 1 and 2, showing SH-EP mCherry-histone-H2B expressing cells transfected with the control siRNA (top panels) undergoing normal mitosis or with the γ-actin siRNA (bottom panels) undergoing abnormal mitosis. Arrows showing uncongressed chromosomes. Time is in h:min:s; Scale bar, 50 µm. (B) A scatter plot showing the duration for prometaphase/metaphase in the control and γ-actin depleted cells, which was measured from nuclear envelope breakdown to the beginning of anaphase. n = the total number of mitotic cells undergoing mitosis analyzed from 3 independent experiments. #Control siRNA cells have more than 203 mitotic cells, however, we only analyzed 203 mitotic cells due to fewer mitotic cells in the γ-actin siRNA cells. Therefore the actual percentage of mitotic abnormalities for the control siRNA cells is lower than 8.37%. #P < 0 .005, statistically significant when comparing the γ-actin siRNA cells to the control siRNA cells.
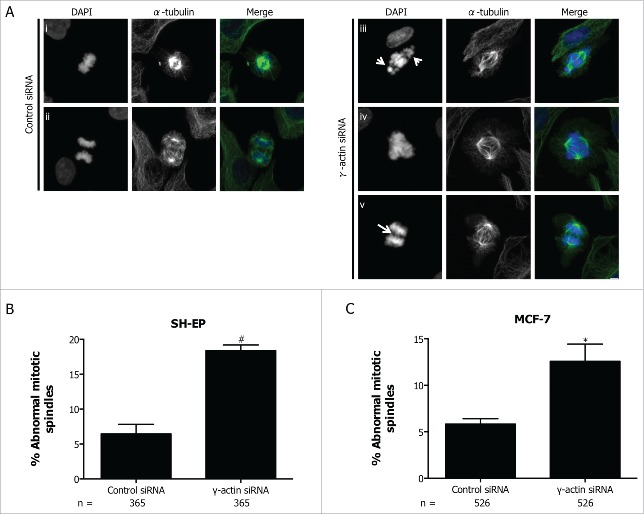
Due to increased chromosome misalignment and missegregation in the γ-actin siRNA cells, mitotic spindle organization was examined to determine whether the observed mitotic defects were associated with an increased number of abnormal mitotic spindles. Indeed, the SH-EP cells treated with γ-actin siRNA displayed more mitotic spindle abnormalities (18.4%, 2.85-fold increase, p = 0.0017) compared to control siRNA (6.5%) (Fig. 8A-B). The majority of the observed spindle defects were bipolar spindles with uncongressed chromosomes (see arrow in Fig. 8A(iii)), with a few multipolar spindles (Fig. 8A(iv)) and anaphase bridges (Fig. 8A(v)). Similar types of spindle defects were detected in the control cells, but occurred at a much lower frequency compared to the γ-actin-depleted cells (Fig. 8B). Increased formation of spindle abnormalities was also observed in MCF-7 cells treated with γ-actin siRNA (12.6%, 2.16-fold increase, p = 0.0254) compared to the control siRNA (5.8%) (Fig. 8C) and was also confirmed with 2 additional γ-actin siRNAs in both SH-EP (Fig. S7B) and MCF-7 cells (Fig. S7C). The two additional siRNAs, γ-actin siRNA duplex 1 and 2 can effectively suppress γ-actin expression in MCF-7 cells (Fig. S1B). These data clearly show that γ-actin is required for mitotic integrity in cancer cells.
Figure 8.
Increased formation of abnormal mitotic spindles in the γ-actin knockdown cells. (A) Confocal images of normal mitotic spindles in control siRNA SH-EP cells (i-ii) and abnormal mitotic spindles in γ-actin siRNA SH-EP cells (iii-v). Cells were stained with α-tubulin to visualize the mitotic spindles and counterstained with DAPI to visualize the chromosomes. Arrows in (iii) shows uncongressed chromosomes in metaphase and arrow in (v) shows anaphase bridge. Confocal images show maximum projections. Scale bar, 5 µm. Quantification of abnormal spindle mitoses in SH-EP (B) and MCF-7 (C) cells transfected with either the control or γ-actin siRNA. The types of mitotic cells that were counted include prometaphase, metaphase, anaphase and telophase. n=the total number of mitotic cells analyzed per condition. Data are mean ± SEM of 3 independent experiments. *P < 0 .05, #P <0 .005, statistically significant when comparing the γ-actin siRNA cells to the control siRNA cells.
