Acute kidney injury (AKI), a rapid loss of renal function, is a major clinical problem associated with a high degree of morbidity and mortality. During AKI, a stressor, such as ischemia-reperfusion (IR), causes tubular cell death, interstitial inflammation and vascular damage. Recovery from AKI depends on the ability of renal epithelial cells to repopulate denuded nephron segments. Surviving epithelial cells de-differentiate, migrate to the injury site, proliferate, and then re-differentiate to establish epithelial polarity and restore kidney function. Recovery from AKI, however, is less complete if the injury is severe or if organ damage preexists. This is particularly true in older individuals, in whom AKI is often followed by a disturbed repair process leading to a higher risk of permanent kidney damage.1
The epidemiologic implications of age and AKI outcome are confirmed by studies in mammalian injury models. One mechanism thought to contribute importantly to the poor repair is the reduction in epithelial proliferation capacity in older kidneys. Unfortunately, injury magnitude and recovery potential are coupled in most experimental injury models in an age-dependent fashion. It has therefore been difficult to dissect, whether increased damage in old kidneys causes a reduction in tubular proliferative potential or vice versa. In order to separate the two mechanisms in our experimental setting, young and old mice were exposed to a small dose of lead-acetate as a non-injurious mitogen resulting in increased epithelial proliferation in young but not in old mice.2 The proliferation disadvantage was accompanied by more senescent cells in old mice, detected by SA-β-galactosidase positivity, γH2.AX+/Ki-67− cells, and higher levels of p16INK4a and p21CIP/WAF1. Thus supporting the concept that age itself and associated cellular senescence reduces the proliferative ability of tubular cells regardless of injury severity. Interestingly, cyclin D1 levels were inversely related to the proliferative potential, being considerably higher in tubular cells of old mice. A similar correlation between cyclin D1 expression and chronological age was found in human kidney biopsies. Cyclin D1 is regarded as a cell cycle promoting protein, which functions as a regulatory subunit of cyclin-dependent kinases required for cell cycle G1/S transition. However, a growing body of evidence suggests additional roles of cyclin D1 acting as a key regulator of DNA damage repair, cell migration, autophagy, mitochondrial biogenesis, glucose metabolism and apoptosis.3 Future studies will have to decipher, whether increased cyclin D1 levels in old kidneys play a role in renal aging and cellular senescence (Fig. 1).
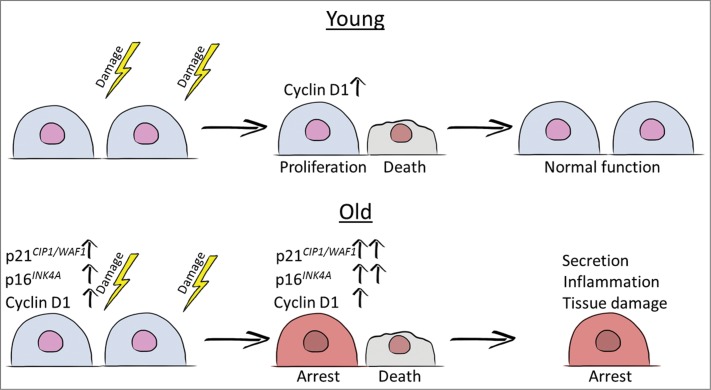
Figure 1.
Cartoon simplifying the stress induced events in young and old tubular cells. If damage is severe tubular cells die and need to be replenished to restore kidney function. While this repair process is efficient in young cells, cellular senescence, as mirrored by expression of cell cycle inhibitors and possibly by enhanced expression of Cyclin D1, disturbs this process in old cells. Changes in the senescent cell secretome may lead to increased inflammation and fibrosis further hindering repair, worsening disease outcome and leading to chronic kidney disease.
Telomere-dependent and stress-induced cellular senescence have been linked to increased severity of renal injury and reduced regeneration in AKI. Telomerase deficient (Terc−/−) mice display severe shortening of telomeres and increased instance of senescence by the fourth generation (G4). Exposure of Terc−/− G4 mice to renal IR extends renal dysfunction and severely perturbs recovery.4 Similarly, loss of stress-induced senescence marker p16INK4a attenuates permanent damage, such as interstitial fibrosis and tubular atrophy and allows for better functional recovery.5 p16INK4a knockout also increases survival of mice after kidney allograft transplantation by decreasing long-term injury.5 Senescent cells seem to play an important role in reducing the regenerative capacity of the tubular epithelium after AKI. However, the link between cellular senescence and progression of kidney disease is more multifaceted, including changes in autocrine and paracrine signaling components of senescent cells.
Senescent cells change their signaling pattern by developing a so-called senescence associated secretory phenotype (SASP). The secretion profile of senescent cells shifts toward an increase in pro-inflammatory cytokines and tumor aggravating growth factors.6 Recent data suggest that SASP may occur in the kidney. After AKI tubular epithelial cells become arrested in G2/M phase leading to an increased secretion of pro-fibrotic factors such as TGF-β and CTGF.7 Inhibition of p53 resulted in cell cycle progression and decreased fibrosis demonstrating a complex relationship between renal tubular cell cycle arrest and kidney disease progression. SASP may prove to play an important role in this relationship, especially in the context of misdirected repair in elderly individuals.
The deleterious effects of senescent cells, especially if mediated through SASP, may reach well beyond a reduction in proliferative capacity. Kidney injury caused by oxidative or cytotoxic stress inflicts DNA or mitochondrial damage increasing the load of senescent cells. Those senescent cells not only hinder recovery by insufficient replacement of damaged cells, but also by increased inflammatory and profibrotic signaling. Altogether, this feeds into a vicious cycle of degeneration. Application of proper tools is of utmost importance when studying potential effects of cellular senescence and SASP in kidney injury. Due to the culture stress, primary tubular epithelial cells (PTEC) taken from young and old mouse kidneys quickly lose their age-related differences, thus external stress is necessary to create a reliable senescent phenotype in vitro. Ten days after γ-irradiation PTEC retain epithelial characteristics, do not proliferate, express markers associated with senescence, and reflect the senescent phenotype of renal epithelial cells in vivo.2 Factors secreted by these cells may offer insight into the progression of AKI to chronic kidney disease. Studying the complex interaction of renal aging, tubular cell senescence, SASP signaling and changes in the AKI response may provide important information for treatment of acute and chronic kidney disease.
References
- 1.Schmitt R, et al.. Am J Kidney Dis 2008; 52:262–71; PMID:18511164; http://dx.doi.org/ 10.1053/j.ajkd.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 2.Berkenkamp B, et al.. PLoS One 2014; 9(2):e88071; PMID:24505380; http://dx.doi.org/ 10.1371/journal.pone.0088071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pestell RG. Am J Pathol 2013; 183:3–9; PMID:23790801; http://dx.doi.org/ 10.1016/j.ajpath.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westhoff JH, et al.. J Am Soc Nephrol 2010; 21:327–36; PMID:19959722; http://dx.doi.org/ 10.1681/ASN.2009010072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun H, et al.. J Am Soc Nephrol 2012; 23:1467–73; PMID:22797186; http://dx.doi.org/ 10.1681/ASN.2011100967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchkonia T, et al.. J Clin Invest 2013; 123(3):966–72; PMID:23454759; http://dx.doi.org/ 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, et al.. Nat Med 2010; 16(5):535–43; 1p following 143; PMID:20436483; http://dx.doi.org/ 10.1038/nm.2144 [DOI] [PMC free article] [PubMed] [Google Scholar]