Abstract
Objective
Some researchers claim that the quality of informed consent of clinical research participants in developing countries is worse than in developed countries. To evaluate this assumption, we reviewed the available data on the quality of consent in both settings.
Methods
We conducted a comprehensive PubMed search, examined bibliographies and literature reviews, and consulted with international experts on informed consent in order to identify studies published from 1966 to 2010 that used quantitative methods, surveyed participants or parents of paediatric participants in actual trials, assessed comprehension and/or voluntariness, and did not involve testing particular consent interventions. Forty-seven studies met these criteria. We compared data about participant comprehension and voluntariness. The paucity of data and variation in study methodology limit comparison and preclude statistical aggregation of the data.
Results and Discussion
This review shows that the assertion that informed consent is worse in developing countries than in developed countries is a simplification of a complex picture. Despite the limitations of comparison, the data suggest that: (1) comprehension of study information varies among participants in both developed and developing countries, and comprehension of randomisation and placebo controlled designs is poorer than comprehension of other aspects of trials in both settings; and (2) participants in developing countries appear to be less likely than those in developed countries to say they can refuse participation in or withdraw from a trial, and are more likely to worry about the consequences of refusal or withdrawal.
Introduction
Many prospective research participants in developing countries have little formal education, lack familiarity with biomedical research and consent procedures, and have limited access to healthcare services. Consequently, it is widely believed that they have more difficulty comprehending study information and providing voluntary consent than do their counterparts in developed countries.1–11 Such views are echoed in ethics guidelines such as those of the Council for International Organizations of Medical Sciences (CIOMS),12 in a report by the National Bioethics Advisory Commission,13 and in the popular press. For instance, a front-page New York Times article framed the problems with comprehension in a trial in the Ivory Coast as a matter of an impenetrable wall between scientific complexity and the ability of locals to understand it—one participant was described as “still not grasp [ing]—even after repeated questioning—what a placebo is or why she might have been given that instead of a real medicine”.14
But what do we know about the quality of informed consent in developing country research? Does available evidence demonstrate that the quality of informed consent from developing country participants is worse than the quality of informed consent from participants in developed nations?
To begin addressing these questions, we reviewed and compared available data on the quality of informed consent from research in both developing and developed countries. We identify similarities and differences between studies of consent in developed and developing countries, highlight gaps in the available data, and make recommendations for future research on the quality of informed consent.
