Abstract
PGRP-S (Tag7) is an innate immunity protein involved in the antimicrobial defense systems, both in insects and in mammals. We have previously shown that Tag7 specifically interacts with several proteins, including Hsp70 and the calcium binding protein S100A4 (Mts1), providing a number of novel cellular functions. Here we show that Tag7–Mts1 complex causes chemotactic migration of lymphocytes, with NK cells being a preferred target. Cells of either innate immunity (neutrophils and monocytes) or acquired immunity (CD4+ and CD8+ lymphocytes) can produce this complex, which confirms the close connection between components of the 2 branches of immune response.
Keywords: chemotaxis, human lymphocytes, Mts1, NK cells, Tag7, Tag7-Mts1 complex
Introduction
Peptidoglycan recognition protein PGRP-S (PGLYRP1, Tag7) is a member of the recently characterized family of pattern recognition molecules. Pattern recognition proteins are involved in the defense mechanisms of innate immunity by interacting with molecules known as pathogen-associated molecular patterns (PAMPs). These proteins contribute to activation of anti-pathogen cells by switching on signals that either induce production and secretion of molecules involved in a direct attack on the pathogen or activate and regulate the components of innate immunity.1,2
PAMPs comprise a variety of microbial molecules, including structural components of the bacterial cell wall, and the proteins that bind to them have been actively studied in regard to their antimicrobial activity.1 These proteins are highly conserved from insects to humans and differ in the specificity of microbial recognition.3,4
Mechanisms of antibacterial defense with the involvement of such proteins have been better elucidated in insects. PGRP-S, the first known protein of this family, was discovered in Drosophila melanogaster. This protein binds to the lysine-rich bacterial cell wall peptidoglycan (PGN), contributing to the killing of Gram-positive bacteria.5,6 As was shown by Jules A. Hoffmann and colleagues, PGRP-S activates through Toll receptor the defense pathway leading to secretion of antimicrobial peptides.7
The antibacterial role of PGRP-S in mammals is less studied. Its murine, bovine, and human homologs have been described in neutrophils, eosinophils, and bone marrow cells.8 We have detected this protein in human T lymphocytes.9,10 Murine PGRP-S contained in neutrophil granules has been shown to inhibit the growth of Gram-positive bacteria in the culture medium.11 PGRP-S-deficient mice are highly vulnerable to bacterial infections, and their neutrophils cannot kill the bacteria they phagocytize.12 Bovine PGRP-S affects a broad spectrum of bacteria. Detailed studies on its mechanism of action have shown that this protein interacts with PAMPs less specifically, and can bind and kill microbes in a PGN-independent manner.13
We were the first to describe this protein in mice and named it as Tag7.14 Our studies on the role of Tag7 in antitumor immunity have shown that human Tag7 is involved in several antitumor defense reactions and that Tag7-transformed cells can be used in cancer immunotherapy.15 Moreover, the presence of this protein on the surface of highly specialized CD4+CD25+ lymphocytes is absolutely required for their ability to recognize and subsequently kill HLA-negative tumor cells.10
According to X-ray crystallographic data, the PGRP-S molecule has 2 binding sites: a highly conserved site for PNP binding and a more variable site for interaction with other ligands.16 The latter may include proteins that form complexes with PGRP-S, and, importantly, such complexes can acquire new, and possibly opposing functions.
As we demonstrated previously, Tag7 specifically interacts in vitro with the major heat shock protein, Hsp70, to form a complex with cytotoxic activity against a broad spectrum of tumor cell lines. Interestingly, both components of this specific complex are not exhibiting this activity separately.9 Stimulation of cytotoxic T lymphocytes by cancer cells leads to the production and secretion of the same complex, which has similar activity against tumor cells.9,17
Moreover, we have found 2 additional proteins that can interact with Tag7: the Ca2+-binding protein S100A4 (Mts1), encoded by the gene that is highly expressed in metastatic tumors and cells of the immune system, and the cochaperone HspBP1, which inhibits the ATPase activity of Hsp70.18-21 Their interaction with Tag7 results in the suppression of cytotoxicity of the Tag–Hsp70 complex.
Chemotaxis is one of the main processes of cellular activity.22 The ability of Tag7 to evoke chemotaxis, has been studied independently by 2 research teams. Their results seem contradictory. According to Liu et al.,11 this protein has no chemoattractant activity, while Mirkina et al.23 have reported that it attracts dendritic cells. To resolve this contradiction, we decided to test for the involvement of Tag7 in the migration of leukocytes. This study is aimed to find out (1) whether Tag7, alone or in complex with other proteins, can cause a chemotactic effect, and if so, (2) whether this effect is cell type specific and (3) takes place under natural conditions.
Results
Interaction between Tag7 and Mts1 produces a chemoattractant factor
We tested whether Tag7 alone could affect the migration of PBMC. Using a Transwell cell culture system, we found that the level of spontaneous cell migration did not exceed 2–3% of the number of cells in the upper compartment, and the addition of Tag7 alone to the lower compartment of the chamber had no detectable effect (Fig. 1A).
Figure 1.
