Abstract
The Foundation for the NIH (FNIH) Sarcopenia Project validated cutpoints for appendicular lean mass (ALM) to identify individuals with functional impairment. We hypothesized the prevalence of sarcopenia and sarcopenic obesity would be similar based on the different FNIH criteria, increase with age, and be associated with risk of impairment limitations. We identified 4,984 subjects ≥60 years from the National Health and Nutrition Examination Surveys 1999–2004. Sarcopenia was defined using: ALM (males<19.75kg; females<15.02kg), and ALM adjusted for body mass index (BMI) (males<0.789; females<0.512). Sarcopenic obesity is defined as subjects fulfilling criteria for sarcopenia and obesity by body fat (men≥25%; females≥35%). Prevalence rates of both sarcopenia and sarcopenic obesity were evaluated with respect to sex, age category (60–69, 70–79, >80years) and race. We assessed the association of physical limitations, basic and instrumental activities of daily living (ADL) and sarcopenia status. The mean age was 70.5 years in males and 71.6 years in females. Half (50.8%, n=2,531) were female, and mean BMI was 28kg/m2 in both sexes. ALM was higher in males than in females (24.1 vs. 16.3; p<0.001) but fat mass was lower (30.9 vs. 42.0;p<0.001). In males, sarcopenia prevalence was 16.0% and 27.8% using the ALM and ALM/BMI criteria. In females, prevalence was 40.5% and 19.3% using the ALM and ALM/BMI criteria. Sarcopenia was associated with a 1.10 [0.86,1.41] and 0.93 [0.74,1.16], and 1.46 [1.10,1.94] and 2.13 [1.41,3.20], risk of physical limitations using the ALM and ALM/BMI definitions in males and females, respectively. Prevalence of sarcopenia and sarcopenic obesity vary greatly, and a uniform definition is needed to identify and characterize these high risk populations.
Keywords: sarcopenia, obesity, body fat, epidemiology, function
1. INTRODUCTION
One of the unfortunate consequences of people living longer[1] is the greater incidence of functional impairment and disability[2]. Impaired function in older adults is associated with a higher risk of institutionalization, mortality, and a compromised quality of life[3–5]. Sarcopenia, defined as the loss of muscle mass and strength with aging, is a strong predictor of adverse outcomes[6]. This syndrome is commonly observed in geriatrics practices and is increasingly recognized as an entity, even in surgical subspecialties[7].
Identifying patients with sarcopenia is critically important in order to target interventions for older adults who are at greatest risk. The standardized definition of sarcopenia varies throughout the literature[8]. Variation occurs because multiple mathematical constructs of the condition have been developed using different age cutoffs or on lower quintiles of a cohort being examined. Racial and ethnic differences in study and referent populations may also contribute to the variation in the prevalence. This creates an inherent challenge in applying cutoffs to populations that may have different characteristics than the one being examined.
The Foundation for the National Institutes of Health (FNIH) in 2014 was developed on the premise that clinically relevant cutpoints are associated with longitudinal adverse outcomes[9]. Based on large cohort studies, this group recognized both muscle strength (as represented by grip strength) and muscle mass, as two important determinants of future function. The purpose of our study was to apply the definitions from the FNIH consortium on a representative cohort of older adults to ascertain the prevalence of sarcopenia and sarcopenic obesity. We hypothesized that the two FNIH definitions of sarcopenia based on muscle mass would provide similar prevalence estimates of sarcopenia and sarcopenic obesity, increase with age, and be associated with functional impairments.
2. METHODS AND MATERIALS
A secondary analysis was conducted using data obtained from the National Health and Nutrition Examination Surveys (NHANES). NHANES is a cross-sectional survey representative of non-institutionalized older adults. NHANES uses a multistage probability sampling design, and is complex, stratified and oversamples minorities and older adults. The results provide excellent external validity to the rest of the United States population. The survey has been conducted by the Centers for Disease Control and Prevention since 1971, and its contents and procedures are fully available online at http://www.cdc.gov/nchs/nhanes.htm (accessed February 2015). For this analysis, we restricted our sample to participants from the 1999–2004 datasets. The study was funded by internal Dartmouth institutional funds. The local Institutional Review Board exempted this study from review due to the de-identified nature of the data.
There were 38,077 subjects screened, of which 31,125 were interviewed and 29,402 were examined in a standardized mobile examination center. Body composition data was ascertained using dual-energy X-ray absorptiometry (DEXA). We limited our analytic cohort to subjects aged 60 years and older, as sarcopenia and sarcopenic obesity are less prevalent and impact functional status less so in younger populations[8]. There were 4,984 subjects of all races (non-Hispanic White, non-Hispanic Black, Hispanic and Other). Subjects were also classified by age group where applicable (60–69.9, 70–79.9, and ≥80years).
All baseline data, including demographics, socioeconomic factors, and co-morbidities were assessed using a self-report questionnaire. All measurements were performed on the right side of the body to the nearest tenth of a centimeter, except where amputations, casts, and other factors prevented such an assessment. Weight was measured using an electronic digital scale, calibrated in kilograms, and height was measured using a stadiometer after deep inhalation. Body mass index (BMI) was calculated as weight (kg) divided by height(m) squared[10]. Waist circumference was measured standing, at the iliac crest, crossing the mid-axillary line, with the measuring tape placed around the trunk.
Functional status was assessed using self-reported questionnaires. All subjects were asked on a scale of 1 to 4 the degree of difficulty in performing a given activity (none to unable to do). Physical limitation (PL) questions, assessing mobility performance, included: walking ¼ mile; walking up 10 steps; stooping, crouching and kneeling; lifting/carrying 10 pounds; walking between rooms on the same floor; and standing up form an armless chair. Subjects were classified as having ‘any difficulty’ if they indicated a response other than ‘no difficulty’ on the questionnaire. Subjects responding with ‘do not do, refused or don’t know’ were classified as having a limitation, in line with our previous analysis[11–13]. Any PL was defined as any difficulty of the aforementioned questions. Basic ADLs included: difficulty getting in and out of bed; using a fork, knife, or drinking from a cup; standing for long periods of time; and dressing yourself. NHANES did not have information on bathing or toileting. Having difficulty with any of these activities indicated basic ADL impairment. Lastly, we were only able to report on three instrumental ADLs[14], including managing money, performing house chores, or preparing meals, as this was the information that NHANES contained, as opposed to the eight well accepted ones[14]. Any physical, basic or instrumental ADL is considered a functional limitation.