Discussion
Although the actin cytoskeleton is known to be involved in centrosome separation and orientation of the mitotic spindles, less is known about the role of the non-muscle actin isoforms, β-actin and γ-actin in mitosis. In this study, we demonstrate for the first time that partial depletion of γ-actin causes abnormal centrosome amplification in cancer cells and induces mitotic defects which lead to a delay in mitosis.
We have previously shown that γ-actin is required in order for a microtubule-targeted agent to induce mitotic arrest.24 However, this requirement is not specific to microtubule-targeted agents, but rather, we now show that γ-actin is required for mitotic arrest induced by a broad class of anti-mitotic agents suggesting that this cytoskeletal protein is essential for mitotic progression. Indeed, we provide evidence that γ-actin is involved either directly or indirectly in regulating mitotic progression by regulating chromosomes alignment and segregation. Among the cell cycle markers examined in this study, aberrant expression of cyclin E was the most pronounced effect observed in the γ-actin depleted cells, where high levels of cyclin E were maintained throughout the untreated and paclitaxel treated γ-actin siRNA cells at 22 h. Overexpression of cyclin E in γ-actin knockdown cells probably reflects the accumulation of cells at G0/G1 cell cycle phase due to a delay in cell proliferation.24 Interestingly, overexpression of the lower molecular weight cyclin E has also been shown to cause centrosome amplification in breast cancer cells.27 In addition, overexpression of either the full length or the lower molecular weight cyclin E caused mitotic progression defects by affecting chromosome alignment and segregation.27,28 Abnormal centrosome amplification and mitotic progression defects were 2 features displayed by γ-actin depleted cancer cells in this study.
One of the hallmarks of cancer cells is the presence of extra centrosomes that induced formation of multipolar spindles and chromosome missegregation which results in mitotic abnormalities.29-31 Supernumerary centrosomes were detected in neuroblastoma and breast cancer cells transfected with the control siRNA, however γ-actin depletion increased the frequency of extra centrosomes in neuroblastoma and breast cancer cells above that of control cells. Suppression of γ-actin induced abnormal centrosome amplification in the cancer cells as γ-actin depletion did not increase the number of normal human fibroblast cells with extra centrosomes. We do know that partial depeletion of γ-actin interferes with centrosome orientation in migrating SH-EP cells.21 However, the occurrence of abnormal centrosome amplification due to γ-actin depletion remains to be fully investigated to determine whether γ-actin depletion causes centriole overduplication, denovo formation of centrioles, centrosome fragmentation or cytokinesis failure.32 The accumulation of γ-actin depleted cells in G0/G1 cell cycle phase may have affected centrosome duplication.