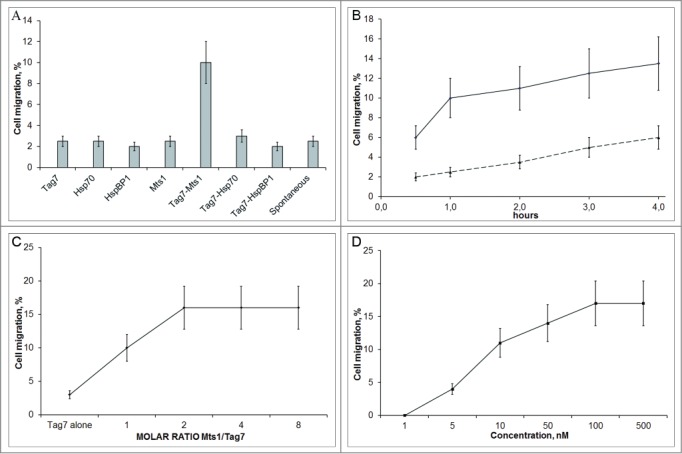
The effect of different test proteins and their complexes on the migration of PBMCs. (A) Tag7–Mts1 attracts PBMCs. Migration was tested in a Transwell system with 2 compartments separated by a polycarbonate membrane with a pore size of 8 µm. PBMCs were resuspended in 200 µl of the RPMI 1640 medium and carefully added to the upper compartment. Proteins and their complexes (10−7 M each) were transferred into the lower chamber of the Transwell system. Standard incubation to allow the formation of the protein complexes was 30 min at 37°C in PBS (pH 7.4). The number of cells in the upper compartment was taken as 100%, and the relative number of cells that migrated to the lower well was estimated after 1 h, using the MTT test. (B) The kinetics of the PMBC migration in the presence of Tag7–Mts1. PBMCs were added to the upper compartment. Components of the complex (10−7M each) were incubated for 30 min at 37°C in RPMI medium and then were added to the lower wells. The number of cells that migrated to the lower well was calculated after various time intervals using the MTT test. (solid line). Spontaneous migration is shown by the dashed line. For the spontaneous migration estimate, the testing RPMI medium was placed in the lower compartment instead of the proteins. (C) The dependence of the PBMC migration on the Mts1/Tag7 molar ratio. Experiment was performed as in B, but the lower wells contained Tag7 (10−8 M) and Mts1 at concentrations of 0 to 8 × 10−8 M. (D) The dependence of the PBMC migration on the concentration of Tag7–Mts1. Experiment was performed as in B, but the lower wells contained Tag7 and Mts1 (10−9 to 10−7 M) at the Mts1/Tag7 molar ratio of 2.
In view of our previous data that Tag7 forms complexes with Hsp70, Mts1 (S100A4), and HspBP1, tests for the cell chemotactic motility were performed with these proteins alone (as controls), as well as after their preincubation with Tag7. Neither these proteins alone nor the Tag7–Hsp70 and Tag7–HspBP1 (10 nM each) had any detectable effect, while the Tag7–Mts1 complex significantly stimulated cell migration (Fig. 1A). The kinetics of chemotaxis (Fig. 1B) showed that all cells sensitive to the Tag–Mts1 complex migrated through the semipermeable membrane within 1 h.
Tag7-Mts1 complex exhibits maximum activity at 1:2 molar ratio
Taking into account that Mts1 is prone to dimerization,24 we varied the Tag7/Mts1 molar ratio with a constant Tag7 concentration (10−8 M) and evaluated PBMC migration with the resulting complexes. The results show that the migration activity reaches a saturation level at the molar ratio of Tag7 to Mts1 in the complex at 1:2 (Fig. 1C). The molar concentrations of the complex indicated below refer to Tag7. The maximum chemotactic effect on PBMCs in the experiments with different concentrations of the complex was achieved at 100 nM Tag7–Mts1 (Fig. 1D).
Natural Killer cells are the most sensitive to Tag7–Mts1
Our next goal was to estimate which particular PBMC subpopulations were attracted by Tag7–Mts1. PBMCs were incubated in the Transwell cell culture system with or without the chemoattractant complex, and the numbers of CD14+, CD4+, CD8+, and CD16+CD56+ cells in its compartments were determined by flow cytometry (Fig. 2A, Supplemental Table 1) On this basis, we calculated the levels (%) of total migration, spontaneous migration, and specific migration caused by Tag7–Mts1 for each of the leukocyte types.
Figure 2.
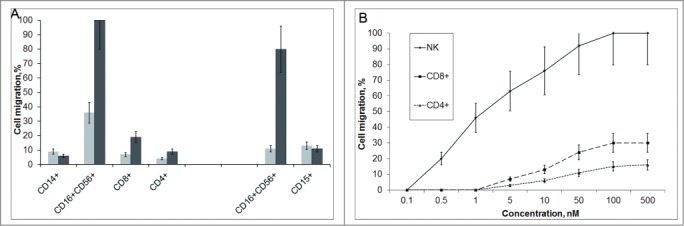
The Tag7–Mts1 comples stimulates migration of CD4+, CD8+, and CD16+CD56+ (NK) cells. (A) On the left panel PBMCs were placed in the upper compartment of the Transwell system, the lower compartment contained Tag7–Mts1 (5 × 10−8 M, Mts1/Tag7 = 2) (black bars). As a control, the RPMI medium was placed in the lower compartment (gray bars). The initial number of cells in the upper compartment was taken as 100%, and the relative number of cells that migrated to the lower compartment was estimated after 1 h by the flow cytometry. On the right panel, the isolated subpopulations of CD16+CD56+ (NK) and CD15+ cells (neutrophils) were used instead of the PBMC. (B) The dependence of migration of NK, CD4+, and CD8+ cell populations isolated using immunomagnetic beads (see Material and Methods) on the concentration of Tag7–Mts1 (Mts1/Tag7 molar ratio = 2). The number of cells in the upper compartment was taken as 100%, and the relative number of cells that migrated to the lower well with a test protein or complex was estimated after 1 h using the MTT test.
The motility of monocytes in the control was low, and it was further reduced when Tag7–Mts1 was added to the lower compartment of the chamber. A positive chemotaxis was recorded for T lymphocytes (CD4+ and CD8+) and especially for the NK cells (CD16+CD56+), which showed the highest motility: every third cell spontaneously migrated through the membrane between the compartments, and all of them migrated to the lower compartment when it contained Tag7–Mts1.
To further validate this effect, we used immunomagnetic beads to isolate a PBMC fraction enriched up to 85% with NK cells. The level of spontaneous cell migration in this subpopulation was lower, while the level of chemotactic migration exceeded it more than sixfold. For comparison, neutrophils (CD15+) in the fraction isolated in the same way showed no higher spontaneous motility than did the NK cells and were not attracted by the Tag7–Mts1 complex.
A more detailed analysis of chemotaxis induced by different Tag7–Mts1 concentrations in the enriched cell subpopulations showed that the motility of NK, CD4+, and CD8+ cells reached a maximum at the same Tag7–Mts1 concentration of 10−7 M (Fig. 2B). However, the concentration causing the onset of directed cell migration proved to be an order of magnitude lower for the NK cells than for the purified T lymphocytes. It is noteworthy that the data on the dependence of chemotaxis from concentration for the CD4+ and CD8+ cell subpopulations are similar to that for the total PBMCs. Such similarity is not surprising, since T lymphocytes comprise the greater part of PBMCs attracted by the Tag7–Mts1 complex, but it provides evidence that the procedure of lymphocyte purification with magnetic beads does not affect the properties of these cells.
