Abstract
Gastric polyps are frequently encountered on endoscopic examinations. While many of these represent true epithelial lesions, some of the polyps may result from underlying stromal or lymphoid proliferations or even heterotopic tissue. Histologic examination is essential for accurate typing of the polyps to predict malignant potential and underlying possible genetic abnormalities. The focus of this review is on gastric hamartomatous polyps, which are relatively rare and diagnostically challenging. Though most of the gastric hamartomatous polyps are benign, certain types are associated with increased malignant potential. These include certain polyps associated with specific genetic familial polyposis syndromes and gastric inverted hamartomatous polyps. Identification of these polyps can result in the prevention or early diagnosis of gastric carcinoma and also help in the identification of family members with polyposis syndromes. The aim of this review is to categorize gastric hamartomatous polyps and aid in the identification of high-risk categories.
Keywords: stomach, hamartomatous polyp, polyposis
Introduction
Gastric polyps are encountered in approximately 1%–6.35% of endoscopies.1–3 Gastric polyps are rarely symptomatic and are usually discovered incidentally on endoscopy.1 The larger gastric polyps may present with bleeding, anemia, obstructive symptoms, and pain.4 The most common types of gastric polyps are fundic gland polyps (FGPs), hyperplastic polyps, and adenomas.3,5,6 Gastric neuroendocrine tumors (carcinoids), infiltrates (such as xanthomas and lymphoproliferative neoplasms), mesenchymal proliferations (such as leiomyomas, gastrointestinal stromal tumors, and inflammatory fibroid polyps), and others can also be present as polyps.2–4 Gastric hamartomatous polyps are uncommon and comprise about 1% of all the stomach polyps.7 Most gastric polyps are difficult to characterize on the basis of endoscopic appearance alone and need histologic characterization for assessment of malignant potential.4 The aim of this review is to characterize the clinical and pathological features of gastric hamartomatous polyps.
Hamartomatous polyps are characterized by disorganized growth of tissue indigenous to the site.4 They can be solitary or syndromic.8 The syndromes commonly associated with gastric hamartomatous polyps are Peutz–Jeghers syndrome (PJS), juvenile polyposis, and phosphatase and tensin homolog (PTEN) hamartoma syndrome (PTHS). In the solitary group, solitary juvenile polyps and Peutz–Jeghers type polyps have been reported.8,9 Also described infrequently are inverted gastric hamartomatous polyps, which are submucosal lesions characterized by inverted growth pattern of gastric glands.7 It is important to distinguish sporadic or solitary polyps from syndromic polyps as the sporadic or solitary polyps generally have a relatively benign course, while those associated with a syndrome have a higher lifetime malignancy risk (though specific gastric cancer risk data are not available).8 An exception in the sporadic group is gastric inverted hamartomatous polyps (GIHPs), which have a 20% risk of being associated with adenocarcinoma.7,10
PJS Polyps
PJS is an autosomal dominant inherited disorder, characterized by a germline mutation in STK11 mutation.11 In 1921, Peutz reported a Dutch family with gastrointestinal polyposis and distinctive pigmentation of the skin and mucous membranes and highlighted the inherited nature of the syndrome.12 In 1949, the combination of intestinal polyposis and pigmentation of the skin and mucous membranes was established as a distinct entity in a publication by Jeghers et al.13 In 1954, Bruwer et al coined the eponym “Peutz–Jeghers syndrome” in the title of his article on this disorder.14 The prevalence of PJS is 1 in 200,000.15 PJS is characterized by mucocutaneous melanosis, gastrointestinal polyposis, intestinal, and extraintestinal cancers.16 The disease may have variable penetrance within the same family.17 Gastrointestinal PJS polyps are most common in the upper jejunum, followed by colon and stomach.11 Gastric polyps occur in approximately 15%–30% of PJS cases.4 Clinical manifestations include intussusception, chronic gastrointestinal bleeding, and anemia. The patients might be subjected to multiple laparotomies, putting them at risk for bowel obstruction.17 On endoscopy, these polyps have a velvety or papillary surface and resemble hyperplastic polyps (Fig. 1A).2 The polyps in colon often have a characteristic morphology with branching smooth muscles covered by hyperplastic epithelium giving rise to a characteristic arborizing (Christmas tree) pattern. Furthermore, lobulated clusters of colonic crypts, a feature that may discriminate them from other polyps such as hyperplastic polyps or juvenile polyps, can be seen.18 However, gastric Peutz–Jeghers polyps often lack the typical arborizing histology pattern and are often not readily distinguished from gastric juvenile polyps or hyperplastic polyps (Fig. 1B).19 Sometimes, displacement of mucin-secreting glands into the submucosa or muscularis propria can mimic well-differentiated adenocarcinoma.20 Malignant transformation of the gastric PJS polyps, though rare, has been reported.21–23 In addition, Defago et al have described carcinoma in situ in a gastric polyp in a patient with PJS.24 Though the overall cancer risk in PJS is highest for colorectal carcinoma, these patients have approximately 29% risk of developing stomach cancer.25 The common extracolonic tumors in these patients include pancreas, breast, ovary (sex cord stromal tumors with annular tubules), testis (Sertoli cell tumors), and cervix (adenoma malignum).20 Per the American College of Gastroenterology (ACG) guidelines, any individual with perioral/buccal pigmentation with at least two histologically confirmed PJS type polyps or family history should be tested for STK11 gene mutation for PJS.26 The phenotype may be more severe in families with a truncating STK11 mutation than a missense mutation.20 The recommended endoscopic surveillance interval for gastrointestinal manifestations is three years and for extraintestinal neoplasms is one year.26
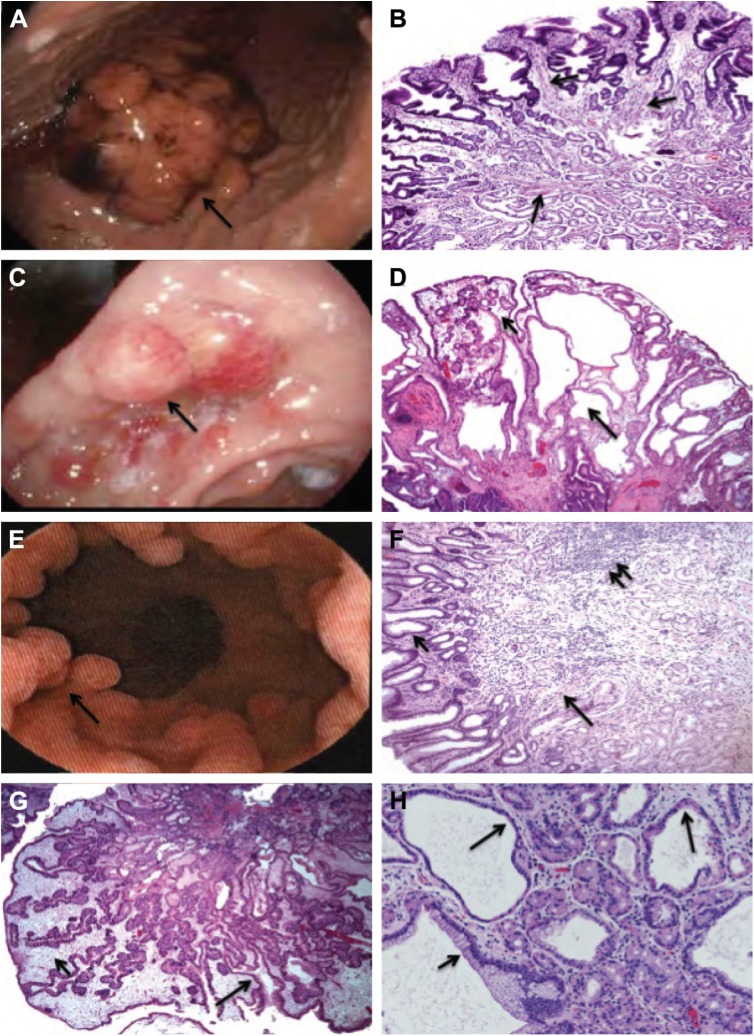
Figure 1.