Body composition measures were assessed using dual energy x-ray absorptiometry (DEXA) QDR-4500 Hologic scanner (Bedford, MA), by trained technicians. Subjects taller than 192.5cm or weighing greater than 136.4kg were excluded from this assessment. All metal objects were removed, except false dentition and hearing aids. Fat mass, lean muscle mass, appendicular skeletal muscle mass of all limbs, and bone mineral content were assessed. Total body fat percent and lean mass percent were determined. All NHANES cycles performed similar operation procedures. Sarcopenia was defined using the two FNIH proposed definitions: ALM and ALM divided by BMI[9]. For men, the cutoffs were <19.75kg and <0.789, and in women, the cutoffs were <15.02kg and <0.512. Obesity was defined using percent body fat using thresholds of 25% for males, and 35% for females (values used in our previous studies)[11, 13, 15, 16]. Additionally, subjects were classified as having obesity using the standard BMI category (≥30kg/m2) with sarcopenia. A diagnosis of sarcopenic obesity was considered if subjects fulfilled criteria for both sarcopenia and obesity using these definitions.
2.1 Statistical Analyses
We followed the policies and procedures as outlined by NHANES and analyzed the data accordingly (http://www.cdc.gov/nchs/nhanes). We present the unweighted counts of each group in Table 1 by sex, age group, and race. All baseline variables are represented as mean (standard error) for continuous variables, and count (weighted percentage) for categorical values. We intentionally stratified our results by sex because of considerable differences in body composition and its outcomes[17]. A t-test of unequal variances compared means between continuous variables and sexes, and a chi-square or Fisher exact test compared respective categorical variables. We identified individuals with sarcopenia, physical, basic ADL, and instrumental ADL limitations based on the ALM and ALM/BMI definitions by age category (≥60years, 60–69.9, 70–79.9, 80+), and by race (non-hispanic white, non-hispanic black, Hispanic, other). We compared prevalence rates within each race and age category using weighted estimates, and determined whether differences existed between definitions using chi-square or Fisher exact tests.
Table 1.
Characteristics of 4,984 Study Subjects of NHANES 1999–2004 Age>60 Years
| Male N=2,453 |
Females N=2,531 |
p-value | |
|---|---|---|---|
| Age, years | 70.5 (0.18) | 71.6 (0.25) | <0.01 |
| Anthropometry | |||
| Weight, kg | 85.1 (0.43) | 72.0 (0.35) | <0.01 |
| Body Mass Index, kg/m2 | 28.2 (0.11) | 28.3 (0.13) | 0.50 |
| High Body Mass Index, % | 629 (29.8) | 837 (33.2) | 0.02 |
| Waist Circumference, cm | 104.4 (0.32) | 96.7 (0.30) | <0.01 |
| Fat Mass, kg | 27.0 (0.21) | 31.1 (0.22) | <0.01 |
| Fat Mass, % | 30.9 (0.12) | 42.0 (0.13) | <0.01 |
| Total Lean Mass, kg | 56.1 (0.23) | 39.5 (0.16) | <0.01 |
| Total Lean Mass, % | 66.5 (0.01) | 55.8 (0.01) | <0.01 |
| Appendicular Lean Mass, kg | 24.1 (0.12) | 16.3 (0.09) | <0.01 |
| ALM/BMI | 0.86 (0.01) | 0.58 (0.01) | <0.01 |
| Co-Morbidities | |||
| Diabetes | 542 (19.8) | 518 (17.2) | 0.06 |
| Coronary Artery Disease | 521 (23.0) | 349 (14.7) | <0.01 |
| Arthritis | 965 (41.0) | 1,414 (57.3) | <0.01 |
| Smoking | |||
| Never | 784 (30.6) | 1,543 (59.1) | |
| Current | 374 (13.9) | 237 (10.3) | <0.01 |
| Former | 1,289 (55.4) | 746 (30.6) |
All values are mean (standard error of mean) or count (%) for continuous variables and counts (weighted percentages) for categorical variables by sex.
Total lean mass includes the entire body skeletal mass excluding bone mineral content. Appendicular lean muscle mass is defined as the lean mass of the sum of all the limbs (arms and legs) excluding bone mineral content.
A p-value represents a t-test of unequal variance (continuous variables) or a chi-square/Fisher exact test (categorical variables) between males and females
High body mass index is defined as ≥30kg/m2
Abbreviations: ALM – Appendicular lean Mass; BMI – Body mass index
Our primary analysis identified the association between each definition of sarcopenia and physical limitations, basic ADLs and instrumental ADLs. To identify this relationship, we created multivariable logistic regression models using NHANES weighting and accounted for the stratified clustered sampling. Subjects were assigned a binary outcome (yes/no) for physical, basic ADL and instrumental ADL limitations. Our primary predictor was whether a person had sarcopenia (yes/no). We performed separate analyses for each sarcopenia definition. Four models were created: Model 1 adjusted for age; Model 2 adjusted for age, race, smoking status (current, former, never); Model 3 adjusted for diabetes and arthritis; and Model 4 additionally adjusted for coronary artery disease. Odds ratios (95% confidence intervals) are presented for each multivariable model.
An exploratory analysis determined the prevalence rate of those with sarcopenia and obesity. Counts and weighted prevalence rates were assessed. We determined the prevalence rates of physical limitations in those with sarcopenia and obesity, and assessed differences between sarcopenic obesity definitions and sex. Within sex prevalence rates and between sex rates were compared using either chi-square or Fisher exact tests between ALM/BMI and ALM definitions using percent body fat for obesity, and ALM with body fat and BMI definitions for obesity, as well. Lastly, we reproduced our above models for the association between physical, basic ADL, and instrumental ADL limitations and sarcopenic obesity. All analyses were performed using STATA v.13 (College Station, TX). A two sided p-value of 0.05 was considered statistically significant.