Furthermore, the presence of extra centrosomes may have led to the increased formation of multipolar spindles in the γ-actin knockdown cells. Interestingly, a previous study has shown that cells with extra centrosomes can overcome the formation of multipolar spindles by clustering their centrosomes.30 To correct for the effect of extra centrosomes, the cells undergo spindle reorganization by initially forming multipolar spindles and then bipolar spindles.29,31 This process requires the activation of the spindle assembly checkpoint (SAC) which subsequently causes prolonged mitotic arrest.29-31 Prolonged mitotic arrest was observed in the γ-actin-depleted cells, indicating that SAC is functional in these cells. The delay in anaphase onset in the γ-actin depleted cells could also be due to the presence of unattached kinetochores.2 Indeed the major type of mitotic abnormality displayed by the γ-actin-depleted cells were bipolar spindles with uncongressed chromosomes indicating the presence of unattached kinetochores, which in turn could have led to prolonged metaphase arrest in the γ-actin-depleted cells. Direct association between γ-actin and the kinetochores has not been established in this study. We do know that γ-actin knockdown results in suppression of interphase microtubule dynamics24 but it is not known if γ-actin knockdown also affects kinetochore microtubule dynamics. We cannot exclude the possibility that γ-actin knockdown may have altered the localization and distribution of proteins such as centromere-associated protein E that binds to the kinetochore during mitosis and is essential for maintaining stable spindle-kinetochore attachment.33 Defects in the localization and expression of kinetochore associated proteins may interefere with spindle-kinetochore binding. Moreover, correct spindle positioning is essential for proper mitotic progression and is regulated by retraction fibers,34 cortex stiffness35 and astral microtubules interactions with cortical actin.36 Actin-rich retraction fibers are projected from the cell membrane of mitotic cells to the stratum and it is believed to be important for correct positioning of the mitotic spindles.34,37,38 Formation of actin-rich retraction fibers was inhibited due to γ-actin depletion and the impact of less retraction fibers on mitotic progression and spindle positioning is unknown and warrants further investigation. Furthermore, we did observe that partial depletion of γ-actin led to γ-actin being prominent in the cell membrane during prometaphase and metaphase. Reorganization of actin filaments to the cell cortex during mitosis is crucial for the interactions between the cell cortex and astral microtubules, which is thought to be important for spindle assembly and correct spindle orientation.3,39 Disruption to the cortical actin perturbs the interaction of astral microtubules to the cortex and affects mitotic spindle orientation.2,36 This redistribution of γ-actin to the cell membrane observed in this study may interfere with astral microtubules tethering to the cell cortex.
This study shows for the first time that γ-actin is required for broad classes of anti-mitotic agents to induce mitotic arrest. Moreover, γ-actin is required to maintain centrosome integrity and regulate mitotic progression. To fully understand the role of γ-actin in maintaining the integrity of the centrosome and mitotic spindles, further studies are required to determine γ-actin's role in centrosome duplication, maturation, spindle pole movement, astral microtubule tethering to the cell cortex, and binding or stabilization of kinetochore-microtubule spindle attachment. If depletion of γ-actin disrupts these processes it would suggest a link between this protein and the mitotic spindle orientation and potentially spindle dynamics.
Materials and Methods
Cell culture
Neuroblastoma SH-EP, SH-EP GFP-βI-tubulin, SH-EP mCherry-histone-H2B expressing cells and the breast cancer MCF-7 cells were maintained as monolayers in Dulbecco's Modified Eagle Medium (Life Technologies) supplemented with 10% fetal calf serum (FCS) and grown in a humidified incubator at 37°C and 5% C02. The human fetal fibroblast MRC-5 cells were maintained as monolayers in MEM media supplemented with 10% fetal calf serum, 0.1% non-essential amino acids (NEAA) (Life Technologies), 0.1% sodium pyruvate (Life Technologies), 0.2% sodium bicarbonate (Life Technologies), 0.1% L-glutamine. All cell lines used were free from mycoplasma contamination.
Construction of the mCherry-histone-H2B expressing plasmid
In order to generate the plasmid coding for mCherry-histone-H2B, the mouse histone-H2B cDNA (accession number BC082774) was excised from an existing cyan fluorescent protein (CFP)-tagged histone-H2B plasmid (kindly provided by Professor Kensaku Mizuno, Department of Biomolecular Sciences, Graduate School of Life Sciences, Tohoku University, Sendai, Japan) by digesting the plasmid with Blg II and EcoR I. Then the histone-H2B cDNA was subcloned into the BspE I and Xho I site of the pcDNA3.1 mCherry vector.