(A) Gastric polyp (4 cm) (arrow) in a 24-year-old man with PJS (STK11 mutation detected). (B) Gastric polyp in PJS (STK11 mutation detected) showing fine branching smooth muscle bands (arrows) between hyperplastic gastric glands. The classic arborizing histology pattern, seen in the intestinal PJS polyps, is often not seen in stomach polyps. (C) Gastric polyps (arrow) in a 25-year-old man with JPS (SMAD4 mutation detected). (D) Gastric polyp in JPS (SMAD4 mutation detected) showing multiple cystic dilated glands (long arrow) in an edematous, inflamed lamina propria (short arrow). (E) Multiple polyps (arrow) in a 36-year-old man with CS (PTEN mutation detected). (F) Gastric polyp in CS (PTEN mutation detected) showing hyperplastic foveolar epithelium (short arrow)-lined polyp with gastric glands embedded in a heterogeneous stroma, consisting of intercalating bands of smooth muscle (long arrow), lymphoid tissue (double arrow), and variable chronic inflammatory infiltrate. (G) Gastric polyp in CCS showing hyperplastic foveolar epithelium (long arrow)-lined polyp with an edematous, inflamed lamina propria (short arrow) mimicking gastric JPS polyps or hyperplastic polyps. (H) FGPs showing dilated glands lined by parietal cells (long arrow) or mucous cells (short arrow).
Solitary PJ polyps are rare. These polyps occur in the later stage of the life and are larger in size than the polyps in PJS.27 The largest polyp described so far has been 15 cm in dimension.27 The endoscopic and histologic appearances are similar to that of syndromic PJ polyps.11 Some authors have suggested that the solitary gastric PJ polyps have less branching of the muscularis mucosae as compared with familial PJS polyps.28 Jin et al also described a proliferation of smooth muscles in the submucosa. The cases of solitary PJ polyps were found negative for STK11 mutation, which is classically detected in PJS.29,30 Solitary PJ polyps have been known to have a good prognosis with no evidence of malignant transformation.22,31 Low-grade intraepithelial neoplasia has been rarely reported in cases of solitary PJ polyps.29,32 However, Burkart et al questioned the existence of true solitary PJ polyps and found a higher association of intestinal and extraintestinal malignancies in these patients. Due to this observation, they proposed that solitary PJ polyps may represent an early or incomplete form of PJS, and these patients may have similar lifetime risk of malignancy.32
Juvenile Polyposis Syndrome Polyps
Juvenile polyposis syndrome (JPS) is the most common heritable gastrointestinal polyposis syndrome with a prevalence of 1 in 100,000–160,000.33 It is an autosomal dominant disorder characterized by a mutation in SMAD4 (also called the MADH4 gene), BMPRA1, or ENG genes.11,19,26 All these genes are a part of the tumor growth factor-β signaling family and directly or indirectly affect the cell growth inhibition and apoptosis. The polyps most commonly affect the colon and rectum (98%), followed by stomach (14%), jejunum/ileum (7%), and duodenum (2%).25 On endoscopic examination, the polyps have a rounded and smooth contour (Fig. 1C), in contrast to the papillary surface of PJ type polyps. On microscopic examination,17 the polyps were found to be characterized by dilated mucous-filled glands in an edematous, inflamed lamina propria (Fig. 1D). These patients have 17%–22% risk of colorectal carcinoma and 10%–21% lifetime risk of gastric and duodenal carcinoma. Patients with SMAD4 mutations should also be screened for hereditary hemorrhagic telangiectasia, especially pulmonary arteriovenous malformation due to the risk of pulmonary hemorrhage.25,34 Lam-Himlin et al found in their study that the features of gastric JP type and PJ type polyps are less specific than their intestinal counterparts, and there may be a significant morphologic overlap between the two groups.19 Studies have shown that SMAD4 mutation is more commonly associated with epithelial phenotype with high crypt density, while BMPRA1 is more frequently associated with stroma-rich phenotype.35 While van Hattem et al found no difference in the association of dysplasia with these two mutations,35 Handra-Luca et al demonstrated that SMAD4 mutations are associated with a more aggressive polyp phenotype.36 Gastric polyposis is more commonly seen in SMAD4 mutation-related JP.34,37 Per the ACG guidelines, any individual with five or more JPs in the colorectum or any number of JPs in the other parts of the gastrointestinal tract should be evaluated for JPS by testing for SMAD4 and BMPR1A gene mutations. In patients testing positive for JPS, endoscopic follow-up is recommended every one to three years.26 Once a disease-causing mutation is identified in a patient with JPS, other family members should also undergo mutation-specific testing to determine whether the disease is present or absent so that appropriate surveillance can be undertaken.26
Solitary JPs are common, encountered in around 2% pediatric population.35 They are common in the colorectum but are rarely reported in the stomach and small bowel as well.8,15 Endoscopically and histologically, they are similar to syndromic JPs.17 These are benign polyps with no associated risk of malignancy. However, multiple JPs (>3) should raise a possibility of JPS as it has a 39% lifetime risk of malignancy and requires surveillance.8,35
PTHS Polyps
PTHS is a group of autosomal dominant inheritable disorders characterized by mutations in the PTEN gene.25 These include Cowden syndrome (CS), Bannayan–Riley–Ruvalcaba syndrome (BRRS), Proteus syndrome, and Proteus-like syndrome.16,38 Mutations in other genes, such as SDH (succinate dehydrogenase B, C, and D), PIK3CA, and AKT1, as well as hypermethylation of KLLN have been identified in a subset of PTEN mutation-negative patients.25,39
Cowden syndrome