3. RESULTS
Sample sizes are indicated in Appendix A and baseline characteristics of the cohort are shown in Table 1. Females had lower waist circumference, muscle mass and ALM mass than males, as well as lower rates of diabetes and coronary artery disease. BMI was similar between the two sexes. Table 2 outlines the overall prevalence of sarcopenia using the two FNIH definitions by age, age group, and race category. Th prevalence of sarcopenia differed by definition type and increased with age in all sexes and races. In both sexes, Hispanics had a higher prevalence of sarcopenia than other races. Individual and composite limitations are presented in Table 3. Significant differences were observed between definitions in physical, basic ADL, and instrumental ADL limitations in both sexes.
Table 2.
Prevalence of Sarcopenia in NHANES 1999–2004 by FNIH Definition
| Overall | Non-Hispanic Whites | Non-Hispanic Blacks |
Hispanics | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Definition | ALM/BMI | ALM | ALM/BMI | ALM | ALM/BMI | ALM | ALM/BMI | ALM | ALM/BMI | ALM |
| Males | ||||||||||
| Age ≥60 | 719 (27.8) | 459 (16.0)† | 404 (27.1) | 258 (14.9) † | 35 (10.0) | 31 (8.5) * | 258 (49.3) | 147 (27.6) * | 22 (44.8) | 23 (38.3) |
| 60–69 years | 239 (20.9) | 98 (8.9) † | 98 (19.8) | 40 (8.2)* | 9 (4.3) | 7 (2.9) | 122 (43.5) | 43 (16.4) * | 10 (43.0) | 8 (24.1) |
| 70–79 years | 282 (32.4) | 186 (19.1) † | 152 (31.2) | 89 (16.5) † | 19 (18.1) | 19 (16.7) | 103(54.8) | 68 (34.6) | 8 (46.2) | 10 (57.9) † |
| ≥80 years | 198 (41.9) | 175 (32.9) † | 154 (42.0) | 129 (31.9) † | 7 (19.1) | 5 (15.6) * | 33 (62.6) | 36(58.5) * | 4 (51.9) | 5 (62.0) * |
| Females | ||||||||||
| Age ≥60 | 577 (19.3) | 1,028 (40.5) † | 281 (19.0) | 627 (41.9) † | 21 (5.9) | 51 (13.1) * | 263 (37.4) | 315 (51.2) * | 12 (19.6) | 35 (54.6) * |
| 60–69 years | 227 (14.1) | 343 (30.0) † | 66 (13.4) | 153 (30.9) * | 8 (3.5) | 16 (6.6) | 150 (32.9) | 159 (41.0) * | 3 (10.7) | 15 (47.3) |
| 70–79 years | 183 (21.6) | 309 (42.6) † | 88 (20.7) | 183 (43.2) * | 9(8.5) | 16 (14.0) | 82 (48.4) | 97 (58.0) | 4 (23.7) | 13 (59.9) * |
| ≥80 years | 167 (27.2) | 376 (61.1) † | 127 (28.0) | 291 (62.3) † | 4 (8.6) | 19 (32.6) | 31 (30.5) | 59 (73.4) | 5 (54.0) | 7 (75.1) |
Abbreviations: BMI – body mass index; FNIH – Foundation for the National Institutes of Health Values represented are counts (weighted prevalences).
Appendicular Lean Muscle (ALM) cutoffs are: men <19.75kg; females <15.02kg
ALM/BMI cutoffs are <0.789 in men and <0.512 in females
All Hispanics include Mexican Americans
p-value <0.01,
p-value<0.05; representing the difference between ALM/BMI and ALM prevalence rates within each category using a chi-square/Fisher exact test. All other values are considered non-significant (p>0.05)
Table 3.
Prevalence of Limitations by Sex and Low Muscle Mass Definition – Males and Females
| Males | Females | |||
|---|---|---|---|---|
| Physical Limitations | ALM/BMI | ALM | ALM/BMI | ALM |
| Walking ¼ mile | 189 (31.3) | 110 (25.1)† | 219 (48.2) | 306 (31.5) † |
| Walking up 10 steps | 139 (23.0) | 88 (19.3) * | 181 (43.1) | 243 (27.7) † |
| Able to stoop, crouch, kneel | 387 (54.4) | 218 (46.4) † | 376 (66.4) | 557 (52.0) † |
| Lift/carry 10 pounds | 160 (20.1) | 128 (23.4) † | 261 (44.7) | 415 (38.9) † |
| Walking between rooms on same floor | 84 (10.0) | 74 (12.1) * | 87 (14.3) | 109 (8.4) |
| Standing up from armless chair | 204 (27.1) | 122 (21.6) † | 211 (36.7) | 281 (25.1) * |
| Any Physical Limitation | 346 (56.3) | 207 (52.3) † | 351 (74.7) | 562 (60.8) † |
| Basic ADL limitation | ALM/BMI | ALM | ALM/BMI | ALM |
| Getting in and out of bed difficulty | 140 (19.1) | 80 (15.7) * | 136 (21.6) | 190 (15.6) * |
| Using fork, knife, drinking from cup | 52 (6.1) | 38 (7.4) * | 41 (6.9) | 72 (6.6) |
| Standing for long periods difficulty | 340 (47.0) | 201 (39.8) † | 312 (59.3) | 492 (47.6) † |
| Dressing yourself difficulty | 108 (15.0) | 59 (11.8) | 99 (15.9) | 150 (12.1) |
| Any Basic ADL limitation | 369 (50.3) | 228 (44.7) † | 336 (62.8) | 534 (51.6) † |
| Instrumental ADLs | ALM/BMI | ALM | ALM/BMI | ALM |
| Managing money | 99 (11.9) | 87 (16.3) * | 85 (13.3) | 164 (13.1) † |
| House chore | 205 (27.6) | 150 (27.1) * | 241 (43.5) | 350 (33.2) † |
| Preparing meals | 130 (16.1) | 107 (18.5) † | 93 (12.9) | 149 (11.4)* |
| Any IADL Limitation | 264 (33.5) | 191 (34.4) † | 267 (47.4) | 409 (37.9) † |
| Any Functional Limitation | 394 (62.3) | 242 (59.1) † | 366 (78.7) | 617 (68.0) † |
Abbreviations: ADL – Activity of Daily Living; BMI – body mass index; FNIH – Foundation for the National Institutes of Health; IADL – Instrumental ADL Values represented are counts (weighted prevalences)
p-values comparing the proportions between either definition
Appendicular Lean Muscle (ALM) cutoffs are: men <19.75kg; females <15.02kg
ALM/BMI cutoffs are <0.789kg in men and <0.512 in females
Any Physical Limitation represents self-reported difficulty performing any of the physical limitations listed above; Any Basic ADL limitations represents self-reported difficulty performing any of the basic ADLs listed above; Any Instrumental ADL limitation represents a self-reported difficulty performing any of the instrumental ADLs listed above; Any Limitation represents a difficulty performing any of the above noted limitations.