Plasmid and siRNA transfection
The neuroblastoma SH-EP cells were stably transfected with the mCherry-histone-H2B plasmid to visualize the chromosomes when performing timelapse fluorescence microscopy. Briefly, the SHEP cells were transfected with 4 µg of the mCherry-histone-H2B plasmid using Lipofectamine 2000 (Life Technologies) according to the manufacturer's instructions. Following 48 h post-transfection the cells were selected in media containing 1 mg/mL G418 (Geneticin, Life Technologies). A pooled population of the SH-EP cells stably transfected with mCherry-histone-H2B were maintained as a monolayer. Generation of the SH-EP GFP-βI-tubulin cells has been previously described.24
SiRNA transfections were carried out as previously described.24 Briefly, SH-EP cells, SH-EP GFP-βI-tubulin or SH-EP mCherry-histone H2B expressing cells were plated and transiently transfected with All Stars negative control siRNA (25 or 100 nM, Qiagen), γ-actin siRNA (encoded by the ACTG1 gene, accession NM_001614) (5′-AAGAGATCGCCGCGCTGGTCA-3′, 100 nM, Qiagen),40 γ-actin siRNA duplex 1 (5′- GAGAAGAUGACUCAGAUU-3′, 25 nM, Dharmacon)41 and γ-actin siRNA duplex 2 (5′-GAGCCGUGUUUCCUUCCAU-3′, 25 nM, Dharmacon)41 using Lipofectamine 2000 (Life Technologies), according to manufacturer's instructions. The human fetal fibroblast MRC-5 cells were transfected with 100 nM of either the All Stars negative control siRNA or γ-actin siRNA, whereas the breast cancer MCF-7 cells were transfected with 10 nM of All Stars negative control siRNA, γ-actin siRNA, γ-actin siRNA duplex 1 or γ-actin siRNA duplex 2.
Immunofluorescence microscopy
Dual staining for β-actin and γ-actin were performed as previously described.21,25 Briefly, the SH-EP cells were transfected with the control or γ-actin siRNAs into 4 well glass chamber slides as described above. Following 72 h incubation the cells were rinsed with 20 mM HEPES/DMEM then fixed with warm 1% paraformaldehyde/DMEM for 15 min then washed with PBS and permeabilized with 100% ice-cold methanol at -20°C for 10 min. Stained with monoclonal antibody against cytoplasmic γ-actin (mAb 2A3, IgG2b)25 and followed by staining with Alexa Fluor 568 anti-mouse IgG2b secondary antibody (Life Technologies). Then dual stained with monoclonal antibody against cytoplasmic β-actin (mAb 4C2, IgG1)25 and Alexa Fluor 488 anti-mouse IgG1 secondary antibody (Life Technologies).
To stain the centrosomes, SH-EP, MCF-7 or MRC-5 siRNA transfected cells were fixed in 100% ice-cold methanol following 72 h post-siRNA transfection, washed with PBS and incubate with 10% FCS/PBS to prevent non-specific binding of antibodies. Followed by incubation with either γ-tubulin primary antibody (Sigma-Aldrich) or pericentrin polyclonal antibody (Covance) and stained with Alexa Fluor 568 anti-rabbit secondary antibody (Life Technologies). Then dual stained with monoclonal antibody against α-tubulin (Sigma-Aldrich) and Alexa Fluor 488 anti-mouse secondary antibody (Life Technologies).
Staining and analysis of mitotic spindles was carried out as previously described.42
Images were acquired using either an Olympus FV1000 confocal microscope (Olympus) equipped with an oil immersion objective 100X 1.4 NA or a Zeiss LSM 780 confocal microscope (Zeiss) with a water/glycerol objective 60X 1.3 NA. A series of 0.4 µm sections were collected using sequential scanning. Images were processed using either the Olympus Fluoview software (Olympus) or ZEN software (Zeiss).
To analyze centrosome amplification, images were viewed using the Olympus Flouview software and centrosome numbers were scored in interphase cells.
Protein detection and quantitation
For cell cycle markers protein expression, the SH-EP GFP-βI-tubulin cells were transfected with control siRNA or γ-actin siRNA for 72 h as described above. The siRNA transfected cells were either untreated or treated with 10 nM paclitaxel for 22 or 70 h to induce G2/M cell cycle arrest. Both the floating and adherent cells were collected. Aliquots of the cells were taken for flow cytometry to confirm that paclitaxel induces G2/M cell cycle arrest and the rest of the cells were lysed in RIPA buffer containing protease inhibitors (1:100-fold, Sigma-Aldrich). For cleaved PARP protein expression, the SH-EP GFP-βI-tubulin siRNA post siRNA transfected cells followed by treatment with increasing concentrations of paclitaxel for 48 h, floating and adherence cells were collected and protein was isolated as described above. For analysis of p21Waf1(Cip1), p27Kip1 and polo-like kinase 1 (PLK1) protein expression, the SH-EP cells transfected with siRNA were examined at 24, 48 or 72 h post trasfection. Cells were harvested at each time point and subject to protein analysis.