Methods: search strategy and selection criteria
We conducted a comprehensive PubMed search using the Medical Subject Headings (MeSH) terms informed consent, comprehension and decision making in combination with clinical trials or randomized controlled trials (box 1). In addition, we examined bibliographies,15 literature reviews16,17 and reference lists from relevant papers, and consulted with international experts on informed consent to clinical research.
Box 1. MeSH terms strategy.
(informed consent[mh] AND (Comprehension[mh] OR decision-making[mh])
AND (randomized controlled trials as topic[mh] OR clinical trial as topic[mh])
AND (Humans[Mesh] AND English[lang]))
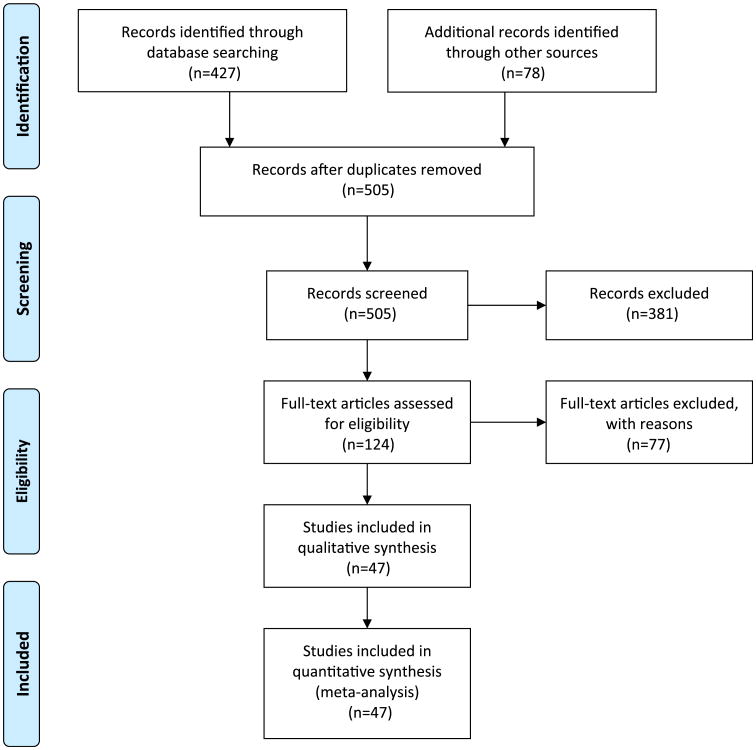
We included studies that met four criteria: (1) used quantitative methods to study informed consent (to allow for comparison of relatively similar data sets); (2) surveyed participants or paediatric participants' parents in actual clinical trials rather than hypothetical scenarios (as we are concerned with what participants understand and how they make decisions in real trials); (3) did not test informed consent interventions aimed at improving its quality (to avoid confounding results); and (4) assessed at least one of two domains critical to measuring the quality of informed consent: comprehension of study information and voluntariness of consent. While some published data on disclosure exist, there are little to no comparable data from non-intervention utilising trials that evaluate understanding and comprehension relative to the quality of disclosure. A total of 427 studies were identified through the PubMed search (figure 1) and 79 from bibliographies, literature reviews, reference lists and consultations with experts. Of those 506 studies, 47 met all four criteria: 18 studies evaluated the quality of informed consent in trials in developing countries and 32 studies evaluated the quality of informed consent in trials in developed countriesi (tables 1 and 2). Identified studies were reviewed by the authors and information extracted regarding the type and location of the clinical trial, the sample size, and the method and timing of assessing informed consent. Data about participants' comprehension of trial information and voluntariness were extracted, including understanding of the purpose and nature of the research, the risks and side effects, and randomisation and placebo controlled design (tables 3–5), as well as perceived pressure and participant knowledge of the right to refuse to enrol or withdraw from a trial (table 6). Direct comparison or meta-analysis of study data was not feasible, as the relevant studies did not employ a uniform methodology or study design.
Figure 1.
PRISMA 2009 flow diagram (adapted from Moher D, Liberati A, Tetziaff J, et al; The PRISMA Group. Preferred reporting items for systemic reviews and meta analyses: the PRISMA statement. PLoS Med 2009;6: e1000097. For more information, visit http://www.prisma-statement.org). This figure is produced in colour in the online journal—please visit the website to view the colour figure.
Table 1. Developing country consent studies.