Hsp70 and HspBP1 block the chemoattractant activity of Tag7–Mts1
To gain an insight into the nature of the observed phenomenon, the formation of a chemoattractant complex by 2 proteins that have no chemoattractant activity, it appeared expedient to find conditions for reducing or eliminating this activity of the complex. The most natural and feasible approach in our case was to use proteins interacting with Tag7. In our previous studies,9,19 the interaction with Tag7 was shown for Hsp70, HspBP1, and Mts1, with Mts1 and HspBP1 binding to the Tag7–Hsp70 complex resulting in the inhibition of its cytotoxic activity. Here, we analyzed the effect of Hsp70 and HspBP1 on the chemoattractant activity of Tag7–Mts1. Aliquots of this complex were incubated with increasing amounts of the third component (Hsp70 or HspBP1) and tested for activity on PBMCs. As shown in Fig. 3A, Hsp70 at an equimolar concentration to the complex had no effect, but its excess over Tag7–Mts1 gradually inhibited the chemoattractant activity of this complex, which dropped to zero when the excess was fivefold. This was accompanied by an increase in its cytotoxic activity (Supplemental Fig. 1). Such a result strongly suggested that Mts1 in the complex was competitively substituted by Hsp70. To test this possibility, we adsorbed Tag7–Mts1 on anti-Tag7 Sepharose, which specifically bound this complex (Supplemental Fig. 2), and then treated the column with a fivefold excess of Hsp70. An analysis of proteins that remain bound to the column revealed the presence of Hsp70 instead of Mts1 (Fig. 3B).
Figure 3.
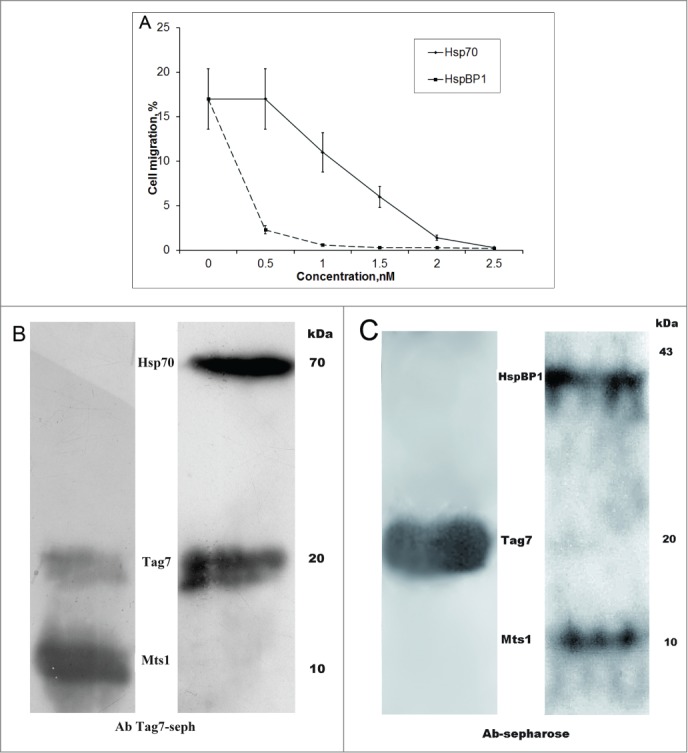
Hsp70 and HspBP1 suppress the effect of Tag7–Mts1 on the cell migration. (A) The Tag7-Mts1 complex (5 × 10−8M, Mts1/Tag7=2 ) was formed in RPMI and incubated for 30 min at 37 °C under sterile conditions. Then, a third protein was added: (squares) HspBP1 and (circles) Hsp70. The mixture was incubated for another 30 min, and assayed for the migration activity toward PBMC, as described previously. (B) Hsp70 displaces Mts1 from the Tag7–Mts1 complex. On the left panel, an aliquot of Tag7-Mts1 (300 ng, Mts1/Tag7 = 2) was absorbed on the anti-Tag7 Sepharose, the bound proteins were eluted with 0.25 M TEA from the column, and were analyzed by immunoblotting with antibodies to Mts1 and Tag7. On the right panel, an aliquot of Tag7-Mts1 (300 ng, Mts1/Tag7 = 2) was absorbed on the anti-Tag7 Sepharose, and then a solution containing Hsp70 (1500 ng) was passed through the column, the bound proteins were eluted with 0.25 M TEA from the column, and were analyzed by immunoblotting with antibodies to Mts1, Tag7, and Hsp70. (C) HspBP1 binds to the Tag7–Mts1 complex. An aliquot of Tag7-Mts1 (300 ng, Mts1/Tag7 = 2) was absorbed on the anti-Tag7 Sepharose, and then a solution containing 600 ng of HspBP1 was passed through the column. The bound proteins were eluted with 0.25 M TEA and analyzed by immunoblotting with antibodies to Tag7 (left panel) or to Mts1 and HspBP1 (right panel).
The addition of HspBP1 to Tag7–Mts1 resulted in an abrupt drop of chemoattractant activity: the ability of the complex to stimulate migration of PMBCs practically disappeared at an equimolar concentration of HspBP1 (Fig. 3A). To reveal the cause of this phenomenon, we repeated the experiment with Tag7–Mts1 adsorbed on anti-Tag7 Sepharose but washed the column with a twofold excess of HspBP1. An analysis of the fraction containing the bound protein showed that it contained not only Tag7 and Mts1 but also HspBP1 (Fig. 3C), and this ternary complex was apparently incapable of causing migration of PBMCs.
Chemoattractant Tag7–Mts1 complex is produced by neutrophils, monocytes, and lymphocytes
We next tested whether such a chemoattractant factor may be naturally produced by cells involved in the immune defense. To this end, PMBCs were incubated for 18 h under standard conditions (see Material and Methods), without activators, and the conditioned medium was then collected and applied onto an anti-Tag7 Sepharose column. The material specifically bound to the column contained both Tag7 and Mts1, with Mts1 being represented by both monomers and tetramers (Fig. 4A); i.e., PMBC proved to release the Tag7–Mts1 complex to the medium. An ELISA analysis of the complex showed that the Mts1/Tag7 ratio was 2:1.