Twenty to 30 µg of protein lysates were separated on 12% SDS-PAGE gel, electrotransfer to nitrocellulose membranes. The membranes were probed with monoclonal antibodies against cyclin A (clone E23.1, Abcam), cyclin B1 (clone V152, Abcam), cyclin D1 (clone DSC-6, Novocastra, Leica BiosystemsS), cyclin E (clone HE12, Abcam), securin (clone DSC-280, Abcam), γ-actin,43 cleaved PARP (Asp214) (clone D64E10, Cell Signaling), PLK1 (clone 208G4, Cell Signaling), p21Waf1(Cip1 (clone DCS560, Cell Signaling) and p27Kip1 (clone 57/Kip1/p27, BD Biosciences) and GAPDH (clone 6C5, Abcam) as a control for equal loading. Proteins were detected by ECL (Pierce, Thermo Scientific) and membranes were scanned using the Typhoon (GE Healthcare). Densitometry analysis was carried out as previously described.23
Cell cycle analysis
For cell cycle analysis, the SH-EP GFP-βI-tubulin siRNA transfected cells were treated with increasing concentrations of either the Eg5 inhibitor III, dimethylenastron (Merck Millipore) or the Aurora A inhibitor, MLN8237 (Alisertib) (Selleck) for 22–70 h. The cells were harvested, washed with PBS and fixed with ice-cold 80% ethanol for 30 minutes on ice and stored at 4°C until analyzed by FACS. Briefly the cells were spun down to remove the ethanol, washed once with PBS/1% Tween-20 and then stained with Propidium Iodide (0.1 mg/mL) (Sigma-Aldrich) and RNase (4 μg/mL) (Roche Diagnostics) in PBS/1% Tween-20 for 45 min at 37°C. Ten thousand cells were analyzed using a FACS Calibur flow cytometer (BD Biosciences) and cell cycle distribution was analyzed using CellQuest (BD Biosciences) software.
Time-lapse Imaging
The SH-EP-mCherry-histone-H2B cells were transfected with either the control or γ-actin siRNA and seeded onto 6 well plates as described above. Following 72 h post-siRNA transfection, images were collected every 5 min for 70 h using a 10x Axioplan objective lens on an Axiovert 200M fluorescent microscope (Zeiss,). Cells were maintained at 37°C with 5% CO2. Image analysis was performed using the Axio Vision software (Zeiss).
Statistical analysis
Statistical analysis was performed using 2 sided, unpaired Student's t-test and values are expressed as mean ± SEM. P < 0 .05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Professor Kensaku Mizuno (Tohoku University, Japan) for providing the CFP-histone H2B plasmid, Professor Peter Gunning (UNSW Australia) for the γ-actin polyclonal antibody, Dr Christine Chaponnier (University of Geneva, Switzerland) for the β-actin and γ-actin monoclonal antibodies, Majorie Liu for her technical assistance and Professor Linda Wordeman (University of Washington, Seattle, WA), Professor Murray Norris (Children's Cancer Institute) and Dr. Patrick Kim (Children's Cancer Institute) for critically reviewing the manuscript.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Author Contributions
S.T.P and M.K designed the study. S.T.P performed the experiments and analyzed the data. S.T.P wrote the manuscript and M.K edited the manuscript.
Funding
This work was supported by the Children's Cancer Institute Australia, which is affiliated with the UNSW Australia and the Sydney Children's Hospitals Network and grants from The Kids' Cancer Project (MK), a National Health and Medical Research Council Fellowship #1058299 (MK), and Cancer Council New South Wales Program Grant (MK). MK is also funded by the Australian Research Council Center of Excellence in Convergent Bio-Nano Science and Technology (project number CE140100036).