p-value <0.01,
p-value<0.05; representing the difference between ALM/BMI and ALM prevalence rates within each category using a chi-square/Fisher exact test. All other values are considered non-significant (p>0.05)
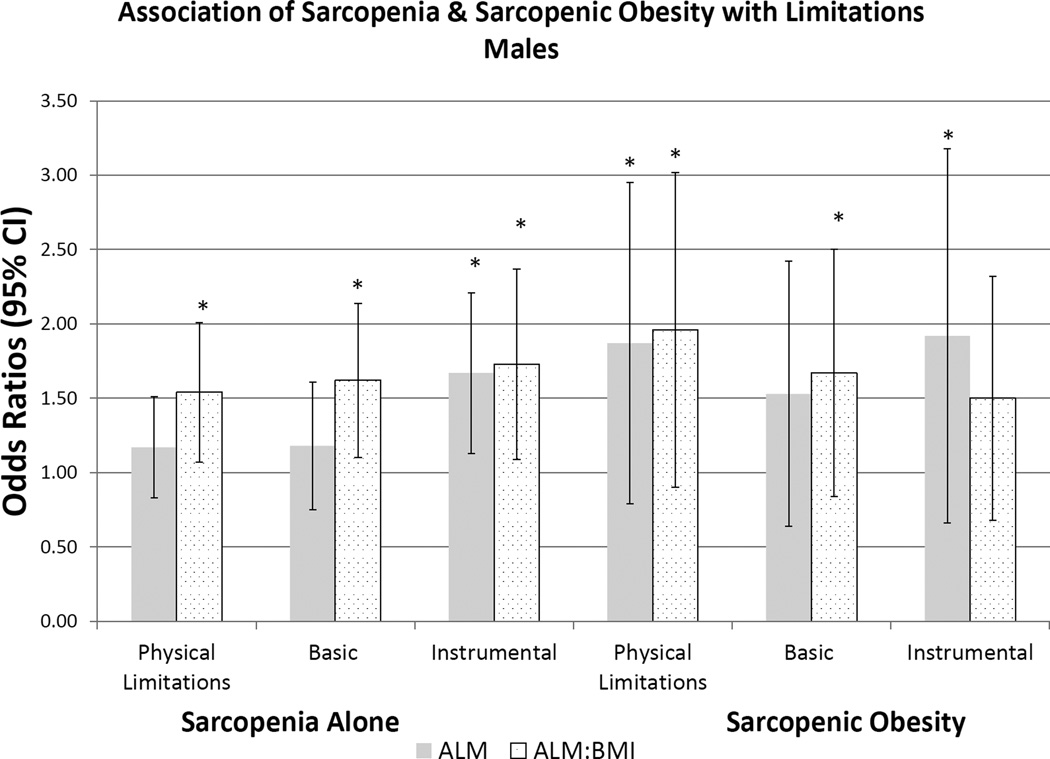
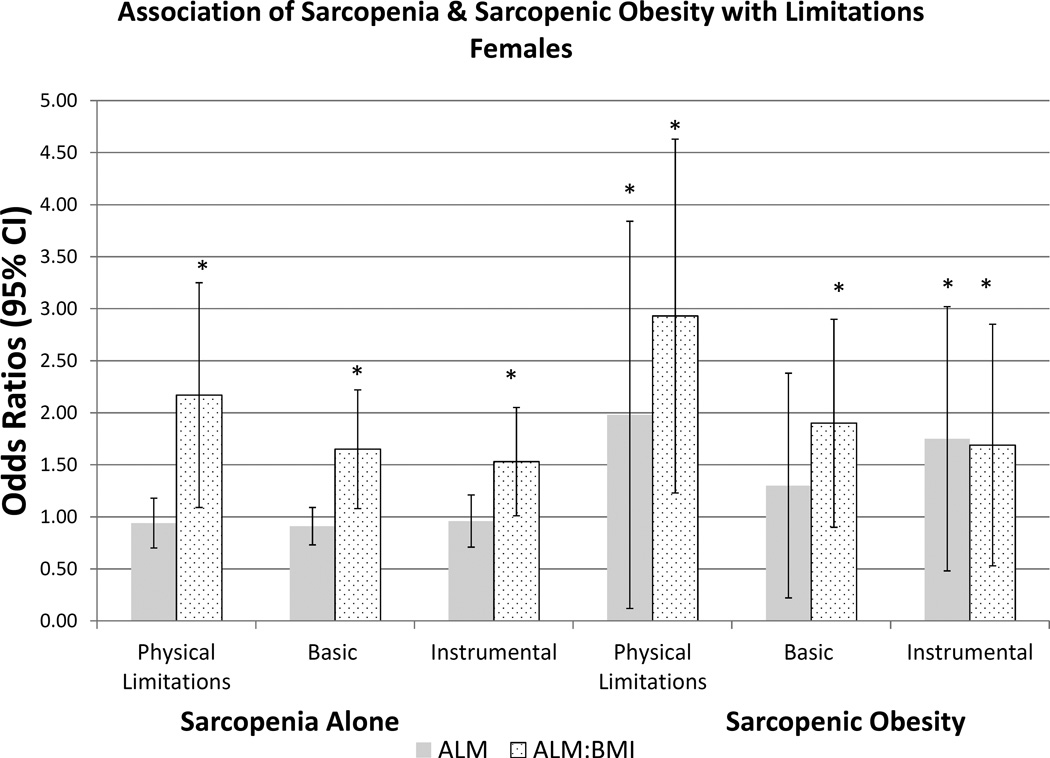
We present the prevalence of sarcopenic obesity in Table 4 using both FNIH definitions for sarcopenia and body composition-defined obesity. Prevalence of sarcopenic obesity was 27.3% and 12.5% using the ALM and ALM/BMI definitions, and 19.1% and 33.5% in men and women, respectively. The prevalence of sarcopenic obesity increased with age based on both definitions, yet the prevalence rate differed by sarcopenia definition for both sexes. The prevalence of sarcopenic obesity using the ALM/BMI definition was lower in females than in males, but higher in females using the ALM definition. Prevalence of physical, basic and instrumental ADL limitations was significantly higher in females than in males. Using a BMI≥30kg/m2, as an obesity measure for sarcopenic obesity, prevalence was markedly low across all sexes. Lastly, we present the multivariable analysis in Figure 1 and Appendix B of the association of sarcopenia or sarcopenic obesity and risk of functional impairments. In males, there are strong associations with functional impairments using the ALM/BMI definition for sarcopenia. The association with sarcopenic obesity in males was also observed in its relation with physical limitations. In females, ALM/BMI was strongly associated with functional impairment with all three domains for both sarcopenia alone, and for sarcopenic obesity.
Table 4.
Prevalence of Sarcopenic Obesity, and Physical Limitations in the United States Population
| Males | Females | Men vs. Females | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sarcopenia | ALM/BMI | ALMa | ALMb | ALM/BMI | ALMa | ALMb | ALM/BMI |
ALM |
ALM |
| Obesity | Body Fat Percent | BMI | Body Fat Percent | BMI | Body Fat Percent | BMI | |||
| p-valuec | p-valuec | p-valued | |||||||
| Age ≥60 | 702 (27.3) | 364 (12.6) † | 3 (0.2)† | 574 (19.1) | 859 (33.5) † | 85 (2.5)† | <0.01 | <0.01 | <0.01 |
| 60–69 years | 232 (20.4) | 75 (6.7) † | -- | 227 (14.1) | 301 (25.1) † | 36 (2.2)† | <0.01 | <0.01 | <0.01 |
| 70–79.9years | 277 (32.2) | 142 (14.9) † | 2 (0.4)† | 182 (21.5) | 263 (36.0) † | 31 (2.6)† | <0.01 | <0.01 | <0.01 |
| ≥80 years | 193 (40.7) | 147 (27.5) † | 1 (0.4)† | 165 (26.9) | 295 (48.0) † | 18 (3.0)† | <0.01 | <0.01 | <0.01 |
| Limitations | |||||||||
| Physical | 338 (56.6) | 168 (54.6) † | 3 (100.0) † | 349 (74.6) | 471(61.6) † | 62 (81.8) † | <0.01 | 0.08 | 0.29 |
| Basic | 360 (50.5) | 186 (46.8) † | 5 (50.5)† | 333 (62.5) | 445 (52.4) † | 50 (48.1)† | <0.01 | 0.16 | 0.93 |
| Instrumental | 257 (33.5) | 152 (24.8) † | 4 (46.5)† | 265 (47.4) | 338 (37.9) † | 42 (46.0)† | <0.01 | 0.31 | 0.99 |
All values represent counts (weighted prevalences). Weighted prevalences are the proportion of those with sarcopenic obesity that have a limitation. Each definition (ALM/BMI and ALM) is combined with an elevated body fat (men >25%, females >35%). Prevalence rates for limitations are the prevalence of each limitations within each category of sarcopenia.