| Authors | Country | Sample | Type of clinical research | Method of evaluation |
|---|---|---|---|---|
| Ellis et al, 201018 | Mali | 89 M and F | Malaria vaccine phase 1 trial | Questionnaire administered after IC document reviewed but before consent |
| Vallely et al, 201020 | Tanzania | 99 F | Placebo controlled trial of HIV vaginal microbicide | Interviews at 4, 24 and 52 weeks |
| Sarkar et al, 200921 | India | 368 Parents | Birth cohort study of diarrhoeal disease | Structured interviews 3–7 months post-trial |
| Oduro et al, 200822 | Ghana | 270 Mothers | Paediatric trials evaluating immune correlates of protection against malaria | Questionnaire administered at end of study |
| Hill et al, 200823 | Ghana | 60 F | Vitamin A supplementation trial | Semi-structured interviews after consent |
| Minnies et al, 200824 | South Africa | 192 Mothers | Paediatric case–control trial of immune correlates against severe childhood TB | Self-administered questionnaire with staff help if necessary, within 1 h of consent |
| Kaewpoonsri et al, 200625 | Thailand | 84 M and F | Malaria drug trials | Interview at third follow-up visit |
| Marshall et al, 200619 | Nigeria | 307 M and F | Genetic studies of hypertension | Interviews administered at variable times usually long after consent |
| Krosin et al, 200626 | Mali | 163 Parents | Paediatric malaria vaccine prevention trial | Questionnaire within 48 h after consent |
| Moodley et al, 200527 | South Africa | 334 M and F | Influenza vaccine trial | Interviews 4–12 months post-trial |
| Pace et al, 200528 | Thailand | 141 M and F | HIV study of IL-2 effectiveness | Interviewers administered survey immediately after consent |
| Pace et al, 200529 | Uganda | 347 Parents | Paediatric malaria treatment study | Interviews immediately after consent |
| Ekouevi et al, 200430 | Côte d'Ivoire | 55 F | HIV mother-to-child transmission prevention trial | Interviews a median of 136 days after consent |
| Joubert et al, 200331 | South Africa | 92 F | Trial of vitamin A for prevention of mother-to-child HIV transmission | Interviews a median of 14 months after consent |
| Lynöe et al, 200132 | Bangladesh | 105 F | Nutritional trial of iron supplements for pregnant women | Interviews after consent |
| Leach et al, 199933 | The Gambia | 137 Mothers | Paediatric trial of Haemophilus influenzae type B conjugate vaccine | Interviews within a week of consent |
| Karim et al, 199834 | South Africa | Evaluation study group: 56 F | Perinatal HIV transmission trial | Questionnaires administered before and after counselling and consent |
| Sensitisation control group: 56 F* | ||||
| Pitisuttithum et al, 199735 | Thailand | 33 M and F | HIV vaccine trial with drug users | Questionnaire before signing consent |
To evaluate the informed consent obtained for the HIV testing that preceded induction into the perinatal transmission trial, researchers administered both pre- and post-counselling questionnaires to an evaluation study group (n=56). A sensitisation control group (n=56) received only post-counselling questionnaires, so as to measure the sensitising effect of the pre-counselling questionnaire given to the evaluation study group.
F, female; IC, informed consent; M, male.
Table 2. Developed country consent studies.
| Authors | Country | Sample | Type of clinical research | Method of evaluation |
|---|---|---|---|---|
| Ellis et al, 201018 | USA | 171 M and F | Malaria vaccine phase I trial | Questionnaire administered after IC document reviewed but before consent |
| Ravina et al, 201036 | USA | 149 M and F | Phase II Parkinson's trial | Self-administered questionnaire at final clinical trial visit |
| Bergenmar et al, 200837 | Sweden | 282 M and F | Phase II and phase III oncology trials | Mail surveys sent within 3 days–2 weeks of consent |
| Knifed et al, 200838 | Canada | 21 M and F | Neuro-oncology trial | Interviews within 1 month of IC |
| Agrawal et al, 200639 | USA | 163 M and F | Phase I oncology trials | Interview immediately after consent |