Figure 4.
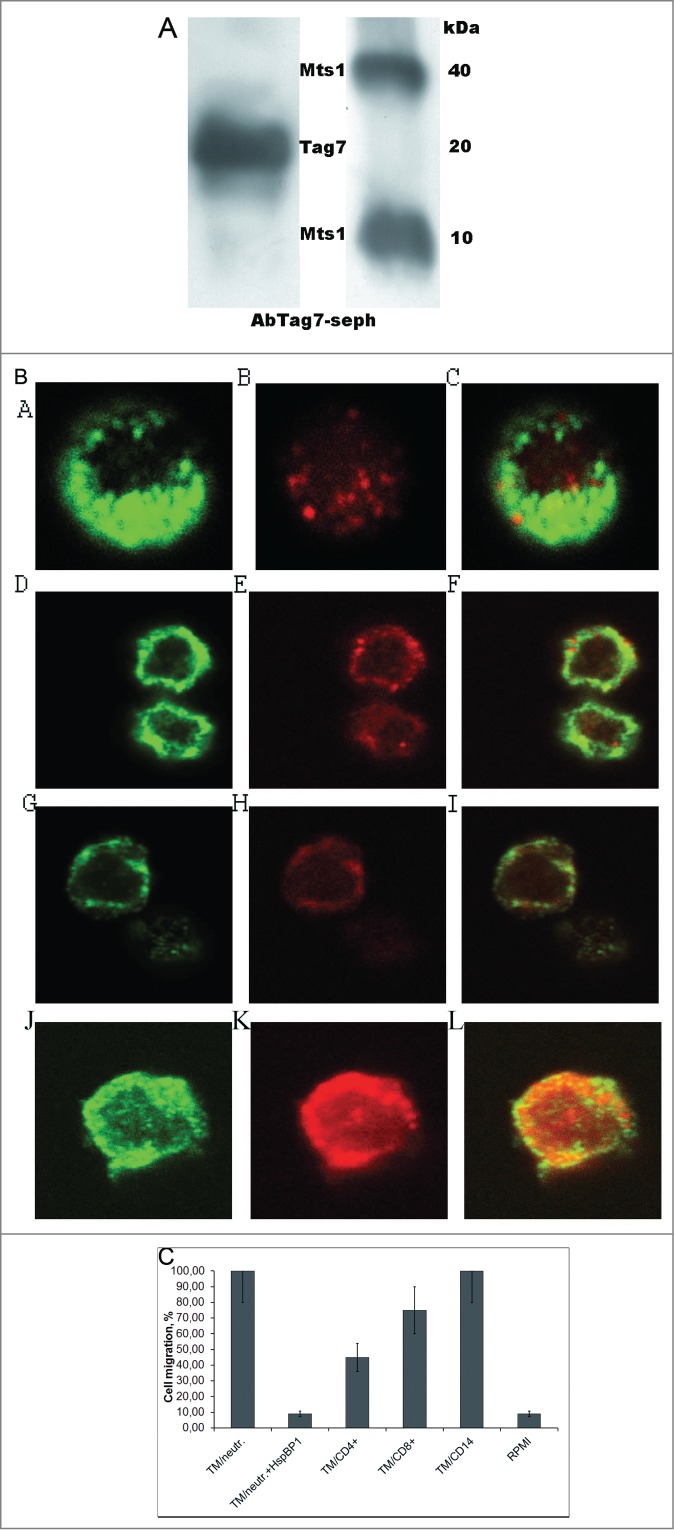
Human leukocytes contain the Tag7–Mts1 complex. (A) Tag7 and Mts1 are contained in the medium conditioned by PBMCs. The PBMC cells were incubated for 18 hours in sterile conditions, than conditioned medium was collected and passed through an anti-Tag7 Sepharose, and the absorbed proteins were eluted and analyzed by immunoblotting with antibodies to Tag7 (on the left) and Mts1 (on the right). (B) Tag7, Mts1, and the Tag7–Mts1 complex are localized in the cytoplasmic granular inclusions in CD4+ and CD8+ lymphocytes and neutrophils. Confocal micrographs of (a–c) neutrophil, (d–f) CD8+ lymphocyte, (g–i) CD4+ lymphocyte, and (j–l) monocyte double-immunostained with (a, d, g, j) rabbit anti-Mst1 antibodies and FITC-conjugated anti-rabbit IgG (green fluorescence) and (b, e, h, k) mouse anti-Tag7 antibodies and phycoerythrin-conjugated anti-mouse IgG (red fluorescence), and (c, f, i, l) merged images of the same cells. Images were taken at ×100 magnification. (C) The effect of Tag7–Mts1 complex (TM) from different sources on the migration of CD56+ cells. Isolated CD56+ cells in 200 µL of RPMI were placed in the upper compartment of the Transwell system while the lower compartment contained immune-adsorbed Tag7–Mts1 from the medium conditioned by neutrophils, CD4+ or CD8+ lymphocytes, monocytes, or RPMI (control). The initial number of cells in the upper compartment was taken as 100%, and the relative number of cells that migrated to the lower compartment was estimated after 1 h using the MTT test.
To determine what particular leukocyte subpopulations contain Mts1 and Tag7, and which cells released the Tag7–Mts1 complex to the medium, we isolated CD14+, CD15+, CD8+, CD4+, and CD16+CD56+ cell fractions using immunomagnetic beads, double-immunostained them for Tag7 and Mts1, and examined these cells under a confocal microscope (Fig. 4B). The results showed that neutrophils contained either Tag7 or Mts1, or both proteins, which were localized in the cytoplasmic granules. The same was observed in monocytes, although Tag7 was not previously detected in these cells.27 Granules with either or both the Tag7 and Mts1 (colocalized with each other) were also found in T lymphocytes, but none of these proteins was detected in NK cells
We then used the approach described above for PMBCs to analyze the medium conditioned by CD14+, CD8+, CD4+, and CD15+ cells (Table 1). All the test samples proved to contain both Tag7 and Mts1, indicating that they were released in complex with each other, but the relative amounts of these proteins in the complex varied between cell subpopulations. The Mts1/Tag7 ratios for neutrophils and monocytes were 2.2 and 2.0, while those for CD4+ and CD8+ lymphocytes, 0.56 and 1.5, respectively. Aliquots of the material bound to the immunosorbent were tested for the ability to attract NK cells. Figure 4C shows that the chemoattractant effect of 4 samples was commensurate to the amount of the Tag7–Mts1 complex that could be formed in each of these cases. Note that the addition of HspBP1 to the sample from neutrophils completely blocked this effect.