p-value represent difference in prevalence rate between both definitions of sarcopenia and obesity based on ALM/BMI and ALM within each sex.
p-value represent difference in prevalence rate between definitions of ALM sarcopenia and obesity (body mass index) between sexes.
-p-value represents difference between males and females for each definition of sarcopenia and obesity.
p-value represents difference between males and females for each definition of sarcopenia and obesity.
p-value <0.01,
p-value<0.05.All other values are considered non-significant (p>0.05)
Abbreviations: ALM: Appendicular lean mass; BMI – Body mass index
Figure 1.
a-legend:
Association of Physical Limitations, Basic and Instrumental Activities of Daily Living with Definition of Sarcopenia in Males. Multivariable logistic regression estimates are represented as Odds Ratios (95% Confidence Intervals). The primary predictor was sarcopenia (yes/no) and sarcopenic obesity (yes/no), respectfully. Referent Category is ‘no sarcopenia’ based on Foundation for the National Institutes for Health definitions, Appendicular Lean Muscle (ALM) cutoffs of: <19.75kg in males and <15.02kg in females; ALM/BMI cutoffs of <0.789kg in men and <0.512 in females. Separate models were created for the primary outcomes of Physical Limitations, Basic ADLs, and Instrumental ADLs. Model 3 is represented, adjusted age, race, smoking status (current, former, never), diabetes and arthritis. * - indicates statistical significance
b-legend:
Association of Physical Limitations, Basic and Instrumental Activities of Daily Living with Definition of Sarcopenia in Females. Multivariable logistic regression estimates are represented as Odds Ratios (95% Confidence Intervals). The primary predictor was sarcopenia (yes/no) and sarcopenic obesity (yes/no), respectfully. Referent Category is ‘no sarcopenia’ based on Foundation for the National Institutes for Health definitions, Appendicular Lean Muscle (ALM) cutoffs of: <19.75kg in males and <15.02kg in females; ALM/BMI cutoffs of <0.789kg in men and <0.512 in females. Separate models were created for the primary outcomes of Physical Limitations, Basic ADLs, and Instrumental ADLs. Model 3 is represented, adjusted age, race, smoking status (current, former, never), diabetes and arthritis. * - indicates statistical significance
4. DISCUSSION
Our results highlight the substantial prevalence of sarcopenia and sarcopenic obesity in a representative cohort of non-institutionalized adults using newly defined criteria for sarcopenia. Our hypothesis that these estimates increase with age was confirmed. However, we rejected our a priori determination that the prevalence rates would not differ by definitions. This study also confirms our previous hypothesis and adds to the body of literature demonstrating the strong association of sarcopenia and sarcopenic obesity with impairment in function[11, 18–26].
