The correct formation and positioning of the cytokinetic ring is key to the maintenance of genomic integrity during mitosis. Even though the events occurring at cytokinetic bridge in telophase have been extensively studied, the regulation of the setting up of the cytokinetic ring that starts as soon as the cell enter anaphase is not yet fully understood. The cytokinetic ring is composed of an actomyosin ring linked to the plasma membrane by scaffold elements such as anillin. Both assembly and contraction of the ring at the equatorial cortex is orchestrated by the local concentration and activation of the small GTPase RhoA. RhoA activation is induced by the central spindle via the centralspinlin dependent recruitment of the RhoGEF Ect2.1 The establishment of the lateral cortical polarity starts as soon as the cell elongates. Early on the various elements of the cytokinetic ring become localized to the lateral membrane and are excluded from the poles. As the cell progress through anaphase they are concentrated at the equatorial cortex and cytokinetic furrow starts to ingress. Many questions remain about the establishment of the cytokinetic ring elements polarization. For instance: how do RhoA and Ect2 accumulate at the equatorial cortex? In addition, it is not well understood how central spindle communicates with the equatorial cortex in anaphase.
Annexin A2 is a phosphotidylinositol (PtdIns) binding protein implicated in vesicular trafficking and the regulation of actin at dynamic membranes.2 In a recent publication, Benaud et al.,3 we have highlighted a role of annexin A2 in early cytokinesis. Removing annexin A2 impedes the formation of the cytokinetic ring and ingression of the plasma membrane, where as the central spindle organization and compaction is not affected, thereby uncoupling the mitotic rearrangement of the actin and the microtubule cytoskeletons (Fig. 1). When annexin A2 is down regulated, the localization of most of the structural elements of the cytokinetic ring is perturbed, indicating that annexin A2 acts upstream, altering regulatory elements. Indeed, we provide evidence that annexin A2 influences the distribution of RhoA at the equatorial cortex. In absence of annexin A2, RhoA is evenly distributed throughout the cortex, which coincide with the decrease in actin polymerization at the equatorial cortex and the presence of F- actin at the pole of the cell. The exact mechanism by which annexin A2 controls the spatial restriction of RhoA localization needs to be further investigated. A first clue is provided by the observation that Ect2 is correctly recruited to the central spindle by the centralspindlin, but the presence of cortical Ect2 is lost. More work need to address the molecular mechanism by which annexin A2 controls cortical Ect2 localization. Several mechanisms of action of annexin A2 can be considered. It could act at the level of an actin dependent traffic between the central spindle and cortex. It may also be involved in the stabilization of the anchorage of the central spindle in close proximity with the equatorial membrane. In addition, whether the absence of cortical Ect2 is sufficient to explain the loss to RhoA polarization or whether annexin A2 influences an additional pathway regulating the localization of RhoA, needs to be clarified.
Figure 1.
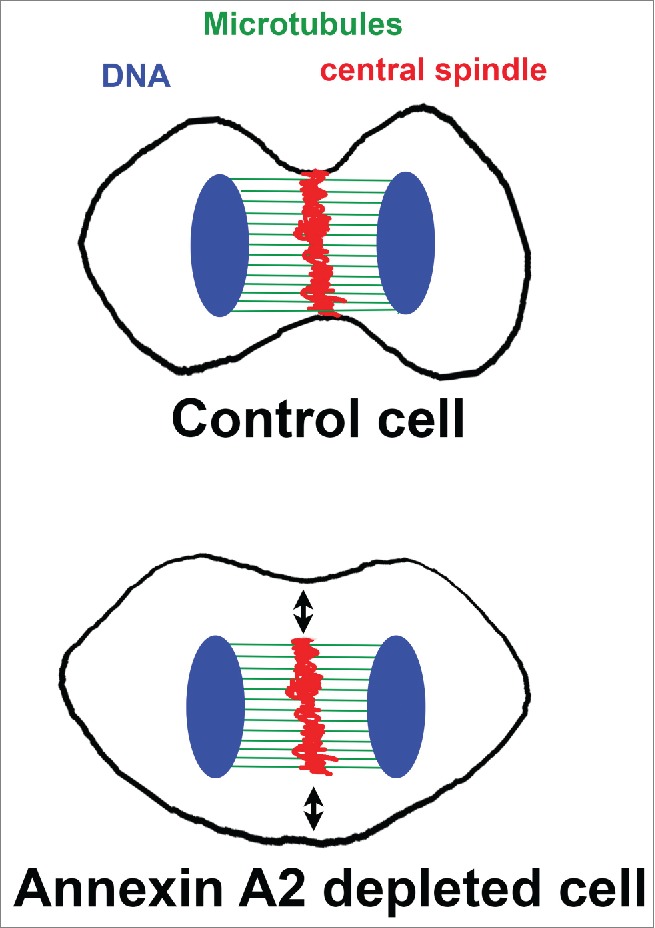
In control cells during anaphase the central spindle is in close proximity with the equatorial plasma membrane. In annexin 2 depleted cells the central spindle did assemble but has lost contact with the plasma membrane (black arrows). (DNA in blue, microtubule in green and midbody in red)
Clearly, the early accumulation of annexin A2 on the lateral cortex and the total absence of equatorial contraction in part of the annexin A2 invalidated cells strongly suggest that annexin A2 acts very early in the establishment of cytokineic ring. The study of annexin A2 biochemical properties as well as its cellular functions in interphase cells and during the establishment of epithelial polarization points out to several complementary mechanisms of action.