Table 1.
Results of ELISA for the contents of Tag7 and Mts1 in the medium conditioned by isolated subpopulations of CD15+, CD8+, CD4+, or CD14+ cells or total PMBCs. The medium (10 mL) was applied onto an anti-Tag7 Sepharose column, and the bound proteins were eluted with 0.25 M TEA.
| Cells | Mts1, nmol | Tag7, nmol | Mts1/Tag7 |
|---|---|---|---|
| CD15+ (neutrophils) | 16.5 | 7.5 | 2.2 |
| CD4+ | 6.7 | 12.0 | 0.56 |
| CD8+ | 13 | 8.7 | 1.5 |
| CD14+ | 14 | 7.0 | 2.0 |
| PMBCs | 0.130 | 0.260 | 0.50 |
Thus, it may be concluded that the chemoattractant factor produced and released ex vivo by neutrophils, monocytes, and T lymphocytes is similar to the Tag7–Mts1 complex formed in vitro from the 2 recombinant proteins. An important fact is that this factor is released in comparable amounts by cells classified as effectors of either innate immunity (neutrophils) or acquired immunity (CD8+ lymphocytes), in the absence of any activators.
Discussion
The results presented above appear to clear up the contra- diction in data on the chemoattractant activity of Tag7 (see Introduction). They show that this protein by itself is not chemotactic for either PMN or PMBCs, while the Tag7–Mts1 complex described in our previous studies can stimulate the migration of lymphocytes.
The functions of the innate immunity protein Tag7 in the immune response are manifold, but the best known is the role it plays in the killing of bacteria due to the ability to recognize PAMPs of the bacterial cell wall.5,7 We have shown that Tag7 is expressed on the surface of CD4+CD25+ lymphocytes, the highly specialized cells of the acquired immunity that participate in the killing of HLA-negative tumor cells by recognizing Hsp70 on their surface.10,18 The latter protein is practically absent on the surface of normal cells but is exposed on tumor cells, thereby serving as an "alarm signal" for effector lymphocytes, and it is Tag7 that recognizes Hsp70 and makes a specific contact with it.
Our data have deepened the insight into the functional activity of Tag7 in the immune defense, showing that it forms stable complexes with Hsp70 and the Ca2+binding protein Mts1,9,18 with the functions of these complexes being completely different from those of their components. Indeed, the Tag7–Hsp70 complex can kill tumor cells, thereby retarding tumor growth, while Tag7 alone has no cytotoxic activity. The main role of Hsp70 is to support the survival of cells (including tumor cells), which is opposite to the function of this binary complex.
In this study, we have uncovered a novel function for the Tag7–Mts1 complex, i.e., its ability to cause migration of lymphocytes. As previously shown for the cytotoxic Tag7–Hsp70 complex, none of Tag7–Mts1 components alone have such an activity. Stimulation of lymphocyte migration in the presence of Tag7–Mts1 is indicative of a new role of Tag7 in the immune defense mechanisms.
A new role in immune defense has also been demonstrated for the second component of this complex, the Mts1 (S100A4) protein of the Ca2+-binding family that participates in various processes of the cell life. Researchers have focused much attention on its role in tumor progression and metastasis,26,27 but the mts1 gene is highly expressed in cells of the immune system, suggesting that this protein may also be involved in the immune defense.26
We have previously found that Mts1 is exposed on the surface of cytotoxic CD4+CD25+ lymphocytes and, together with Tag7, participates in recognition and killing of tumor cells with surface expression of Hsp70. The data presented above show that Mts1 in complex with Tag7 also functions as an attractant for lymphocytes. Similar effect was found for Hsp70 protein located on the surface of cancer cells. Multhoff and colleagues has found that Hsp70 on the surface of cancer cells is able to attract NK cells.28
The Tag7–Mts1 complex is not a chemokine: its components lack the structure (fold) named Greek key, which is shared by classic chemokines,29,30 and the complex acquires chemoattractant properties only after it folds into a specific quaternary structure. Moreover, no cell activation is required for its production and transmission of the chemotactic signal. According to our observations, quiescent cells release Tag7–Mts1 without any stimulation.
This complex is naturally produced mainly by myeloid phagocytes. This is not surprising for neutrophils, since cytoplasmic granules in these cells contain both Tag731 and Mts1.32 Monocytes were not previously found to contain Tag7,32 but the results presented above show that this protein may be present in monocytes (confocal microscopy) and that the medium conditioned by these cells contains the Tag7–Mts1 complex.
We do not suggest/think that every neutrophil or monocyte releases Tag7–Mts1, but it is apparent that none of them is attracted by this complex. However, Tag7–Mts1 has proved to be a powerful chemoattractant for NK cells, classic effector lymphocytes of innate and antitumor immunity. As for T-lymphocytes, they show a certain dualism in this respect, which is due to their heterogeneity. Some CD4+ and CD8+ cell populations may release Tag7–Mts1, while others may be attracted by this complex, with the motility of Tag7–Mts1-sensitive lymphocytes being comparable to that of NK cells: both migrate through the membrane of the Transwell microchemotaxis chamber at the same rate, and their maximum motility is achieved at the same Tag7–Mts1 concentration.