As anticipated, the prevalence rates and multivariable modeling findings differed by sex. Most importantly we found differences in prevalence rates by sarcopenia definition. Prevalence rates by age and ethnicity also varied. One notable exception was observed in non-Hispanic blacks; however, we suspect the lack of statistical differences between such prevalence rates was due to low study power in this subgroup. Physical limitations in both sexes were high in those with both sarcopenia and sarcopenic obesity. Our findings parallel those of others that have demonstrated relationships with these subtypes and impact on physical function.
Using the ALM definition alone, no associations with physical limitations were observed in either sex. In contrast, impairment in instrumental ADL was observed, particularly in males. A shift from using muscle mass to muscle function (or strength) has been advocated as the prime determinant of identifying and studying sarcopenia[9]. A direct and strong causal pathway from mass to strength to function cannot be assumed. Only a subset demonstrates significant weakness that is associated with low muscle mass[9]. We also acknowledge that of the 3 instrumental ADLs in NHANES, household chores and preparing meals may be more dependent on muscle than the other cognitive ADLs, suggesting that these odds ratios may be somewhat higher than would otherwise be expected. While speculative, our results may reflect the phenomenon that ALM may not ideally reflect the degree of impairment in function.
Unfortunately there are no standard definitions for defining obesity. Different approaches that have been used include measures using DEXA, bioelectrical impedance, CT or magnetic resonance imaging. We used well known cutoffs proposed by the World Health Organization using body fat[15] as opposed to using other anthropometric measures such as waist circumference. Previous studies have categorized subjects as having sarcopenic obesity based on BMI[27] and we believe this may lead to bias. Our results suggest that using BMI leads to a markedly lower prevalence rate across both sexes. BMI is not a measure of fat, and has been proven to have poor sensitivity, incorrectly assessing adiposity in >50% of subjects, particularly in older adults[28]. BMI also accounts for muscle mass but not muscle strength and those with an elevated BMI may not fulfill criteria for sarcopenia based on ALM criterion. The modeling used to derive the FNIH thresholds proposed that lean mass be adjusted for body mass, as lean mass leads to the least amount of heterogeneity of future associations with incident mobility limitations[29]. Adjusting for BMI was meant to account for the degree of obesity, particularly in males. The results between definitions for those with sarcopenic obesity differ, both in prevalence and strengths of association. Our study used DEXA to accurately measure body fat which is recommended for body composition analysis[6]. We also observed that rates of sarcopenic obesity paralleled those of sarcopenia in both sexes, suggesting that the majority of persons were classified as having obesity by DEXA. When attempting to identify those with sarcopenic obesity, if DEXA is available, ALM alone should be considered for ascertainment of this entity.
The FNIH consensus agreed to certain cutoffs for the identification of sarcopenia. Importantly, these thresholds are based on the referent populations which have specific baseline characteristics. Measures of ALM are continuous in nature and introducing or altering a specific cutpoint may dramatically alter prevalence[30]. By dichotomizing ALM, subjects just above or just below the threshold may have similar risks to their counterpart but are not identified to be at high long-term risk. Clinicians should be aware of the potential for both over- and underdiagnosis of this clinical condition.
The NHANES datasets have several important limitations. First, the data are cross-sectional and only support associations and not causality. Therefore, increased adiposity and sarcopenia could lead to disability. Alternatively, physical disability could result in muscle loss. Second, the results are highly dependent on the sampling approach and the limited set of survey variables. Third, the survey assesses only non-institutionalized older adults, omitting persons that have higher degrees of sarcopenia, including nursing home residents or those with severe disabilities. The findings are likely an under-representation of the true prevalence of these subgroups. Each population differs, including those of other developed nations, making external validity difficult. Fourth, this data set does not account for weight cycling (alterations in a person’s body composition over the life cycle), deconditioning, and other causes of functional decline[31]. We recognize that self-reported data was used and future analyses would be improved by using standardized functional assessments and/or objective standard measures of physical performance to limit bias. The study relied on definitions used in our previous studies[11–13] and thus may introduce minimal bias in non-response rates, all of which are accounted after using NHANES’ analytical methods. NHANES lacked a complete set of functional variables reducing the generalizability and interpretation of our findings.
An important consideration is that subjects with obesity tend to acquire muscle reserves during the lifespan that allows them to compensate for their body habitus[32]. Our results may not reflect these individuals since the loss in the absolute quantity of muscle mass may not cross the sarcopenia threshold. Furthermore, fat infiltration may occur[26], particularly in those with sarcopenic obesity, leading to an underestimation of rates. FNIH also suggested the use of grip strength as a measure of muscle strength, or gait speed as its surrogate to identify individuals at risk for clinical weakness. Unfortunately, NHANES 1999–2004 does not have any strength or walking speed data and is a limitation of this current study. Future studies should consider using these joint measures of strength and muscle mass not only to define prevalence rates but also to observe concordance between such measures in the assessment of an individual.
Our findings have considerable relevance in light of the recently published FNIH guidelines for sarcopenia. This is a commonly found geriatric syndrome in both sexes with high prevalence, irrespective of the definition used. By identifying subjects with sarcopenia, clinicians and researchers will be able to develop interventions to target this condition, reduce the risk of frailty, and preventing its occurrence and consequences. Further refinement of the criteria, particularly in ascertaining those with sarcopenic obesity is warranted to improve clinical identification and target appropriate care to those at risk.