References
- 1.Heng YW, Koh CG. Actin cytoskeleton dynamics and the cell division cycle. Int J Biochem Cell Biol 2010; 42:1622-33; PMID:20412868; http://dx.doi.org/ 10.1016/j.biocel.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 2.Rankin KE, Wordeman L. Long astral microtubules uncouple mitotic spindles from the cytokinetic furrow. J Cell Biol 2010; 190:35-43; PMID:20603328; http://dx.doi.org/ 10.1083/jcb.201004017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer RE, Sullivan DS, Huffaker T, Koshland D. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast, Saccharomyces cerevisiae. J Cell Biol 1992; 119:583-93; PMID:1400594; http://dx.doi.org/ 10.1083/jcb.119.3.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandquist JC, Kita AM, Bement WM. And the dead shall rise: actin and myosin return to the spindle. Dev Cell 2011; 21:410-9; PMID:21920311; http://dx.doi.org/ 10.1016/j.devcel.2011.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber KL, Sokac AM, Berg JS, Cheney RE, Bement WM. A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature 2004; 431:325-9; PMID:15372037; http://dx.doi.org/ 10.1038/nature02834 [DOI] [PubMed] [Google Scholar]
- 6.Woolner S, O'Brien LL, Wiese C, Bement WM. Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol 2008; 182:77-88; PMID:18606852; http://dx.doi.org/ 10.1083/jcb.200804062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabian L, Forer A. Possible roles of actin and myosin during anaphase chromosome movements in locust spermatocytes. Protoplasma 2007; 231:201-13; PMID:17922265; http://dx.doi.org/ 10.1007/s00709-007-0262-y [DOI] [PubMed] [Google Scholar]
- 8.Forer A, Spurck T, Pickett-Heaps JD. Actin and myosin inhibitors block elongation of kinetochore fibre stubs in metaphase crane-fly spermatocytes. Protoplasma 2007; 232:79-85; PMID:18094930; http://dx.doi.org/ 10.1007/s00709-007-0265-8 [DOI] [PubMed] [Google Scholar]
- 9.Robinson RW, Snyder JA. Localization of myosin II to chromosome arms and spindle fibers in PtK1 cells: a possible role for an actomyosin system in mitosis. Protoplasma 2005; 225:113-22; PMID:15868218; http://dx.doi.org/ 10.1007/s00709-005-0085-7 [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell 2004; 117:361-72; PMID:15109496; http://dx.doi.org/ 10.1016/S0092-8674(04)00341-1 [DOI] [PubMed] [Google Scholar]
- 11.Silverman-Gavrila RV, Forer A. Myosin localization during meiosis I of crane-fly spermatocytes gives indications about its role in division. Cell Motility Cytoskeleton 2003; 55:97-113; http://dx.doi.org/ 10.1002/cm.10112 [DOI] [PubMed] [Google Scholar]
- 12.Carreno S, Kouranti I, Glusman ES, Fuller MT, Echard A, Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J Cell Biol 2008; 180:739-46; PMID:18283112; http://dx.doi.org/ 10.1083/jcb.200709161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunda P, Pelling AE, Liu T, Baum B. Moesin Controls Cortical Rigidity, Cell Rounding, and Spindle Morphogenesis during Mitosis. Curr Biol 2008; 18:91-101; PMID:18207738; http://dx.doi.org/ 10.1016/j.cub.2007.12.051 [DOI] [PubMed] [Google Scholar]
- 14.Solinet S, Mahmud K, Stewman SF, Ben El Kadhi K, Decelle B, Talje L, Ma A, Kwok BH, Carreno S. The actin-binding ERM protein Moesin binds to and stabilizes microtubules at the cell cortex. J Cell Biol 2013; 202:251-60; PMID:23857773; http://dx.doi.org/ 10.1083/jcb.201304052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac M-H. Spindle Positioning in Mouse Oocytes Relies on a Dynamic Meshwork of Actin Filaments. Curr Biol 2008; 18:1514-9; PMID:18848445; http://dx.doi.org/ 10.1016/j.cub.2008.08.044 [DOI] [PubMed] [Google Scholar]