| Franck et al, 200740 | UK | 109 Parents | 25 Different paediatric studies | Questionnaire taken immediately after and 3 months after consent |
| Marshall et al, 200619 | USA | 348 M and F | Genetic studies of hypertension | Interviews long and variably after consent |
| Gammelgaard et al, 200441 | Denmark | 103 M and F | Acute myocardial infarction trials | Mail survey sent to participants in the study 3 weeks after IC |
| Kodish et al, 200442 | USA | 137 Parents | Paediatric leukaemia trial | Parent pairs interviewed within 48 h of consent |
| Lynöe et al, 200443 | Sweden | 44 M and F | Chronic haemodialysis trials | Mail survey about 1 week after disclosure of information |
| Criscione et al, 200344 | USA | 30 M and F | Rheumatoid arthritis trial | Questionnaire 1–4 weeks after consent |
| Kupst et al, 200345 | USA | 20 Parents | Paediatric oncology trials | Interviews 1 month after IC |
| Pope et al, 200346 | Canada | 190 M and F | Cardiology, ophthalmology and rheumatology trials | Mail survey 2–5 months after consent |
| Schats et al, 200347 | The Netherlands | 37 M and F | Subarachnoid haemorrhage emergency management trials | Interviews 7–31 months after IC (median of 20 months) |
| Simon et al, 200348 | USA | Majority English speakers: 60 parents | Paediatric oncology trials | Parents interviewed 48 h after consent |
| Minority English Speakers: 27 parents | ||||
| Minority non-English speakers: 21 parents | ||||
| Joffe et al, 200149 | USA | 207 M and F | Oncology trials, phase I, II and III | Mail survey 1–2 weeks after consent |
| Daugherty et al, 200050 | USA | 144 M and F | Phase I oncology trials | Interviews within 1 week of first administration of investigational treatment |
| Hietanen et al, 200051 | Finland | 261 F | Oncology trial of tamoxifen | Mail survey 5–17 months after consent |
| Montgomery et al, 199852 | UK | 158 M and F | 3 In-house and 3 multi-centre anaesthesia trials | Mail survey up to 24 months after consent |
| Van Stuijvenberg et al, 199853 | The Netherlands | 181 Parents | Paediatric trial of ibuprofen for febrile convulsions | Mail survey up to 2–3 years after consent |
| ACHRE, 199654 | USA | 570 M and F | Oncology and cardiology trials | Brief interviews followed by in-depth interviews |
| Harrison et al, 199555 | USA | 71 M and F | HIV vaccine trial | Self-administered questionnaire after disclosure and before consent |
| Harth et al, 199556 | Australia | 62 Parents | Paediatric trial of oral asthma drug | Self-administered questionnaire 6–9 months after entered trial |
| Estey et al, 199457 | Canada | 29 M and F | Not specified | Interviews 1–6 weeks after consent |
| Miller et al, 199458 | USA | 168 M and F | Trial of analgesic drugs | Interviews 30–90 days after entered trial |
| Lynöe et al, 199159 | Sweden | 43 F | Gynaecology trial of antiphlogistic drugs for fallopian tube inflammation | Mail survey 18 months after study |
| Benson et al, 198560 | USA | Depression study: 24 M and F | Antidepressant trial and antipsychotic trial | Interviews immediately following IC |
| Schizophrenia study: 24 M | ||||
| Penman et al, 198461 | USA | 144 M and F | Oncology trials, phase II and III | Interviews 1–3 weeks after consent |
| Riecken et al, 198262 | USA | 112 M* | 50 Different trials | Interviews within 10 weeks of consent |
| Howard et al, 198163 | USA | 64 M and F | Cardiology trial of β-blockers (BHAT) for acute myocardial infarction | Interviews 2 weeks–15 months after consent |
| Bergler et al, 198064 | USA | 39 M | Hypertension trial of hydrochlorothiazide versus propranolol | Interviews and quizzes just after consent; repeated 3 months later |
The trial involved 156 participants, but only 112 indicated that they were aware that they were participating in a trial, and therefore only 112 were asked questions about voluntariness. ACHRE, Advisory Committee on Human Radiation Experiments; F, female; IC, informed consent; M, male.
Table 3. Understanding of research nature and purpose*.