FNIH criteria of sarcopenia applied to a representative cohort suggest elevated, but varying prevalence rates in the US population. Sarcopenia is associated with functional limitations in both sexes, however, longitudinal studies are necessary to validate these definitions in US based-cohorts.
Acknowledgments
Funding: Funded in part by the Department of Medicine, Geisel School of Medicine at Dartmouth, and the Dartmouth Centers for Health and Aging. Support was also provided by the Dartmouth Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement Number U48DP005018 from the Centers for Disease Control and Prevention. The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
We thank Lydia Gill, BS, for her editing.
FINANCIAL DISCLOSURE
Dr. Batsis receives funding from Health Resources Services Administration (UB4HP19206-01-00) for medical geriatric teaching, the Junior Faculty Career Development Award, the Department of Medicine, Dartmouth-Hitchcock Medical Center, and the Dartmouth Centers for Health and Aging
Dr. Bartels receives funding from the National Institute of Mental Health (K12 HS0217695 (AHRQ), NIMH: T32 MH073553, R01 MH078052, R01 MH089811; R24 MH102794 CDC U48DP005018
Dr. Lopez-Jimenez: n/a
ABBREVIATIONS
- ADL
activities of daily living
- ALM
appendicular lean mass
- BMI
body mass index
- DEXA
dual-energy X-ray absorptiometry
- FNIH
Foundations for the National Institutes of Health
- NHANES
National Health and Nutrition Examination Surveys
- PL
Physical Limitations
Appendix A
Unweighted Sample Sizes for Adults Aged 18 Years and Older by Sex, Age, and Race: NHANES 1999–2004
| Categories by Age | All | Non-Hispanic White | Non-Hispanic Black | All Hispanics | Other |
|---|---|---|---|---|---|
| Males | 7,564 | 3,630 | 1,537 | 2,117 | 280 |
| Age ≥60 years | 2,453 | 1,427 | 386 | 579 | 61 |
| 60–69 years | 1,061 | 490 | 223 | 317 | 31 |
| 70–79 years | 857 | 513 | 123 | 201 | 20 |
| ≥80 years | 535 | 424 | 40 | 61 | 10 |
| Females | 8,307 | 3,915 | 1,689 | 2,394 | 309 |
| Age ≥60 years | 2,531 | 1,419 | 425 | 623 | 64 |
| 60–69 years | 1,115 | 497 | 223 | 317 | 31 |
| 70–79 years | 778 | 439 | 135 | 181 | 23 |
| ≥80 years | 638 | 483 | 63 | 81 | 11 |
Values represent individual subjects
All Hispanics include Mexican Americans
Appendix B
Association of Physical Limitations, Basic and Instrumental Activities of Daily Living with Definition of Sarcopenia
| SARCOPENIA ALONE | SARCOPENIA WITH OBESITY (Body Fat) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALM Definition | ALM/BMI Definition | ALM Definition | ALM/BMI Definition | |||||||||
| MALES |
Physical Limitations |
Basic ADLs |
Instrumental ADLs |
Physical Limitations |
Basic ADLs |
Instrumental ADLs |
Physical Limitations |
Basic ADLs |
Instrumental ADLs |
Physical Limitations |
Basic ADLs |
Instrumental ADLs |
| Model 1 | 1.08 [0.84,1.39] |
1.02 [0.77,1.35] |
1.52 [1.19,1.94] |
1.59 [1.22,2.07] |
1.68 [1.29,2.19] |
1.84 [1.36,2.49] |
1.72 [1.13,2.62] |
1.40 [0.93,2.11] |
1.78 [1.12,2.83] |
2.02 [1.36,2.98] |
1.83 [1.28,2.60] |
1.61 [1.08,2.40] |
| Model 2 | 1.04 [0.82,1.32] |
0.97 [0.74,1.27] |
1.42 [1.10,1.82] |
1.60 [1.22,2.11] |
1.71 [1.30,2.23] |
1.85 [1.35,2.52] |
1.72 [1.11,2.66] |
1.41 [0.93,2.12] |
1.81 [1.13,2.91] |
2.10 [1.38,3.21] |
1.94 [1.34,2.80] |
1.75 [1.15,2.68] |
| Model 3 | 1.17 [0.91,1.51] |
1.18 [0.86,1.61] |
1.67 [1.26,2.21] |
1.54 [1.18,2.01] |
1.62 [1.23,2.14] |
1.73 [1.26,2.37] |
1.87 [1.18,2.95] |
1.53 [0.96,2.42] |
1.92 [1.16,3.18] |
1.96 [1.27,3.02] |
1.67 [1.12,2.50] |
1.50 [0.96,2.32] |
| Model 4 | 1.10 [0.86,1.41] |
1.15 [0.82,1.61] |
1.64 [1.23,2.20] |
1.46 [1.10,1.94] |
1.58 [1.19,2.10] |
1.70 [1.24,2.34] |
1.72 [1.09,2.71] |
1.50 [0.92,2.43] |
1.89 [1.15,3.13] |
1.87 [1.17,2.98] |
1.68 [1.09,2.58] |
1.51 [0.96,2.35] |
| FEMALES | ||||||||||||
| Model 1 | 0.88 [0.71,1.09] |
0.83 [0.68,1.01] |
0.88 [0.72,1.06] |
2.10 [1.42,3.11] |
1.65 [1.23,2.21] |
1.59 [1.18,2.13] |
1.65 [0.89,3.08] |
1.15 [0.62,2.12] |
1.51 [0.83,2.74] |
2.77 [1.77,4.34] |
1.99 [1.39,2.83] |
1.85 [1.16,2.96] |
| Model 2 | 0.89 [0.71,1.11] |
0.83 [0.69,0.99] |
0.87 [0.70,1.07] |
2.24 [1.49,3.37] |
1.75 [1.30,2.36] |
1.64 [1.22,2.19] |
1.81 [0.96,3.44] |
1.26 [0.68,2.34] |
1.61 [0.89,2.91] |
3.09 [1.93,4.94] |
2.23 [1.52,3.28] |
1.98 [1.24,3.17] |
| Model 3 | 0.94 [0.75,1.18] |
0.91 [0.77,1.09] |
0.96 [0.77,1.21] |
2.17 [1.46,3.25] |
1.65 [1.23,2.22] |
1.53 [1.14,2.05] |
1.98 [1.02,3.84] |
1.30 [0.72,2.38] |
1.75 [1.02,3.02] |
2.93 [1.86,4.63] |
1.90 [1.24,2.90] |
1.69 [1.00,2.85] |
| Model 4 | 0.93 [0.74,1.16] |
0.89 [0.75,1.05] |
0.93 [0.75,1.17] |
2.13 [1.41,3.20] |
1.61 [1.18,2.18] |
1.43 [1.06,1.94] |
2.01 [1.02,1.05] |
1.31 [0.72,2.38] |
1.80 [0.97,3.36] |
2.94 [1.86,4.64] |
1.92 [1.26,2.92] |
1.68 [0.97,2.89] |
Multivariable logistic regression estimates are represented as Odds Ratios (95% Confidence Intervals). The primary predictor was sarcopenia (yes/no). Referent Category is ‘no sarcopenia’ based on Foundation for the National Institutes for Health definitions, Appendicular Lean Muscle (ALM) cutoffs of: <19.75kg in males and <15.02kg in females; ALM/BMI cutoffs of <0.789kg in men and <0.512 in females. Separate models were created for the primary outcomes of Physical Limitations, Basic ADLs, and Instrumental ADLs.