The diagnosis of CS is made based on the International Cowden Consortium criteria, which were modified by the National Comprehensive Cancer Network.11 The incidence of CS in the general population is 1 in 200,000 and more than 90% patients present in the adult life by the late third decade.38 Mucocutaneous hamartomas (trichilemmomas, acral keratosis, and papillomatous lesions) are pathognomonic features of CS.38 Macrocephaly and Lhermitte–Duclos disease or dysplastic cerebellar gangliocytoma are two other features considered specific for CS and are included in the major criteria.40,41 These patients have an increased risk of breast, thyroid, and endometrial carcinoma, which are the other major criteria.40 Patients with CS also have a predisposition to benign hamartomatous outgrowths such as lipomas, arteriovenous malformations, fibrocystic breast disease, benign thyroid nodules, multiple uterine leiomyomas (fibroids) and/or bicornuate uterus, and gastrointestinal polyps.40 Diffuse esophageal glycogenic acanthosis is present in more than 80% of CS patients and may be diagnostic for CS in the presence of other benign gastrointestinal polyposis.17,42,43 Gastrointestinal polyps occur in up to 50% of patients with CS with a wide variety of endoscopic and histologic features, including adenomatous, inflammatory, hyperplastic, lymphoid, ganglioneuromatous, and leiomyomatous polyps (Fig. 1E and F).11,44 The majority of CS patients (>50%) have two or more different polyp histologies.44 Though most studies describe polyps in CS being colonic, gastric polyps are present in almost all patients with CS and are usually numerous with a variable appearance.42,45 Depending on the major histologic component, they can be smooth contoured or have a hyperplastic/papillary configuration endoscopically. The polyps in the stomach are commonly misdiagnosed as hyperplastic hamartomatous polyps.42,45 Though dysplasia has not been reported in gastric polyps in CS, patients with CS and gastric cancer have been reported.46–48 The risk of colorectal carcinoma in CS is 7%–15%, and 1 in 100 patients with CS may develop gastric malignancy.11
Individuals with multiple gastrointestinal hamartomas or ganglioneuromas should be evaluated for PTEN gene mutation. The recommended gastrointestinal surveillance for patients with PTEN gene mutation is colonoscopy and esophagogastroduodenoscopy examination beginning at the age of 15 years and repeated every two years or two to three years,26 though different suggestions has been suggested.49
Bannayan–Riley–Ruvalcaba syndrome
BRRS is another PTEN-related congenital autosomal dominant syndrome characterized by macrocephaly, penile freckling, slowed psychomotor development along with multiple cutaneous, and visceral hamartomas such as lipomas and hemangiomas. Diagnostic criteria for BRRS have not been set but are based heavily on the presence of the above cardinal clinical features along with PTEN mutation. Intestinal polyposis affects approximately 45% of the affected individuals, and the polyp characteristics are similar to CS.17,50 Owing to the similarity of germline mutations in the PTEN gene, it has been proposed that CS and BRRS may be the same syndrome along a broad spectrum. The various mutations or deletions in the different regions of the PTEN gene may confer the varying risk for developing BRRS versus CS.17
Proteus syndrome and Proteus-like syndromes
Proteus syndrome is a complex, highly variable disorder characterized by disproportionate growth of skin, skeleton, and central nervous system tissue.16,38 The specific diagnostic criteria include connective tissue nevi, epidermal nevi, dysregulated adipose tissue, vascular malformation, and facial phenotype.51 Proteus-like syndrome is undefined but refers to individuals with significant clinical features of Proteus syndrome who do not meet the diagnostic criteria for Proteus syndrome.38 The gastrointestinal findings reported in Proteus syndrome include rectal polyps, colonic lipomatosis, and gastrointestinal hemangiomas.51 The rectal polyps described by Lamireau et al had an inflammatory polyp morphology with foci of ossification.52
Hereditary Mixed Polyposis Syndrome Polyps
Hereditary mixed polyposis syndrome (HMPS) is a rare gastrointestinal polyposis syndrome that was originally described in a large Ashkenazi Jewish family with multiple colorectal polyps and cancer.53,54 It has been mapped to a locus on chromosome 15q13.3–q14 in a number of families with HMPS, but the exact underlying mechanism still remains clear. Recently, a duplication of approximately 40 kb upstream of the gremlin 1 (GREM1) gene that encodes the secreted bone morphogenetic protein (BMP) antagonist at chromosome 15 was found to be associated with HMPS.55,56 Increased GREM1 expression is predicted to cause reduced BMP pathway activity, a mechanism that also underlies tumorigenesis in juvenile polyposis of the large bowel.55 The mean age of polyp occurrence in one family was 28 years. Polyps in HMPS patients have various different morphologies, including atypical juvenile polyps, hyperplastic polyps, and adenomas.57 These glands may show a strikingly serrated morphology mimicking serrated adenoma/polyp.20 HMPS may be misdiagnosed as JPS or serrated polyposis syndrome and vice versa. Though HMPS is rare and the exact course of polyp progression is not known, they are thought to follow a hyperplastic to juvenile to Peutz–Jeghers, then adenomatous and finally carcinoma sequence.57 This disease appears to affect only the colon and does not involve the stomach or small intestine; no other accompanying extraintestinal manifestation have been currently described.17 Genetic testing for GREM1 mutation and expression might be considered in families with adenomatous and hamartomatous polyposis in which an etiology cannot be determined. Based on the current knowledge of this entity, management should probably be similar to that for familial adenomatous polyposis (FAP).26 Of note, a most recent study demonstrated that the prevalence of GREM1 mutation among Lynch syndrome Ashkenazim is 0.7%, and one mutation carrier was found who fulfills the Amsterdam criteria for Lynch syndrome.56 The relationship between GREM1 mutation and Lynch syndrome is still unclear.