- 16.Li H, Guo F, Rubinstein B, Li R. Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat Cell Biol 2008; 10:1301-8; PMID:18836438; http://dx.doi.org/ 10.1038/ncb1788 [DOI] [PubMed] [Google Scholar]
- 17.Schuh M, Ellenberg J. A New Model for Asymmetric Spindle Positioning in Mouse Oocytes. Curr Biol 2008; 18:1986-92; PMID:19062278; http://dx.doi.org/ 10.1016/j.cub.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 18.Cao J, Crest J, Fasulo B, Sullivan W. Cortical Actin Dynamics Facilitate Early-Stage Centrosome Separation. Curr Biol 2010; 20:770-6; PMID:20409712; http://dx.doi.org/ 10.1016/j.cub.2010.02.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uzbekov R, Kireyev I, Prigent C. Centrosome separation: respective role of microtubules and actin filaments. Biol Cell 2002; 94:275-88; PMID:12489696; http://dx.doi.org/ 10.1016/S0248-4900(02)01202-9 [DOI] [PubMed] [Google Scholar]
- 20.Silverman-Gavrila R, Forer A. Evidence that actin and myosin are involved in the poleward flux of tubulin in metaphase kinetochore microtubules of crane-fly spermatocytes. J Cell Sci 2000; 113:597-609; PMID:10652253 [DOI] [PubMed] [Google Scholar]
- 21.Shum MS, Pasquier E, Po'uha ST, O'Neill GM, Chaponnier C, Gunning PW, Kavallaris M. gamma-Actin regulates cell migration and modulates the ROCK signaling pathway. FASEB J 2011; 25:4423-33; PMID:21908715; http://dx.doi.org/ 10.1096/fj.11-185447 [DOI] [PubMed] [Google Scholar]
- 22.Pasquier E, Tuset MP, Sinnappan S, Carnell M, Macmillan A, Kavallaris M. gamma-Actin plays a key role in endothelial cell motility and neovessel maintenance. Vasc Cell 2015; 7:2; PMID:25705373; http://dx.doi.org/ 10.1186/s13221-014-0027-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verrills NM, Po'uha ST, Liu ML, Liaw TY, Larsen MR, Ivery MT, Marshall GM, Gunning PW, Kavallaris M. Alterations in gamma-actin and tubulin-targeted drug resistance in childhood leukemia. J Natl Cancer Inst 2006; 98:1363-74; PMID:17018783; http://dx.doi.org/ 10.1093/jnci/djj372 [DOI] [PubMed] [Google Scholar]
- 24.Po'uha ST, Honore S, Braguer D, Kavallaris M. Partial depletion of gamma-actin suppresses microtubule dynamics. Cytoskeleton (Hoboken) 2013; 70:148-60; PMID:23335583; http://dx.doi.org/ 10.1002/cm.21096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dugina V, Zwaenepoel I, Gabbiani G, Clement S, Chaponnier C. Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J Cell Sci 2009; 122:2980-8; PMID:19638415; http://dx.doi.org/ 10.1242/jcs.041970 [DOI] [PubMed] [Google Scholar]
- 26.Marxer M, Ma HT, Man WY, Poon RY. p53 deficiency enhances mitotic arrest and slippage induced by pharmacological inhibition of Aurora kinases. Oncogene 2014; 33:3550-60; PMID:23955083; http://dx.doi.org/ 10.1038/onc.2013.325 [DOI] [PubMed] [Google Scholar]
- 27.Bagheri-Yarmand R, Biernacka A, Hunt KK, Keyomarsi K. Low molecular weight cyclin E overexpression shortens mitosis, leading to chromosome missegregation and centrosome amplification. Cancer Res 2010; 70:5074-84; PMID:20530685; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keck JM, Summers MK, Tedesco D, Ekholm-Reed S, Chuang L-C, Jackson PK, Reed SI. Cyclin E overexpression impairs progression through mitosis by inhibiting APCCdh1. J Cell Biol 2007; 178:371-85; PMID:17664332; http://dx.doi.org/ 10.1083/jcb.200703202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW. Centrosome Amplification Can Initiate Tumorigenesis in Flies. Cell 2008; 133:1032-42; PMID:18555779; http://dx.doi.org/ 10.1016/j.cell.2008.