Model 1: Adjusted for age
Model 2: Adjusted for age (Model 1), race + smoking status (current, former, never)
Model 3: Adjusted for Model 2 co-variates, and diabetes and arthritis
Model 4: Adjusted for Model 3 co-variates and coronary artery disease.
Estimates in bold face are statistically significant
Footnotes
Work was presented in part at the 2015 International Conference of Frailty & Sarcopenia, Boston, MA
REFERENCES
- 1.Lubitz J, Cai L, Kramarow E, Lentzner H. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003;349:1048–1055. doi: 10.1056/NEJMsa020614. [DOI] [PubMed] [Google Scholar]
- 2.Dunlop DD, Hughes SL, Manheim LM. Disability in activities of daily living: patterns of change and a hierarchy of disability. Am J Public Health. 1997;87:378–383. doi: 10.2105/ajph.87.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Ann Intern Med. 2012;156:131–140. doi: 10.1059/0003-4819-156-2-201201170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guralnik JM, LaCroix AZ, Branch LG, Kasl SV, Wallace RB. Morbidity and disability in older persons in the years prior to death. Am J Public Health. 1991;81:443–447. doi: 10.2105/ajph.81.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jette AM, Branch LG. Impairment and disability in the aged. J Chronic Dis. 1985;38:59–65. doi: 10.1016/0021-9681(85)90008-6. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age and Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Y, Karvellas CJ, Baracos V, Williams DC, Khadaroo RG, Acute C, et al. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014;156:521–527. doi: 10.1016/j.surg.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy x-ray absorptiometry data from the national health and nutrition examination survey 1999–2004. J Am Geriatr Soc. 2013;61:974–980. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- 9.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser: World Health Organization; 1995. pp. 1–452. [PubMed] [Google Scholar]
- 11.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 12.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Lopez-Jimenez F. Normal weight obesity and functional outcomes in older adults. Eur J Intern Med. 2014;25:517–522. doi: 10.1016/j.ejim.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez-Jimenez F. Normal weight obesity and mortality in United States subjects >/=60 years of age (from the Third National Health and Nutrition Examination Survey) Am J Cardiol. 2013;112:1592–1598. doi: 10.1016/j.amjcard.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 15.Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin Pharmacol Ther. 2007;82:509–524. doi: 10.1038/sj.clpt.6100355. [DOI] [PubMed] [Google Scholar]
- 16.Batsis JA, Singh S, Lopez-Jimenez F. Anthropometric measurements and survival in older americans: results from the third national health and nutrition examination survey. J Nutr Health Aging. 2014;18:123–130. doi: 10.1007/s12603-013-0366-3. [DOI] [PubMed] [Google Scholar]
- 17.Heo M, Faith MS, Pietrobelli A, Heymsfield SB. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. Am J Clin Nutr. 2012;95:594–602. doi: 10.3945/ajcn.111.025171. [DOI] [PubMed] [Google Scholar]
- 18.Aubertin-Leheudre M, Lord C, Goulet ED, Khalil A, Dionne IJ. Effect of sarcopenia on cardiovascular disease risk factors in obese postmenopausal women. Obesity (Silver Spring) 2006;14:2277–2283. doi: 10.1038/oby.2006.267. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts Instrumental Activities of Daily Living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 20.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 21.Janssen I. Skeletal Muscle Cutpoints Associated with Elevated Physical Disability Risk in Older Men and Women. Am J Epidemiol. 2004;159:413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 22.Jarosz PA, Bellar A. Sarcopenic obesity: an emerging cause of frailty in older adults. Geriatr Nurs. 2009;30:64–70. doi: 10.1016/j.gerinurse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes. 2009;33:885–892. doi: 10.1038/ijo.2009.130. [DOI] [PubMed] [Google Scholar]
- 24.Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, et al. Sarcopenic Obesity: Prevalence and Association With Metabolic Syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA) Diabetes Care. 2010;33:1652–1654. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: A new category of obesity in the elderly. Nutrition Metabolism and Cardiovascular Diseases. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Current Opinion in Clinical Nutrition and Metabolic Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batsis JA, Lopez-Jimenez F. Cardiovascular risk assessment--from individual risk prediction to estimation of global risk and change in risk in the population. BMC Med. 2010;8:29. doi: 10.1186/1741-7015-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy RA, Patel KV, Kritchevsky SB, Houston DK, Newman AB, Koster A, et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc. 2014;62:1476–1483. doi: 10.1111/jgs.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: The health, aging and body composition study. J Am Geriatric Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]