We have shown that chemoattractant Tag7–Mts1 complex is produced in the cells of both the innate (neutrophils, monocytes) and acquired (subpopulation of CD4+, CD8+ lymphocytes) immune system. We cannot claim that these complexes are identical. Proteins and their complexes can be subjected to various modifications in different cells, including post-translational modifications. Several glycosylation sites are known for Tag7 protein,16 and Mts1 can be phosphorylated by PKC.33 In addition, our results demonstrated that Mts1 in a leukocyte conditioned medium can be in 2 alternative forms, monomeric and tetrameric, and these properties of Mts1, possibly, could affect the chemotactic activity of the Tag7–Mts1 complex. It is also worth noting that other proteins could interact with the Tag7–Mts1 complex. Our data demonstrate that Hsp70 and HspBP1 proteins can bind to this complex, and potentially reduce its chemoattractant properties. Perhaps, there are other proteins that can modulate the activity of the Tag7–Mts1 complex as well.
Nevertheless, the complexes secreted by cells from the different branches of the immune system, have an identical functional activity, which correlated well with the activity of the complexes reconstituted from the recombinant proteins at the same ratio of Tag7 to Mts1, similar to that from the tested supernatants.
We have also shown that the Tag7–Mts1 complex induces motility of the subpopulations of NK, and CD4+ and CD8+ lymphocytes. These data suggest a presence on the surface of these cells, belonging to different branches of the immune response, of functionally identical receptors that can interact with the Tag7-Mts1 complex. The study of the nature of these receptors will be the subject of further research.
Thus, the detection of a new chemoattractant function of Tag7, a protein of the innate immunity branch, shows that it can participate in the regulation of both branches of the immune response, suggesting that they have much in common.
Material and Methods
Cells. Human peripheral blood mononuclear cells (PBMCs) were isolated as described9 to purify the CD16+CD56+, CD14+, CD8+, and CD4+ cell subpopulations by means of negative selection, and the CD15+ subpopulation, by positive selection using immunomagnetic beads (Dynal Biotech ASA, Norway) according to the manufacturer's protocol. Total PBMCs and the above cell subpopulations (4 × 106 cells/mL) were incubated in RPMI-1640 with 5% fetal calf serum at 37°C for 18 h, and the cells were removed by centrifugation to collect the conditioned medium. Human materials were used according to the regulations of Ministry of Health of Russian Federation.
Proteins and protein complexes. Recombinant Tag7 was obtained using the pET-28 plasmid (Novagen, Madison, WI) encoding a fragment (aa 22–196) of human Tag7 (kindly provided by Dr. Andreas Bracher, Max Planck Institute of Biochemistry, Martinsried, Germany). Human 70-kDa heat shock protein 1A (Hsp70, GenBank accession no. NM_005345) was subcloned in pQE-30 (Qiagen, Germany) and expressed in Escherichia coli M15 [pREP4] (Qiagen). Human HspBP1 (GenBank accession number NM_012267) was subcloned in pET-28a. The proteins were purified on Ni-NTA agarose (Qiagen) according to the manufacturer's protocol. Human Mts1 was subcloned in pQE-30 (Qiagen), expressed in Escherichia coli M15 [pREP4] (Qiagen), and isolated and purified as described.34 Protein complexes were formed under standard conditions, by incubating their components in PBS, pH 7.4, for 30 min at 37°C. To isolate the Tag7–Mts1 complex from the medium conditioned by neutrophils, monocytes, or CD4+, CD8+ T lymphocytes, the medium was applied onto an anti-Tag7 Sepharose column (see below) and, after washing the column with PBS–0.5 M NaCl and PBS alone, bound proteins were eluted with 0.25 M triethylamine (TEA) (Sigma), pH12.5.
Antibodies, immunoadsorption, and immunoblotting. R-Phycoerythrin-conjugated antibodies to CD4+, CD8+, and CD56+ cells were from Caltag Laboratories (Burlingame, CA); rabbit polyclonal anti-Mts1 (S100A4) antibodies (Ab-8), from NeoMarkers (Fremont, CA); and rabbit anti-HspBP1 antibodies, from Delta Biolabs (Campbell, CA). Rabbit and mouse anti-Tag7 antibodies were raised as described.9 Anti-Tag7 resin was prepared by coupling the antibodies to CNBr-activated Sepharose 4B (Amersham Biosciences) according to the manufacturer's protocol.
Proteins eluted from the column were dried, resolved by 10–15% SDS-PAGE, and blotted onto a PVDF membrane (GE Healthcare Ltd., UK). The membrane was blocked with 1% non fat dry milk and incubated with primary antibodies (anti-Mts1, anti-Tag7, anti-Hsp70, or anti-HspBP1) and horseradish peroxidase-conjugated secondary antibodies (GE Healthcare). Proteins were visualized using ECL Plus detection reagents (GE Healthcare) according to the manufacturer's protocol. The contents of Mts133 and Tag720 in samples were measured by a ELISA.
Cell migration activity
Migration assays were performed in a Transwell microchemotaxis chamber cell culture system (Costar). All assays were done in triplicate. The upper and lower compartments were separated by a tissue culture polycarbonate membrane (polyvinyl-pyrrolidone free; Nucleopore, Pleasanton, CA), 6.5 mm in diameter, with a pore size of 8μm. Proteins were placed in a total volume of 600 μl of RPMI 1640 medium in the lower compartment. Then, PBMC or CD56-positively sorted cells were resuspended in 200 μl of RPMI 1640 medium and carefully added to the upper compartment. After a 1-h incubation period in a humidified incubator at 37°C and 5% CO2, the cell suspension in the lower compartment was harvested, and cell viability was determined by using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). To each well 0.5 mg/ml of MTT was added. The samples were incubated in the absence of light for 3 h, after which the medium was removed. The precipitates were resuspended in 150 µl of DMSO. The absorbance was measured on a plate reader (Bio-Rad Laboratories) at 530 nm. The calibration curve was plotted using PBMC dilutions within a range of 5–500 × 103 cells/ml. The number of cells in the upper compartment was taken as 100%.
Flow cytometry. The cells were fixed with 1% paraformaldehyde (Sigma) and stained with appropriate antibodies at room temperature. The samples (at least 104 cells each) were analyzed with an Epics Elite flow cytometer (Coulter, Marseille, France) in the logarithmic channel of fluorescence. The data were processed with Immuno-4 (version 4.02; Coulter) for single-parameter histograms or with EXPO32 software (Applied Cytometry Systems, Sheffield, United Kingdom).