Cronkhite–Canada Syndrome Polyps
Though not really hereditary, multiple gastrointestinal polyps have been reported in Cronkhite–Canada syndrome (CCS). This rare protein-losing gastroenterocolopathy syndrome also has other clinical features, including chronic diarrhea, malnutrition, onycholysis, alopecia, and skin hyperpigmentation.58 The majority of patients (>80%) are diagnosed at the age of 50 years or older, and the mean age at presentation is 59–63.5 years.59,60 The extremely rare pediatric cases reported actually have features of infantile juvenile polyposis.61,62 CCS has a poor prognosis, and the five-year mortality rate can be up to 50% if it is untreated or if treatment is delayed or inadequate. Although there is no standard therapy, limited success has been reported with antibiotics, steroids, nutritional therapy, 5-aminosalicylate acid, histamine H2 receptor antagonists, antitumor necrosis factor-α agents, immunomodulators, and eradication of Helicobacter pylori.60 The precise mechanism of CCS remains elusive, but no convincing familial predisposition has been identified and to date no germline mutations have been associated with this disorder. Recent studies favor an autoimmune process characterized by immune dysregulation,63,64 and therefore, steroids are considered the mainstay of medical treatment, although the recommended dose and duration of their use have varied widely in the literature.60
CCS polyps constitute less than 0.1% of the gastric polyps and resemble JPS polyps or hyperplastic polyps histologically characterized by expanded edematous lamina propria containing a predominantly mononuclear inflammatory cell infiltrate and tortuous, dilated to cystic glands/foveolae or crypts (Fig. 1G).1,59 Interestingly, in contradistinction to other GI polyposis syndromes, the intervening endoscopically/macroscopically spared nonaffected mucosa in CCS is histologically affected and shows lamina propria edema and inflammatory cell infiltration, as well as gland/crypt dilation and distortion.59,65 Usually, the polyps in CCS are diffuse throughout the entire gastrointestinal tract with esophagus sparing and are nonneoplastic; however, adenomatous transformation or dysplasia has been reported.65,66 There have been reports that colonic carcinomas can arise in the CCS polyps of colon.66,67 Gastric adenocarcinomas have also been reported in patients with CC polyps.68–70 At present, there is still no widely accepted algorithm or specific criteria on the diagnosis of CCS. The presence of diffuse gastrointestinal polyposis, characteristic histology in both endoscopically abnormal and abnormal mucosa, as well as relevant supporting clinical findings, including typical ectodermal changes, are the current cornerstones of diagnosis.59
Fundic Gland Polyps
FGPs, the most common polyp types (13%–77% of all gastric polyps),2 have been included in the hamartomatous group by some authors. Sporadic fundic gland polys have been associated with chronic proton pump inhibitor use.71 Endoscopically, the polyps have a smooth, glassy, and transparent appearance and are usually multiple and present in the fundic region of the stomach.2 Histologically, these polyps are characterized by dilated glands lined by parietal cells or mucous cells (Fig. 1H).2 Dysplasia in sporadic FGPs is uncommon, and malignant transformation is rare. Levy and Bhattacharya reported low-grade dysplasia in 0.3% of all their FGPs with no progression to high-grade dysplasia or adenocarcinoma on follow-up.71 There are occasional reports of high-grade dysplasia in sporadic FGPs.72 FGPs are present in >80% of patients with FAP. Bianchi et al have found dysplasia in 41% of the FGPs associated with FAP.73 The frequency of dysplasia was higher (62%) in the study by Arnason et al.74 The frequency of high-grade dysplasia was similar in the two groups (3%–4%). Despite higher frequency of dysplasia in FAP-associated FGPs, gastric adenocarcinoma arising in FGPs is rare in FAP.75 FAP-associated gastric polyps can show a hybrid phenotype, including foveolar hyperplastic, pyloric gland, and intestinal types.74,76 In our experience, these hybrid polyps can also resemble PJ type polyps morphologically. Dysplasia in FGPs should prompt a clinician to examine the patient for possible FAP or its variants. The recommended follow-up for patients who test positive for adenomatous polyposis coli (APC) gene mutation is endoscopic gastrointestinal surveillance every one to two years.26
Gastric Inverted Hamartomatous Polyps
GIHPs are a distinct entity characterized by submucosal growth of hypertrophic glands with cystic dilatation.10,77 They are distinct from the other types of hamartomatous polyps, which have an exophytic configuration contrary to the endophytic nature of these polyps.78 On endoscopic examination, these are reported as solitary submucosal masses.79 On endoscopy, extrusion of milky mucinous material from the surface of the lesion and calcifications from the biopsy site may provide a clue to diagnosis.79,80 On histology, there is cystic proliferation of glands, which may be accompanied by smooth muscle proliferation, and formation of ectopic duct-like structures has also been reported.81,82 In addition, fibroblastic and neural proliferation may also be seen with glandular elements.79 Diagnosis of GIHP is difficult without pathologic examination and may mimic ectopic pancreas on endoscopy and endosonography.82 Certain features have been suggested on endoscopic ultrasound imaging, such as hyperechoic lesions with hypoechoic spots, which might be suggestive of GIHPs.79 En bloc removal is recommended in lesions >2 cm due to the associated malignant risk (up to 20% risk of malignancy).83,84 Though it is rare, Hirasaki et al have reported a case of GHIP associated with signet ring cell carcinoma.85
Other Syndromes Associated Hamartomatous Polyps
Other less common hereditary hamartomatous polyposis syndromes include Gorlin syndrome, neurofibromatosis type 1 (NF-1), and multiple endocrine neoplasia type 2b (MEN 2B).17 Gastric polyposis has also been reported in McCune–Albright syndrome (MAS), a rare genetic disorder caused by postzygotic-activating mutation of the GNAS gene. MAS is characterized by the classical triad of skin hyperpigmentation (café-au-lait spots), polyostotic fibrous dysplasia, and endocrine dysfunctions, notably precocious puberty, hyperthyroidism, growth hormone excess, hyperprolactinemia, and hypercortisolism. The reported gastric polyp histology is similar to that of PJS.86 Recently, the study on somatic GNAS mutation in GI tumors of PJS patients revealed that GNAS is not involved in the pathogenesis of GI tumors in PJS.87 However, it is not clear whether gastric polyps are indeed a specific manifestation of these syndromes, though one would assume it might be possible. Further studies and more cases are needed to establish these relationships.
Conclusion
Hamartomatous polyps in the stomach are rare entities with variable clinical, endoscopic, and histologic features, genetic alterations, and risks of malignancy (Table 1). Multiple hamartomatous polyps should alert the pathologist and the clinician of the possibility of a hereditary polyp/cancer syndrome. There is an overlap in the morphologic features of various hamartomatous polyps, and molecular genetic testing is essential to establish the definitive diagnosis in the proper clinical context. Though the risk of malignant transformation is rare in hamartomatous polyps, some polyps including GIHPs and polyps in syndromes including PJS and JPS have a higher risk of malignant transformation. Collaboration between the pathologist, clinician, and genetic counselor is essential in making a diagnosis of polyposis syndromes. This is beneficial not only to patients for initiation of early surveillance but also for testing of family members for polyposis syndromes.
