Maintenance of haematopoietic stem/progenitor cells not only relies on their intrinsic genetic program but also on interactions with their cellular microenvironment, called “niche”, which is required to safeguard stemness. The niche concept proposed by Schofield in 1978 while studying mouse haematopoiesis came to reality with works on Drosophila germ cells and has been extended to other tissues and organisms.1 Remarkably, the identification of a haematopoietic niche in Drosophila promoted the use of this model organism to investigate the fundamental processes regulating haematopoiesis.
Indeed thanks to the phylogenetic conservation of several aspects of blood cell development from fly to mammals, a Drosophila larval haematopoietic organ called the lymph gland (LG) has become a popular model to study the intrinsic and extrinsic mechanisms controlling blood system homeostasis. In third instar larva, this organ is composed of multiple lobes: the posterior ones contain almost exclusively blood cell progenitors (prohemocytes), whereas the anterior/primary lobes contain prohemocytes in the Medullary Zone that give rise to differentiated hemocytes in the Cortical Zone (Fig. 1). At their posterior tip, primary lobes also present a small cluster of cells, the Posterior Signalling Centre (PSC), expressing the EBF transcription factor Collier (Col), the HOX factor Antennapedia (Antp) and different signalling molecules including Hedgehog (Hh). Importantly, the PSC was proposed to act as a niche required for progenitor fate maintenance in the LG.2,3 Notably it was shown that col mutant larvae, which lack a PSC, exhibit massive prohemocyte differentiation and that inhibiting Hh signalling pathway in the prohemocytes promote their differentiation. Moreover, several reports showed that modulating PSC size/activity affects the balance between progenitor and differentiated blood cells.
Figure 1.
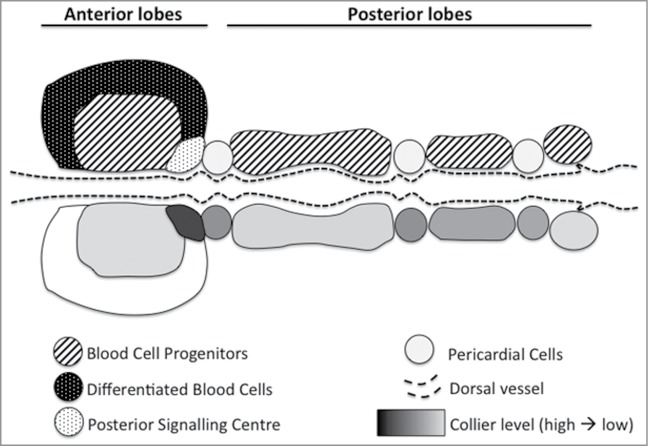
The Drosophila lymph gland. Upper part: schematic representation of lymph gland organisation. The anterior and posterior lobes are arranged on either side of the dorsal vessel and separated by (non-haematopoietic) pericardial cells. Lower part: EBF/Collier expression level in the different compartments is shown in gray scale.
In contrast with this model, our recent results show that blood cell progenitor maintenance is independent of the PSC, strongly undermining the notion that the PSC is a niche.4 Using a cell ablation strategy, we generated LG deprived of PSC, yet these “niche-less” LG harboured a normal proportion of prohemocytes, which remained quiescent and did not display any sign of differentiation. To circumvent possible shortcomings of this strategy, we also re-assessed the phenotypes of col and Antp mutant larvae, which are both devoid of PSC. While massive differentiation was observed in col LG, Antp LG maintained their pool of progenitors. These results suggested that col acts outside of the PSC to sustain prohemocyte fate. Indeed, beside its high expression in the PSC, col is expressed at low level in the prohemocytes and inhibiting col expression in the prohemocytes is sufficient to cause their differentiation even in the presence of PSC.4 These findings lead to a paradigm shift concerning Drosophila haematopoiesis: we propose that the PSC is not the LG haematopoietic niche and that Col directly promotes blood cell progenitor maintenance independently of its requirement for PSC development.
It will be interesting now to decipher the upstream mechanisms controlling col expression within blood cell progenitors and to tackle the potential role of EBF factors in mammalian HSCs. There are also pending questions concerning Col mechanism of action. What are its target genes in the prohemocytes versus the PSC? Is their regulation dependent on Col level or cell type specific partners? Which Col targets participate in blood “stemness”? Along that line, a recent report revealed that, contrary to the common belief, blood cell progenitors are present in Drosophila adults and seem to originate from larval LG cells expressing col.5 col may therefore also contribute to adult haematopoiesis by maintaining these blood cells undifferentiated during larval life.
Although the PSC is not required for blood cell progenitor maintenance, it is not neutral for LG homeostasis. First, PSC-less larvae fail to differentiate a specialised blood cell type following wasp infestation,2,5 indicating that the PSC is important for mounting a proper immune response. Second, in light of previous publications, one could speculate that the PSC is not only a source of pro-maintenance factors but also of pro-differentiation molecules. In the absence of PSC both signals are removed and LG development seems sufficiently robust to result in minimally altered blood cell homeostasis whereas modifying PSC activity may tilt the balance of pro/anti-differentiating molecules and affect haematopoiesis. Tallying with its name, the PSC may thus be an important signalling centre that integrates developmental and environmental cues to fine-tune blood cell production.
Clearly, our findings challenge the idea that the PSC is the haematopoietic niche for LG prohemocytes. Where and what is the niche then? To answer this question, it will be interesting to study the presence of other sources of Hh than the PSC as this signalling seems crucial for prohemocyte maintenance.3 Also, differentiated blood cells feedback on prohemocytes to keep them undifferentiated.6 In addition, a compact extracellular matrix (ECM) is observed around the Medullary Zone/prohemocytes and modulating ECM composition affects prohemocyte maintenance.7 Furthermore, one could speculate that cells of the cardiac tube that are lining the LG participate in prohemocyte maintenance. As for mammalian HSC, the LG niche may be a complex structure with contribution from different cell types. Future studies, by decrypting this complexity, should bring valuable insights into the microenvironmental interactions controlling haematopoietic progenitor behaviour.
References
- 1.Scadden DT. Cell 2014; 157:41-50; PMID:24679525; http://dx.doi.org/ 10.1016/j.cell.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krzemien J, et al.. Nature 2007; 446:325-8; PMID:17361184; http://dx.doi.org/ 10.1038/nature05650 [DOI] [PubMed] [Google Scholar]
- 3.Mandal L, et al.. Nature 2007; 446:320-4; PMID:17361183; http://dx.doi.org/ 10.1038/nature05585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benmimoun B, et al.. Proc Natl Acad Sci U S A 2015; 112:9052-7; PMID:26150488; http://dx.doi.org/ 10.1073/pnas.1423967112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh S, et al.. Dev Cell 2015; 33:478-88; PMID:25959225; http://dx.doi.org/ 10.1016/j.devcel.2015.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mondal BC, et al.. Cell 2011; 147:1589-600; PMID:22196733; http://dx.doi.org/ 10.1016/j.cell.2011.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigorian M, et al.. Dev Biol 2013; 384:301-12; PMID:23510717; http://dx.doi.org/ 10.1016/j.ydbio.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]