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon M, Godinho SA, Chandhok NS, Ganem NJ, Azioune A, Thery M, Pellman D. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev 2008; 22:2189-203; PMID:18662975; http://dx.doi.org/ 10.1101/gad.1700908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z, Loncarek J, Khodjakov A, Rieder CL. Extra centrosomes and/or chromosomes prolong mitosis in human cells. Nat Cell Biol 2008; 10:748-51; PMID:18469805; http://dx.doi.org/ 10.1038/ncb1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderhub SJ, Kramer A, Maier B. Centrosome amplification in tumorigenesis. Cancer Lett 2012; 322:8-17; PMID:22342684; http://dx.doi.org/ 10.1016/j.canlet.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 33.Wood KW, Chua P, Sutton D, Jackson JR. Centromere-Associated Protein E: A Motor That Puts the Brakes on the Mitotic Checkpoint. Clinical Cancer Res 2008; 14:7588-92; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4443 [DOI] [PubMed] [Google Scholar]
- 34.Thery M, Bornens M. Cell shape and cell division. Curr Opin Cell Biol 2006; 18:648-57; PMID:17046223; http://dx.doi.org/ 10.1016/j.ceb.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 35.Thery M, Bornens M. Get round and stiff for mitosis. HFSP J 2008; 2:65-71; PMID:19404473; http://dx.doi.org/ 10.2976/1.2895661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thery M, Racine V, Pepin A, Piel M, Chen Y, Sibarita JB, Bornens M. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol 2005; 7:947-53; PMID:16179950; http://dx.doi.org/ 10.1038/ncb1307 [DOI] [PubMed] [Google Scholar]
- 37.Fink J, Carpi N, Betz T, Betard A, Chebah M, Azioune A, Bornens M, Sykes C, Fetler L, Cuvelier D, et al.. External forces control mitotic spindle positioning. Nat Cell Biol 2010; 13:771-8; http://dx.doi.org/ 10.1038/ncb2269 [DOI] [PubMed] [Google Scholar]
- 38.Thery M, Jimenez-Dalmaroni A, Racine V, Bornens M, Julicher F. Experimental and theoretical study of mitotic spindle orientation. Nature 2007; 447:493-6; PMID:17495931; http://dx.doi.org/ 10.1038/nature05786 [DOI] [PubMed] [Google Scholar]
- 39.Tame MA, Raaijmakers JA, van den Broek B, Lindqvist A, Jalink K, Medema RH. Astral microtubules control redistribution of dynein at the cell cortex to facilitate spindle positioning. Cell Cycle 2014; 13:1162-70; PMID:24553118; http://dx.doi.org/ 10.4161/cc.28031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci 2001; 114:4557-65; PMID:11792820 [DOI] [PubMed] [Google Scholar]
- 41.Baranwal S, Naydenov NG, Harris G, Dugina V, Morgan KG, Chaponnier C, Ivanov AI. Nonredundant roles of cytoplasmic β- and gamma-actin isoforms in regulation of epithelial apical junctions. Mol Biol Cell 2012; 23:3542-53; PMID:22855531; http://dx.doi.org/ 10.1091/mbc.E12-02-0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Po'uha ST, Shum MSY, Goebel A, Bernard O, Kavallaris M. LIM-kinase 2, a regulator of actin dynamics, is involved in mitotic spindle integrity and sensitivity to microtubule-destabilizing drugs. Oncogene 2009; 29:597-607; PMID:19881550; http://dx.doi.org/ 10.1038/onc.2009.367 [DOI] [PubMed] [Google Scholar]
- 43.Schevzov G, Vrhovski B, Bryce NS, Elmir S, Qiu MR, O'Neill G M, Yang N, Verrills NM, Kavallaris M, Gunning PW. Tissue-specific tropomyosin isoform composition. J Histochem Cytochem 2005; 53:557-70; PMID:15872049; http://dx.doi.org/ 10.1369/jhc.4A6505.2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.