Table 1.
Syndromic gastric hamartomatous polyps.
| GENETICS | COMMON LOCATION OF POLYPS | ENDOSCOPIC FEATURES | HISTOLOGIC FEATURES | RISK OF GASTRIC CANCER | OTHER FEATURES OF THE SYNDROME | |
|---|---|---|---|---|---|---|
| Peutz-Jeghers Syndrome | STK11/LKB1 mutation | Jejunum, colon, stomach | Velvety, papillary surface | “Christmas tree”-like with branching smooth muscle bundles covered by hyperplastic epithelium | 15%–29% | Mucocutaneous melanosis, sex cord stromal tumors with annular tubules of ovary, Sertoli cell tumor of testis, adenoma malignum of cervix, other tumors of breast and pancreas |
| Juvenile polyposis | SMAD4, BMPRA1, or ENG mutation | Colon, rectum, stomach | Smooth, round surface | Dilated mucous-filled glands in an edematous, inflamed lamina propria | 10%–21% | May be associated with hereditary hemorrhagic telangiectasia |
| PTEN hamartoma syndrome | PTEN mutation | Colon, stomach | Variable | Adenomatous, inflammatory, hyperplastic, lymphoid, ganglioneuromatous, leiomyomatous or mixed morphologies | 1% | Mucocutaneous hamartomas, Lhermitte Duclos disease, macrocephaly, breast, thyroid and uterine tumors |
| Hereditary mixed polyposis syndrome | GREM1 mutation | Colon | Variable | Variable | Unclear | Variable, commonly seen in Ashkenazi Jewish family |
| Cronkhite-Canada syndrome | Likely immune-mediated | Throughout the GI tract | Inflammatory or hyperplastic appearing polyps with abnormal or normal intervening mucosa | Edematous, inflamed lamina propria and tortuous, dilated to cystic glands/foveolae or crypts | Rare | Alopecia, cutaneous pigmentation, onycholysis, chronic diarrhea, etc. |
Footnotes
ACADEMIC EDITOR: Melpakkam Srinivas, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 239 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived the concepts: MV and XZ. Analyzed the data: MV. Wrote the first draft of the manuscript: MV. Contributed to the writing of the manuscript: MV, XY and XZ. Agree with manuscript results and conclusions: MV, XY and XZ. Jointly developed the structure and arguments for the paper: XY. Made critical revisions and approved final version: MV, XY and XZ. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Oberhuber G, Stolte M. Gastric polyps: an update of their pathology and biological significance. Virchows Arch. 2000;437(6):581–590. doi: 10.1007/s004280000330. [DOI] [PubMed] [Google Scholar]
- 2.Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol. 2009;6(6):331–341. doi: 10.1038/nrgastro.2009.70. [DOI] [PubMed] [Google Scholar]
- 3.Carmack SW, Genta RM, Schuler CM, Saboorian MH. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009;104(6):1524–1532. doi: 10.1038/ajg.2009.139. [DOI] [PubMed] [Google Scholar]
- 4.Islam RS, Patel NC, Lam-Himlin D, Nguyen CC. Gastric polyps: a review of clinical, endoscopic, and histopathologic features and management decisions. Gastroenterol Hepatol (N Y) 2013;9(10):640–651. [PMC free article] [PubMed] [Google Scholar]
- 5.Borch K, Skarsgard J, Franzen L, Mardh S, Rehfeld JF. Benign gastric polyps: morphological and functional origin. Dig Dis Sci. 2003;48(7):1292–1297. doi: 10.1023/a:1024150924457. [DOI] [PubMed] [Google Scholar]
- 6.Sonnenberg A, Genta RM. Prevalence of benign gastric polyps in a large pathology database. Dig Liver Dis. 2015;47(2):164–169. doi: 10.1016/j.dld.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Odashima M, Otaka M, Nanjo H, et al. Hamartomatous inverted polyp successfully treated by endoscopic submucosal dissection. Intern Med. 2008;47(4):259–262. doi: 10.2169/internalmedicine.47.0360. [DOI] [PubMed] [Google Scholar]
- 8.Enestvedt BK, Chandrasekhara V, Ginsberg GG. Endoscopic ultrasonographic assessment of gastric polyps and endoscopic mucosal resection. Curr Gastroenterol Rep. 2012;14(6):497–503. doi: 10.1007/s11894-012-0292-2. [DOI] [PubMed] [Google Scholar]
- 9.Jin JS, Yu JK, Tsao TY, Lin LF. Solitary gastric Peutz-Jeghers type stomach polyp mimicking a malignant gastric tumor. World J Gastroenterol. 2012;18(15):1845–1848. doi: 10.3748/wjg.v18.i15.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh K, Tsuchigame T, Matsukawa T, Takahashi M, Honma K, Ishimaru Y. Unusual gastric polyp showing submucosal proliferation of glands: case report and literature review. J Gastroenterol. 1998;33(5):720–723. doi: 10.1007/s005350050161. [DOI] [PubMed] [Google Scholar]
- 11.Jung I, Gurzu S, Turdean GS. Current status of familial gastrointestinal polyposis syndromes. World J Gastrointest Oncol. 2015;7(11):347–355. doi: 10.4251/wjgo.v7.i11.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peutz JLA. Over een zeer merkwaardige, gecombineerde familiaire pollyposis van de sligmliezen van den tractus intestinalis met die van de neuskeelholte en gepaard met eigenaardige pigmentaties van huid-en slijmvliezen (Very remarkable case of familial polyposis of mucous membrane of intestinal tract and nasopharynx accompanied by peculiar pigmentations of skin and mucous membrane; in Dutch) Nederl Maandschr v Geneesk. 1921;10:134–146. [Google Scholar]
- 13.Jeghers H, Mc KV, Katz KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J Med. 1949;241(25):993. doi: 10.1056/NEJM194912222412501. illust; passim. [DOI] [PubMed] [Google Scholar]
- 14.Bruwer A, Bargen JA, Kierland RR. Surface pigmentation and generalized intestinal polyposis; (Peutz-Jeghers syndrome) Proc Staff Meet Mayo Clin. 1954;29(6):168–171. [PubMed] [Google Scholar]