Microscopy. CD4+, CD56+, CD8+, CD15+, and CD14+ cells were washed with 2 portions of PBS, fixed and permeabilized using a FIX & PERM kit (Caltag Laboratories), and stained for Tag7 with mouse anti-Tag7 antibodies and Alexa 633-conjugated anti-mouse IgG, and for Mts1 with rabbit anti-S100A4 antibodies and Alexa 546-conjugated anti-rabbit IgG. Fluorescence images were obtained with a Leica TCS SP2 confocal microscope, analyzed with Leica confocal software, and prepared in Photoshop CE (Adobe Systems).
Statistical analysis
A Student t test for means (paired 2 samples) was used for calculating significance, and p values <0.05 were considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This work was supported by Russian Science Foundation grant № 15–14–00031 (paragraphs 3, 4, 5 of Results), RAS Program for Molecular and Cellular Biology (paragraphs 1, 2 of Results).
References
- 1.Hoffmann JA. The immune response of Drosophila. Nature 2003; 426:33-8; PMID:14603309; http://dx.doi.org/ 10.1038/nature02021 [DOI] [PubMed] [Google Scholar]
- 2.Imler JL, Zheng L. Biology of Toll receptors: lessons from insects and mammals. J Leukoc Biol 2004; 75: 18-26; PMID:12960276; http://dx.doi.org/ 10.1189/jlb.0403160 [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science 1999; 284: 1313-8; PMID:10334979; http://dx.doi.org/ 10.1126/science.284.5418.1313 [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20:197-216; PMID:11861602; http://dx.doi.org/ 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- 5.Kang D, Liu G, Lundström A, Gelius E, Steiner H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc Natl Acad Sci U S A 1998; 95: 10078-82; PMID:9707603; http://dx.doi.org/ 10.1073/pnas.95.17.10078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci U S A 2000; 97: 13772-7; PMID:11106397; http://dx.doi.org/ 10.1073/pnas.97.25.13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature; 2001; 414:756-9; PMID:11742401; http://dx.doi.org/ 10.1038/414756a [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Xu Z, Gupta D, Dziarski R. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J Biol Chem 2001; 276:34686-94; PMID:11461926; http://dx.doi.org/ 10.1074/jbc.M105566200 [DOI] [PubMed] [Google Scholar]
- 9.Sashchenko LP, Dukhanina EA, Yashin DV, Shatalov YV, Romanova EA, Korobko EV, Demin AV, Lukyanova TI, Kabanova OD, Khaidukov SV, et al. Peptidoglycan recognition protein tag7 forms a cytotoxic complex with heat shock protein 70 in solution and in lymphocytes. J Biol Chem 2004; 279: 2117-24; PMID:14585845; http://dx.doi.org/ 10.1074/jbc.M307513200 [DOI] [PubMed] [Google Scholar]
- 10.Sashchenko LP, Dukhanina EA, Shatalov YV, Yashin DV, Lukyanova TI, Kabanova OD, Romanova EA, Khaidukov SV, Galkin AV, Gnuchev NV, Georgiev GP. Cytotoxic T lymphocytes carrying a pattern recognition protein Tag7 can detect evasive, HLA-negative but Hsp70-exposing tumor cells, thereby ensuring FasL/Fas-mediated contact killing. Blood 2007; 110: 1997-2004; PMID:17551095; http://dx.doi.org/ 10.1182/blood-2006-12-064444 [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Gelius E, Liu G, Steiner H, Dziarski R. Mammalian peptidoglycan recognition protein binds peptidoglycan with high affinity, is expressed in neutrophils, and inhibits bacterial growth. J Biol Chem 2000; 275: 24490-9; PMID:10827080; http://dx.doi.org/ 10.1074/jbc.M001239200 [DOI] [PubMed] [Google Scholar]
- 12.Dziarski R, Platt KA, Gelius E, Steiner H, Gupta D. Defect in neutrophil killing and increased susceptibility to infection with nonpathogenic gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood 2003; 102: 689-97; PMID:12649138; http://dx.doi.org/ 10.1182/blood-2002-12-3853 [DOI] [PubMed] [Google Scholar]
- 13.Tydell CC, Yuan J, Tran P, Selsted ME. Bovine peptidoglycan recognition protein-S: antimicrobial activity, localization, secretion, and binding properties. J Immunol 2006; 176: 1154-62; PMID:16394004; http://dx.doi.org/ 10.4049/jimmunol.176.2.1154 [DOI] [PubMed] [Google Scholar]
- 14.Kustikova OS, Kiselev SL, Borodulina OR, Senin VM, Afanas'eva AV, Kabishev AA. Cloning of the tag7 gene expressed in metastatic mouse tumors. Genetika 1996; 32:621-8; PMID:8755036 [PubMed] [Google Scholar]
- 15.Larin SS, Korobko EV, Kustikova OS, Borodulina OR, Raikhlin NT, Brisgalov IP, Georgiev GP, Kiselev SL. Immunotherapy with autologous tumor cells engineered to secrete Tag7/PGRP, an innate immunity recognition molecule. J Gene Med 2004; 6: 798-808.PMID: 15241787; http://dx.doi.org/ 10.1002/jgm.560 [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Dube D, Sinha M, Yadav S, Kaur P, Sharma S, Singh TP. Structural insights into the dual strategy of recognition by peptidoglycan recognition protein, PGRP-S: structure of the ternary complex of PGRP-S with lipopolysaccharide and stearic acid. PLoS One 2013; 8: e53756; PMID:23326499; http://dx.doi.org/ 10.1371/journal.pone.0053756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dukhanina EA, Yashin DV, Lukjanova TI, Romanova EA, Kabanova OD, Shatalov YV, Sashchenko LP, Gnuchev NV. Administration of the cytotoxic complex Tag7-Hsp70 to mice with transplanted tumors inhibits tumor growth. Dokl Biol Sci 2007; 414: 246-8; PMID:17668634; http://dx.doi.org/ 10.1134/S0012496607030222 [DOI] [PubMed] [Google Scholar]
