Centromere is a chromatin structure positioning the site of kinetochore assembly. Faithful chromosome segregation relies on the presence of only one centromere per sister chromatid, the recruitment of kinetochore proteins and the establishment/maintenance of centromeric cohesion until anaphase. Centromeres are epigenetically defined by the loading of the histone H3 variant CENH3, which is mediated by specific chaperones that have diverged throughout evolution. Centromeres display specific features in their organization at the chromatin level, i.e. particular intermingled CENH3- and H3-containing nucleosomes and highly repeated DNA sequences. Pericentromeric heterochromatin is necessary for centromere identity and maintenance, specifying CENH3 localization and establishing centromeric cohesion.1 Beside these features, the function of the centromere in the context of the 3D nuclear architecture has been poorly investigated. In the plant model Arabidopsis thaliana, centromeres are spatially distributed within the nucleus and are embedded in chromocentres mainly constituted by pericentromeric heterochromatin located at the nuclear periphery.2 Using functional and cellular approaches, we were able to demonstrate that the small GIP proteins are key actors involved in the regulation of the centromere structure in Arabidopsis.3
The GIP proteins (namely GIP1 and GIP2) were initially characterized by our team as partners of the gamma-tubulin complex protein 3 in Arabidopsis.4 These proteins are conserved throughout evolution and share more than 50% of similarity with their homologues in humans and fission yeast, called MOZART1 and Mitotic spindle organiZing proTein1 (MZT1), respectively. The analyses of Arabidopsis gip1gip2 knocked down mutants revealed severe and pleiotropic developmental phenotypes, suggesting that GIPs may be involved in the regulation of various cellular processes. First, we characterized GIPs as regulators of the recruitment of the gamma-tubulin complexes at microtubule nucleation sites, notably at the nuclear envelope before mitosis onset. We observed that GIP-GFP fusions accumulated as punctuated structures on both sides of the nuclear envelope, at the outer nuclear membrane close to radiating microtubules and close to the inner membrane at centromeres.5 Due to this centromeric localization, GIP proteins were relevant candidates for the investigation of centromere regulation.
We demonstrated that GIP1 and GIP2 formed a protein complex with CENH3 by co-immuno-precipitation experiments and we showed that GIP-GFP fusions colocalized with CENH3 in interphase nuclei. The overexpression of CENH3 increased the recruitment of GIP-GFP proteins at the centromere. A decreased amount of CENH3 was observed at centromeres and in nuclear extracts of gip1gip2 mutants. Altogether, these data strongly argue in favor of a significant role of GIPs for CENH3 loading and/or maintenance at the centromere. Indeed, even though the amount of Kinetochore Null 2 (KNL2)6 - the upstream component for CENH3 loading - was increased in the gip1gip2 mutants, low levels of CENH3 were observed at the centromere. Moreover, the reduced CENP-C recruitment at the centromere in the gip1gip2 mutants emphasizes the importance of GIPs for centromere maintenance and kinetochore maturation.
As centromere maintenance is also ensured by centromeric cohesion, we showed that both centromeric and pericentromeric cohesions were impaired in gip1gip2 in FISH analyses using specific probes on 2C and 4C flow sorted nuclei. Our data were corroborated by an impaired recruitment of the Structural Maintenance of Chromosome 3 (SMC3) cohesin subunit at the centromere in gip1gip2 as well as by a reduced level of the Chromosome Transmission Fidelity 7 (CTF7) shown to be involved in the establishment of cohesion.3 As KNL2 conditions the epigenetic environment for centromere maintenance,6 its localization outside centromeric regions in gip1gip2 suggests that the heterochromatin boundary of the centromere is compromised. This could also explain why centromeric maintenance is altered in gip1gip2, due to the altered pericentromeric heterochromatin environment. Interestingly, the absence of heterochromatin at the centromere boundary in S. cerevisiae, which is devoid of GIP homolog, suggests an alternative pathway for regulating centromeric functions in budding yeast. For other fungi, plants and metazoans, GIP/MZT1 may participate in centromere cohesion and maintenance in conserved mechanisms.
We assume that specific GIP localization in the nuclear envelope environment is essential to coordinate centromeric functions, such as cohesion and CENH3 loading and maintenance for proper kinetochore assembly (Fig. 1). The dynamics of GIP proteins at the nuclear envelope may reflect their regulatory roles in distinct complexes: at the outer nuclear membrane, they facilitate the recruitment of microtubule nucleation complexes and close to the inner membrane they allow the formation of stable centromeric complexes for kinetochore assembly before mitotic entry. As both nuclear and chromocentre architectures are disturbed in gip1gip2,5 it would be interesting to evaluate the role of GIPs in centromere anchoring at the nuclear envelope.
Figure 1.
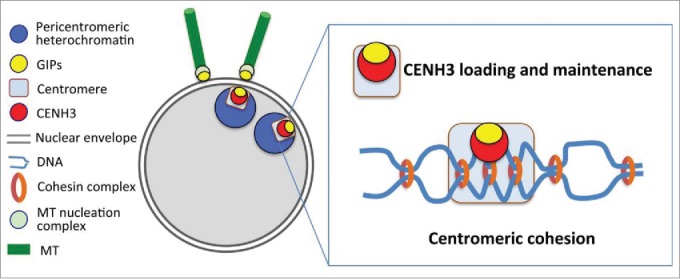
GIP/MZT1 proteins coordinate centromere functions and the establishment of the microtubule (MT) network at the nuclear envelope. GIPs are regulatory proteins, specific of gamma-tubulin complexes at the outer nuclear membrane and of centromeric complexes close to the inner nuclear membrane. At the centromere, they contribute to CENH3 loading and maintenance and ensure centromeric cohesion.
Facing such new mechanisms of centromere regulation, there still remain unanswered questions. Beside the fact that no CENH3 chaperone has been identified in plants so far, are GIPs involved in the regulation of a possible chaperone complex? Since non-coding centromeric RNA stabilizes the centromere/kinetochore in plants (for a review, see7), are GIPs involved in such a process? Further studies should shed new light on the role of GIP/MZ1 proteins in centromere biology.
References
- 1.Fukagawa T, et al.. Dev Cell 2014; 30:496-508; PMID:25203206; http://dx.doi.org/ 10.1016/j.devcel.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fransz P, et al.. Proc Natl Acad Sci U S A 2002; 99:14584-9; PMID:12384572; http://dx.doi.org/ 10.1073/pnas.212325299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batzenschlager M, et al.. Proc Natl Acad Sci U S A 2015; 112:8656-60; PMID:26124146; http://dx.doi.org/ 10.1073/pnas.1506351112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janski N, et al.. Plant Cell 2012; 24:1171-87; PMID:22427335; http://dx.doi.org/ 10.1105/tpc.111.094904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batzenschlager M, et al Front Plant Sci 2013; 4:480; PMID:24348487; http://dx.doi.org/ 10.3389/fpls.2013.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lermontova I, et al.. Plant Cell 2013; 25:3389-404; PMID:24014547; http://dx.doi.org/ 10.1105/tpc.113.114736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lermontova I, et al.. Plant J 2015; 83:4-17; PMID:25976696; http://dx.doi.org/ 10.1111/tpj.12875 [DOI] [PubMed] [Google Scholar]