- 15.Adolph VR, Bernabe K. Polyps in children. Clin Colon Rectal Surg. 2008;21(4):280–285. doi: 10.1055/s-0028-1089943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jelsig AM, Qvist N, Brusgaard K, Nielsen CB, Hansen TP, Ousager LB. Hamartomatous polyposis syndromes: a review. Orphanet J Rare Dis. 2014;9:101. doi: 10.1186/1750-1172-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreibman IR, Baker M, Amos C, McGarrity TJ. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol. 2005;100(2):476–490. doi: 10.1111/j.1572-0241.2005.40237.x. [DOI] [PubMed] [Google Scholar]
- 18.Tse JY, Wu S, Shinagare SA, et al. Peutz-Jeghers syndrome: a critical look at colonic Peutz-Jeghers polyps. Mod Pathol. 2013;26(9):1235–1240. doi: 10.1038/modpathol.2013.44. [DOI] [PubMed] [Google Scholar]
- 19.Lam-Himlin D, Park JY, Cornish TC, Shi C, Montgomery E. Morphologic characterization of syndromic gastric polyps. Am J Surg Pathol. 2010;34(11):1656–1662. doi: 10.1097/PAS.0b013e3181f2b1f1. [DOI] [PubMed] [Google Scholar]
- 20.Jass JR. Colorectal polyposes: from phenotype to diagnosis. Pathol Res Pract. 2008;204(7):431–447. doi: 10.1016/j.prp.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Song SH, Kim KW, Kim WH, et al. Gastrointestinal cancers in a Peutz-Jeghers syndrome family: a case report. Clin Endosc. 2013;46(5):572–575. doi: 10.5946/ce.2013.46.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Tovar J, Gamallo C. Gastric diffuse hamartomatous polyposis as unique manifestation of Peutz-Jeghers syndrome. Acta Chir Belg. 2014;114(6):424–426. [PubMed] [Google Scholar]
- 23.Bujanda L, Beguiristain A, Villar JM, et al. Gastric adenocarcinoma in hamartomatous polyp in Peutz-Jeghers syndrome. Gastroenterol Hepatol. 1996;19(9):452–455. [PubMed] [Google Scholar]
- 24.Defago MR, Higa AL, Campra JL, et al. Carcinoma in situ arising in a gastric hamartomatous polyp in a patient with Peutz-Jeghers syndrome. Endoscopy. 1996;28(2):267. doi: 10.1055/s-2007-1005447. [DOI] [PubMed] [Google Scholar]
- 25.Campos FG, Figueiredo MN, Martinez CA. Colorectal cancer risk in hamartomatous polyposis syndromes. World J Gastrointest Surg. 2015;7(3):25–32. doi: 10.4240/wjgs.v7.i3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223–262. doi: 10.1038/ajg.2014.435. quiz263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lunca S, Porumb V, Velenciuc N, Ferariu D, Dimofte G. Giant solitary gastric Peutz-Jeghers polyp mimicking a malignant gastric tumor: the largest described in literature. J Gastrointestin Liver Dis. 2014;23(3):321–324. doi: 10.15403/jgld.2014.1121.233.vpb2. [DOI] [PubMed] [Google Scholar]
- 28.Kuwano H, Takano H, Sugimachi K. Solitary Peutz-Jeghers type polyp of the stomach in the absence of familial polyposis coli in a teenage boy. Endoscopy. 1989;21(4):188–190. doi: 10.1055/s-2007-1012939. [DOI] [PubMed] [Google Scholar]
- 29.Harbaum L, Geigl JB, Volkholz H, et al. Sporadic gastric Peutz-Jeghers polyp with intraepithelial neoplasia. APMIS. 2009;117(12):941–943. doi: 10.1111/j.1600-0463.2009.02549.x. [DOI] [PubMed] [Google Scholar]
- 30.Kitaoka F, Shiogama T, Mizutani A, et al. A solitary Peutz-Jeghers-type hamartomatous polyp in the duodenum. A case report including results of mutation analysis. Digestion. 2004;69(2):79–82. doi: 10.1159/000077392. [DOI] [PubMed] [Google Scholar]
- 31.Oncel M, Remzi FH, Church JM, Goldblum JR, Zutshi M, Fazio VW. Course and follow-up of solitary Peutz-Jeghers polyps: a case series. Int J Colorectal Dis. 2003;18(1):33–35. doi: 10.1007/s00384-002-0411-x. [DOI] [PubMed] [Google Scholar]
- 32.Burkart AL, Sheridan T, Lewin M, Fenton H, Ali NJ, Montgomery E. Do sporadic Peutz-Jeghers polyps exist? Experience of a large teaching hospital. Am J Surg Pathol. 2007;31(8):1209–1214. doi: 10.1097/PAS.0b013e3180339944. [DOI] [PubMed] [Google Scholar]
- 33.Latchford AR, Neale K, Phillips RK, Clark SK. Juvenile polyposis syndrome: a study of genotype, phenotype, and long-term outcome. Dis Colon Rectum. 2012;55(10):1038–1043. doi: 10.1097/DCR.0b013e31826278b3. [DOI] [PubMed] [Google Scholar]
- 34.Soer E, de Vos Tot Nederveen Cappel WH, Ligtenberg MJ, et al. Massive gastric polyposis associated with a germline SMAD4 gene mutation. Fam Cancer. 2015;14(4):569–573. doi: 10.1007/s10689-015-9822-z. [DOI] [PubMed] [Google Scholar]
- 35.van Hattem WA, Langeveld D, de Leng WW, et al. Histologic variations in juvenile polyp phenotype correlate with genetic defect underlying juvenile polyposis. Am J Surg Pathol. 2011;35(4):530–536. doi: 10.1097/PAS.0b013e318211cae1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Handra-Luca A, Condroyer C, de Moncuit C, et al. Vessels morphology in SMAD4 and BMPR1A-related juvenile polyposis. Am J Med Genet A. 2005;138A(2):113–117. doi: 10.1002/ajmg.a.30897. [DOI] [PubMed] [Google Scholar]
- 37.Friedl W, Uhlhaas S, Schulmann K, et al. Juvenile polyposis: massive gastric polyposis is more common in MADH4 mutation carriers than in BMPR1A mutation carriers. Hum Genet. 2002;111(1):108–111. doi: 10.1007/s00439-002-0748-9. [DOI] [PubMed] [Google Scholar]
- 38.Ngeow J, Eng C. PTEN hamartoma tumor syndrome: clinical risk assessment and management protocol. Methods. 2015;7(7—78):11–19. doi: 10.1016/j.ymeth.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Mester J, Eng C. Cowden syndrome: recognizing and managing a not-so-rare hereditary cancer syndrome. J Surg Oncol. 2015;111(1):125–130. doi: 10.1002/jso.23735. [DOI] [PubMed] [Google Scholar]
- 40.Zbuk KM, Eng C. Hamartomatous polyposis syndromes. Nat Clin Pract Gastroenterol Hepatol. 2007;4(9):492–502. doi: 10.1038/ncpgasthep0902. [DOI] [PubMed] [Google Scholar]