- 18.Dukhanina EA, Kabanova OD, Lukyanova TI, Shatalov YV, Yashin DV, Romanova EA, Gnuchev NV, Galkin AV, Georgiev GP, Sashchenko LP. Opposite roles of metastasin (S100A4) in two potentially tumoricidal mechanisms involving human lymphocyte protein Tag7 and Hsp70. Proc Natl Acad Sci U S A 2009; 106: 13963-7; PMID:19666596; http://dx.doi.org/ 10.1073/pnas.0900116106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dukhanina EA, Yashin DV, Galkin AV, Sashchenko LP. Unexpected deeds of familiar proteins: Interplay of Hsp70, PGRP-S/Tag7 and S100A4/Mts1 in host vs. cancer combat. Cell Cycle 2010; 9: 676-82; PMID:20107319; http://dx.doi.org/ 10.4161/cc.9.4.10782 [DOI] [PubMed] [Google Scholar]
- 20.Yashin DV, Dukhanina EA, Kabanova OD, Romanova EA, Lukyanova TI, Tonevitskii AG, Raynes DA, Gnuchev NV, Guerriero V, Georgiev GP, Sashchenko LP. The heat shock-binding protein (HspBP1) protects cells against the cytotoxic action of the Tag7-Hsp70 complex. J Biol Chem 2011; 286: 10258-64; PMID:21247889; http://dx.doi.org/ 10.1074/jbc.M110.163436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yashin DV, Dukhanina EA, Kabanova OD, Romanova EA, Lukyanova TI, Tonevitskii AG, Belogurov AA, Raynes DA, Sheludchenkov AA, Gnuchev NV, et al. Extracellular HspBP1 inhibits formation of a cytotoxic Tag7-Hsp70 complex in vitro and in human serum. Biochimie 2012; 94: 203-6; PMID:22037021; http://dx.doi.org/ 10.1016/j.biochi.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 22.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology 2005;116(1):1-12; PMID:16108812; http://dx.doi.org/ 10.1111/j.1365-2567.2005.02183.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirkina I, Rot A, Kiselev S, Aversa G, Schweighoffer T Mammalian. Peptidoglycan recognition protein is chemotactic for human polymorphonuclear leukocytes and promotes maturation of human dendritic cells. Clin Invest Med 2004; 27: 27D, Th20.11. [Google Scholar]
- 24.Malashkevich VN, Dulyaninova NG, Ramagopal UA, Liriano MA, Varney KM, Knight D, Brenowitz M, Weber DJ, Almo SC, Bresnick AR. Phenothiazines inhibit S100A4 function by inducing protein oligomerization. Proc Natl Acad Sci U S A 2010; 107: 8605-10; PMID:20421509; http://dx.doi.org/ 10.1073/pnas.0913660107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tydell CC, Yount N, Tran D, Yuan J, Selsted ME. Isolation, characterization, and antimicrobial properties of bovine oligosaccharide-binding protein. A microbicidal granule protein of eosinophils and neutrophils. J Biol Chem 2002; 277: 19658-64; PMID:11880375; http://dx.doi.org/ 10.1074/jbc.M200659200 [DOI] [PubMed] [Google Scholar]
- 26.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 proteins. Curr Mol Med 2013 Jan;13(1):24-57; PMID:22834835 [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra SK, Siddique HR, Saleem M. S100A4 calcium-binding protein is key player in tumor progression and metastasis: preclinical and clinical evidence. Cancer Metastasis Rev 2012; 31: 163-72; PMID:22109080; http://dx.doi.org/ 10.1007/s10555-011-9338-4 [DOI] [PubMed] [Google Scholar]
- 28.Hantschel M, Pfister K, Jordan A, Scholz R, Andreesen R, Schmitz G, Schmetzer H, Hiddemann W, Multhoff G. Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones 2000; 5: 438-42; PMID:11189449; http://dx.doi.org/ 10.1379/1466-1268(2000)005%3c0438:HPMEOP%3e2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan R, Wang Q, Sundberg EJ, Mariuzza RA. Crystal structure of human peptidoglycan recognition protein S (PGRP-S) at 1.70 A resolution. J Mol Biol 2005; 347: 683-91; PMID:15769462; http://dx.doi.org/ 10.1016/j.jmb.2005.01.070 [DOI] [PubMed] [Google Scholar]
- 30.Vallely KM, Rustandi RR, Ellis KC, Varlamova O, Bresnick AR, Weber DJ. Solution structure of human Mts1 (S100A4) as determined by NMR spectroscopy. Biochemistry 2002; 41: 12670-80; PMID:12379109; http://dx.doi.org/ 10.1021/bi020365r [DOI] [PubMed] [Google Scholar]
- 31.Tydell CC, Yuan J, Tran P, Selsted ME. Bovine peptidoglycan recognition protein-S: antimicrobial activity, localization, secretion, and binding properties. J Immunol 2006; 176: 1154-62; PMID:16394004; http://dx.doi.org/ 10.4049/jimmunol.176.2.1154 [DOI] [PubMed] [Google Scholar]
- 32.Dukhanina EA, Lukyanova TI, Romanova EA, Dukhanin AS, Sashchenko LP. Comparative analysis of secretion of S100A4 metastatic marker by immune and tumor cells. Bull Exp Biol Med 2008; 145: 78-80; PMID:19024009; http://dx.doi.org/ 10.1007/s10517-008-0003-z [DOI] [PubMed] [Google Scholar]
- 33.Kriajevska M, Fischer-Larsen M, Moertz E, Vorm O, Tulchinsky E, Grigorian M, Ambartsumian N, Lukanidin E. Liprin β 1, a member of the family of LAR transmembrane tyrosine phosphatase-interacting proteins, is a new target for the metastasis-associated protein S100A4 (Mts1). J Biol Chem 2002; 277: 5229-35; PMID:11836260; http://dx.doi.org/ 10.1074/jbc.M110976200 [DOI] [PubMed] [Google Scholar]
- 34.Tarabykina S, Kriajevska M, Scott DJ, Hill TJ, Lafitte D, Derrick PJ, Dodson GG, Lukanidin E, Bronstein I. Heterocomplex formation between metastasis-related protein S100A4 (Mts1) and S100A1 as revealed by the yeast two-hybrid system. FEBS Lett 2000; 475: 187-91; PMID:10869553; http://dx.doi.org/ 10.1016/S0014-5793(00)01652-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