PtdIns lipids are enriched at the lateral plasma membrane at the site of furrow ingression.4 The cytokinetic ring effectors Anillin, RhoA and Ect2 can all bind directly PtdIns(4,5)P2 in vitro via PH and polybasic domains which are required for their localization to the equatorial membrane. However, whether their affinity for PtdIns(4,5)P2 in vivo is sufficient to explain alone their membranous localization remains to be determined. As mitotic cells progress through anaphase the initial large lateral band of recruitment of cortical cytokinetic elements narrows progressively to the site of furrow ingression. How the clustering at the site of constriction occurs remains an open question. In vivo, annexin A2 is recruited at PtdIns(4,5)P2 membrane domains.2 Annexin A2 displays a PtdIns(4,5)P2 aggregating property creating membrane platforms. Furthermore, tetrameric annexin A2-S100A10 binds PtdIns(4,5)P2 with high affinity and specificity than the monomeric form.2,5 It is interesting to note that the annexin A2 mutant unable to form tetramer does not rescue the cytokinetic phenotype resulting from annexin A2 depletion. A role for annexin A2 in the establishment and maintenance of an equatorial PtdIns lipid domain and scaffolding RhoA and Ect2 is an attractive possibility.
The role of PtdIns and Rab11 membrane trafficking in late cytokinesis has been highly documented.4 Dynamic Rab11 vesicle trafficking can also be observed by live imaging both at the pole and equatorial membranes in early anaphase (our unpublished observation). It would therefore be interesting to investigate whether these recycling endosomes participate in the establishment of specific lateral membrane domain through lipid or cortical elements delivery in early anaphase. Annexin A2 is involved in polarized trafficking of Rab11 recycling endosomes to apical domain of epithelial cells.2 Therefore, further investigations should address the implication of annexin A2 in a Rab11-dependent delivery of RhoA containing vesicles to the forming equatorial membrane in early cytokinesis.
In addition to the central spindle, equatorial microtubules are also believed to contribute to the formation and position of the cleavage furrow. Astral microtubules can recruit RhoA to the equatorial cortex and the centalspinlin has also been detected at the tip of astral microtubules near the equatorial cortex.6 Interestingly, concomitant with defective equatorial membrane ingression, we have observed abundant and longer equatorial microtubules in annexin A2 depleted cell (unpublished observations). This suggests loss of spatial information in absence of annexin A2. Annexin A2 may play a role in the stabilization of equatorial astral microtubules, via the establishment of membrane microdomains or specific cortical composition allowing the anchorage of astral microtubules at the equatorial cortex. It could thereby influences RhoA and cortical Ect2 localization.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Fededa JP, Gerlich DW. Nat Cell Biol 2012; 14:440-7; PMID:22552143; http://dx.doi.org/ 10.1038/ncb2482 [DOI] [PubMed] [Google Scholar]
- 2.Grieve AG MS, Hayes MJ. Int J Cell Biol 2012; 2012:852430; PMID:22505935; http://dx.doi.org/ 10.1155/2012/852430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benaud C, et al.. EMBO Rep 2015; 16:481-9; PMID:25712672; http://dx.doi.org/ 10.15252/embr.201440015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cauvin C, Echard A. Biochim Biophys Acta 2015; 1851:832-43; PMID:25449648; http://dx.doi.org/ 10.1016/j.bbalip.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 5.Gokhale NA, et al. J Biol Chem 2005; 280:42831-40; PMID:16230353; http://dx.doi.org/ 10.1074/jbc.M508129200 [DOI] [PubMed] [Google Scholar]
- 6.Nishimura Y, Yonemura S. J Cell Sci 2006; 119:104-14; PMID:16352658; http://dx.doi.org/ 10.1242/jcs.02737 [DOI] [PubMed] [Google Scholar]