- 41.Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105(21):1607–1616. doi: 10.1093/jnci/djt277. [DOI] [PubMed] [Google Scholar]
- 42.Coriat R, Mozer M, Caux F, et al. Endoscopic findings in Cowden syndrome. Endoscopy. 2011;43(8):723–726. doi: 10.1055/s-0030-1256342. [DOI] [PubMed] [Google Scholar]
- 43.Kay PS, Soetikno RM, Mindelzun R, Young HS. Diffuse esophageal glycogenic acanthosis: an endoscopic marker of Cowden’s disease. Am J Gastroenterol. 1997;92(6):1038–1040. [PubMed] [Google Scholar]
- 44.Stanich PP, Owens VL, Sweetser S, et al. Colonic polyposis and neoplasia in Cowden syndrome. Mayo Clin Proc. 2011;86(6):489–492. doi: 10.4065/mcp.2010.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levi Z, Baris HN, Kedar I, et al. Upper and lower gastrointestinal findings in PTEN mutation-positive Cowden syndrome patients participating in an Active Surveillance Program. Clin Transl Gastroenterol. 2011;2:e5. doi: 10.1038/ctg.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Thihli K, Palma L, Marcus V, et al. A case of Cowden’s syndrome presenting with gastric carcinomas and gastrointestinal polyposis. Nat Clin Pract Gastroenterol Hepatol. 2009;6(3):184–189. doi: 10.1038/ncpgasthep1359. [DOI] [PubMed] [Google Scholar]
- 47.Hamby LS, Lee EY, Schwartz RW. Parathyroid adenoma and gastric carcinoma as manifestations of Cowdens disease. Surgery. 1995;118(1):115–117. doi: 10.1016/s0039-6060(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 48.Marques M, Ramalho R, Baldaque-Silva F, Macedo G. Novel mutation identified in Cowden syndrome presenting as a gastric adenocarcinoma. Clin Res Hepatol Gastroenterol. 2013;37(6):e131–e132. doi: 10.1016/j.clinre.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Heald B, Burke CA, Kalady M, Eng C. ACG guidelines on management of PTEN-hamartoma tumor syndrome: does the evidence support so much so young? Am J Gastroenterol. 2015;110(12):1733–1734. doi: 10.1038/ajg.2015.368. [DOI] [PubMed] [Google Scholar]
- 50.Mester J, Charis E. PTEN hamartoma tumor syndrome. Handb Clin Neurol. 2015;132:129–137. doi: 10.1016/B978-0-444-62702-5.00009-3. [DOI] [PubMed] [Google Scholar]
- 51.Farajzadeh S, Zahedi MJ, Moghaddam SD. A new gastrointestinal finding in proteus syndrome: report of a case of multiple colonic hemangiomas. Int J Dermatol. 2006;45(2):135–138. doi: 10.1111/j.1365-4632.2004.02353.x. [DOI] [PubMed] [Google Scholar]
- 52.Lamireau T, Le Bail B, Sarlangue J, Vergnes P, Lacombe D. Rectal polyps in proteus syndrome. J Pediatr Gastroenterol Nutr. 1993;17(1):115. doi: 10.1097/00005176-199307000-00023. [DOI] [PubMed] [Google Scholar]
- 53.Jaeger EE, Woodford-Richens KL, Lockett M, et al. An ancestral Ashkenazi haplotype at the HMPS/CRAC1 locus on 15q13-q14 is associated with hereditary mixed polyposis syndrome. Am J Hum Genet. 2003;72(5):1261–1267. doi: 10.1086/375144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomlinson I, Rahman N, Frayling I, et al. Inherited susceptibility to colorectal adenomas and carcinomas: evidence for a new predisposition gene on 15q14-q22. Gastroenterology. 1999;116(4):789–795. doi: 10.1016/s0016-5085(99)70061-2. [DOI] [PubMed] [Google Scholar]
- 55.Jaeger E, Leedham S, Lewis A, et al. Hereditary mixed polyposis syndrome is caused by a 40-kb upstream duplication that leads to increased and ectopic expression of the BMP antagonist GREM1. Nat Genet. 2012;44(6):699–703. doi: 10.1038/ng.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laitman Y, Jaeger E, Katz L, Tomlinson I, Friedman E. GREM1 germline mutation screening in Ashkenazi Jewish patients with familial colorectal cancer. Genet Res (Camb) 2015;97:e11. doi: 10.1017/S0016672315000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitelaw SC, Murday VA, Tomlinson IP, et al. Clinical and molecular features of the hereditary mixed polyposis syndrome. Gastroenterology. 1997;112(2):327–334. doi: 10.1053/gast.1997.v112.pm9024286. [DOI] [PubMed] [Google Scholar]
- 58.Shussman N, Wexner SD. Colorectal polyps and polyposis syndromes. Gastroenterol Rep (Oxf) 2014;2(1):1–15. doi: 10.1093/gastro/got041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slavik T, Montgomery EA. Cronkhite-Canada syndrome six decades on: the many faces of an enigmatic disease. J Clin Pathol. 2014;67(10):891–897. doi: 10.1136/jclinpath-2014-202488. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe C, Komoto S, Tomita K, et al. Endoscopic and clinical evaluation of treatment and prognosis of Cronkhite-Canada syndrome: a Japanese nationwide survey. J Gastroenterol. 2016;51(4):327–336. doi: 10.1007/s00535-015-1107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kucukaydin M, Patiroglu TE, Okur H, Icer M. Infantile Cronkhite-Canada syndrome?—Case report. Eur J Pediatr Surg. 1992;2(5):295–297. doi: 10.1055/s-2008-1063463. [DOI] [PubMed] [Google Scholar]
- 62.de Silva DG, Fernando AD, Law FM, Premarathne M, Liyanarachchi DS. Infantile Cronkhite-Canada syndrome. Indian J Pediatr. 1997;64(2):261–266. doi: 10.1007/BF02752461. [DOI] [PubMed] [Google Scholar]
- 63.Sweetser S, Ahlquist DA, Osborn NK, et al. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: support for autoimmunity. Dig Dis Sci. 2012;57(2):496–502. doi: 10.1007/s10620-011-1874-9. [DOI] [PubMed] [Google Scholar]
- 64.Wen XH, Wang L, Wang YX, Qian JM. Cronkhite-Canada syndrome: report of six cases and review of literature. World J Gastroenterol. 2014;20(23):7518–7522. doi: 10.3748/wjg.v20.i23.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bettington M, Brown IS, Kumarasinghe MP, de Boer B, Bettington A, Rosty C. The challenging diagnosis of Cronkhite-Canada syndrome in the upper gastrointestinal tract: a series of 7 cases with clinical follow-up. Am J Surg Pathol. 2014;38(2):215–223. doi: 10.1097/PAS.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 66.Murai N, Fukuzaki T, Nakamura T, et al. Cronkhite-Canada syndrome associated with colon cancer: report of a case. Surg Today. 1993;23(9):825–829. doi: 10.1007/BF00311628. [DOI] [PubMed] [Google Scholar]
- 67.Zhu X, Shi H, Zhou X, et al. A case of recurrent Cronkhite-Canada syndrome containing colon cancer. Int Surg. 2015;100(3):402–407. doi: 10.9738/INTSURG-D-14-00003.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ito M, Matsumoto S, Takayama T, et al. Cronkhite-Canada syndrome associated with esophageal and gastric cancers: report of a case. Surg Today. 2015;45(6):777–782. doi: 10.1007/s00595-014-0977-2. [DOI] [PubMed] [Google Scholar]
- 69.Watari J, Morita T, Sakurai J, et al. Endoscopically treated Cronkhite-Canada syndrome associated with minute intramucosal gastric cancer: an analysis of molecular pathology. Dig Endosc. 2011;23(4):319–323. doi: 10.1111/j.1443-1661.2011.01150.x. [DOI] [PubMed] [Google Scholar]
- 70.Karasawa H, Miura K, Ishida K, et al. Cronkhite-Canada syndrome complicated with huge intramucosal gastric cancer. Gastric Cancer. 2009;12(2):113–117. doi: 10.1007/s10120-009-0506-y. [DOI] [PubMed] [Google Scholar]
- 71.Levy MD, Bhattacharya B. Sporadic fundic gland polyps with low-grade dysplasia: a large case series evaluating pathologic and immunohistochemical findings and clinical behavior. Am J Clin Pathol. 2015;144(4):592–600. doi: 10.1309/AJCPGK8QTYPUQJYL. [DOI] [PubMed] [Google Scholar]
- 72.Jalving M, Koornstra JJ, Gotz JM, et al. High-grade dysplasia in sporadic fundic gland polyps: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2003;15(11):1229–1233. doi: 10.1097/00042737-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 73.Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6(2):180–185. doi: 10.1016/j.cgh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 74.Arnason T, Liang WY, Alfaro E, et al. Morphology and natural history of familial adenomatous polyposis-associated dysplastic fundic gland polyps. Histopathology. 2014;65(3):353–362. doi: 10.1111/his.12393. [DOI] [PubMed] [Google Scholar]
- 75.Garrean S, Hering J, Saied A, Jani J, Espat NJ. Gastric adenocarcinoma arising from fundic gland polyps in a patient with familial adenomatous polyposis syndrome. Am Surg. 2008;74(1):79–83. [PubMed] [Google Scholar]
- 76.Wood LD, Salaria SN, Cruise MW, Giardiello FM, Montgomery EA. Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol. 2014;38(3):389–393. doi: 10.1097/PAS.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee YH, Joo MK, Lee BJ, et al. Inverted hyperplastic polyp in stomach: a case report and literature review. Korean J Gastroenterol. 2016;67(2):98–102. doi: 10.4166/kjg.2016.67.2.98. [DOI] [PubMed] [Google Scholar]
- 78.Aoki M, Yoshida M, Saikawa Y, et al. Diagnosis and treatment of a gastric hamartomatous inverted polyp: report of a case. Surg Today. 2004;34(6):532–536. doi: 10.1007/s00595-004-2761-1. [DOI] [PubMed] [Google Scholar]
- 79.Mori H, Kobara H, Tsushimi T, et al. Two rare gastric hamartomatous inverted polyp cases suggest the pathogenesis of growth. World J Gastroenterol. 2014;20(19):5918–5923. doi: 10.3748/wjg.v20.i19.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamashita M, Hirokawa M, Nakasono M, et al. Gastric inverted hyperplastic polyp. Report of four cases and relation to gastritis cystica profunda. APMIS. 2002;110(10):717–723. doi: 10.1034/j.1600-0463.2002.1101005.x. [DOI] [PubMed] [Google Scholar]
- 81.Ono S, Kamoshida T, Hiroshima Y, et al. A case of early gastric cancer accompanied by a hamartomatous inverted polyp and successfully managed with endoscopic submucosal dissection. Endoscopy. 2007;39(suppl 1):E202. doi: 10.1055/s-2007-966482. [DOI] [PubMed] [Google Scholar]
- 82.Yang TC, Hou MC, Chen PH, Liao WC, Li AF. Gastric hamartomatous inverted polyp mimicking ectopic pancreas on endoscopy and endosonography. Endoscopy. 2014;46(suppl 1 UCTN):E119–E120. doi: 10.1055/s-0034-1364888. [DOI] [PubMed] [Google Scholar]
- 83.Oh SJ, Oh CA, Kim DH, et al. Adenocarcinoma derived from gastric hamartomatous polyps. J Korean Surg Soc. 2011;81(6):419–422. doi: 10.4174/jkss.2011.81.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuoka J, Itaba S, Makihara Y, et al. Three cases of pedunculated gastric hamartomatous inverted polyps resected endoscopically. Nihon Shokakibyo Gakkai Zasshi. 2015;112(6):1030–1036. doi: 10.11405/nisshoshi.112.1030. [DOI] [PubMed] [Google Scholar]
- 85.Hirasaki S, Tanimizu M, Nasu J, Kataoka J, Matsubara M, Suzuki S. A case of gastric hamartomatous inverted polyp (submucosal tumor type) accompanied by gastric cancer. Nihon Shokakibyo Gakkai Zasshi. 2006;103(7):833–838. [PubMed] [Google Scholar]
- 86.Zacharin M, Bajpai A, Chow CW, et al. Gastrointestinal polyps in McCune Albright syndrome. J Med Genet. 2011;48(7):458–461. doi: 10.1136/jmg.2010.086330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korsse SE, Peppelenbosch MP, Smits R, van Veelen W. GNAS is not involved in gastrointestinal tumour formation in Peutz-Jeghers syndrome. Fam Cancer. 2013;12(3):581–582. doi: 10.1007/s10689-013-9602-6. [DOI] [PubMed] [Google Scholar]