| Developed country studies | Developing country studies | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Author | Country | Understood purpose | Author | Country | Understood purpose |
| Knifed et al, 200838 | Canada | 100% | Leach et al, 199933 | The Gambia | 90% |
| Ravina et al, 201036 | USA | 92.6% | Pace et al, 200528 | Thailand | 88% |
| Franck et al, 200740 | UK | 85% | Minnies et al, 200824 | South Africa | 80.6% |
| Howard et al, 198163 | USA | 80% | Pace et al, 200529 | Uganda | 80% |
| Miller et al, 199458 | USA | 73% | Kaewpoonsri et al, 200625 | Thailand | 50% |
| Van Stuijvenberg et al, 199853 | The Netherlands | 53% | Sarkar et al, 200921 | India | 43% |
| Marshall et al, 200619 | USA | 41% | Marshall et al, 200619 | Nigeria | 39% |
| Benson et al, 198560 | USA | 37% each in depression and schizophrenia studies | Krosin et al, 200626 | Mali | 26% |
| Daugherty et al, 200050 | USA | 31% | Joubert et al, 200331 | South Africa | 28%, but 40% knew the substance being tested was vitamin A |
| Harth et al, 199556 | Australia | 13% | |||
| Riecken et al, 198262 | USA | 10% | |||
|
| |||||
| Developed country studies | Developing country studies | ||||
|
|
|
||||
| Author | Country | Understood nature | Author | Country | Understood nature |
|
| |||||
| Knifed et al, 200838 | Canada | 100% | Ekouevi et al, 200430 | Côte d'Ivoire | 95%e100% |
| Criscione et al, 200344 | USA | 100% | Moodley et al, 200527 | South Africa | 95% |
| Hietanen et al, 200051 | Finland | 100% | Minnies et al, 200824 | South Africa | 85.4% knew was research; 36.7% knew there were no immediate benefits |
| Lynöe et al, 199159 | Sweden | 98% | Vallely et al, 201020 | Tanzania | 77% knew gel may not prevent HIV |
| Howard et al, 198163 | USA | 92% | Hill et al, 200823 | Ghana | 75% knew was research, but 93% thought trial capsules were a ‘medicine or vitamin’ |
| Ravina et al, 201036 | USA | 89% understood drugs were experimental† | Lynöe et al, 200132 | Bangladesh | 47% |
| Ellis 201018 | USA | 85% | Krosin et al, 200626 | 26% | |
| Penman et al, 198461 | USA | 78% | |||
| Gammelgaard et al, 200441 | Denmark | 72% | |||
| Riecken et al, 198262 | USA | 72% | |||
| Bergenmar et al, 200837 | Sweden | ∼70% | |||
| Kupst et al, 200345 | USA | 55% | |||
| Schats et al, 200347 | The Netherlands | 38% | |||
| Joffe et al, 200149 | USA | 30% | |||
Arranged from highest to lowest %.
Yet only 57% knew that participation in the study was not part of usual Parkinson's disease treatment.
ACHRE, Advisory Committee on Human Radiation Experiments.
Table 5. Understanding of study design and randomisation*.
| Developed country studies | Developing country studies | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Author | Country | Understood study design | Author | Country | Understood study design |
| Van Stuijvenberg et al, 199853 | The Netherlands | 88% placebo design | Moodley et al, 200527 | South Africa | 49% knew they had a 50% chance of receiving placebo; 19% understood placebo |
| Criscione et al, 200344 | USA | 87% placebo design | Hill et al, 200823 | Ghana | 13% understood ‘not all trial capsules were the same’ |
| Howard et al, 198163 | USA | 86% double blind design | Leach et al, 199933 | The Gambia | 10% placebo design† |
| Harrison et al, 199555 | USA | 79% | |||
| Pope et al, 200346 | Canada | 76% placebo design | |||
| Bergler et al, 198064 | USA | 64% (at start), 28% (3 months later) | |||
|
| |||||
| Developed country studies | Developing country studies | ||||
|
|
|
||||
| Author | Country | Understood randomisation | Author | Country | Understood randomisation |
|
| |||||
| Ravina et al, 201036 | USA | 90%, yet only 67% understood there was a 1 in 3 chance of receiving placebo | Ellis et al, 201018 | Mali | 80% of adults 90% of parents |
| Simon et al, 200348 | USA | 68% of majority English speakers | Krosin et al, 200626 | Mali | 68% |
| 26% of minority English speakers | |||||
| 14% of minority non-English speakers | |||||
| Bergenmar et al, 200837 | Sweden | 85% | Pace et al, 200528 | Thailand | 31% |
| Gammelgaard et al, 200441 | Denmark | 79% | Moodley et al, 200527 | South Africa | 21% |
| Criscione et al, 200344 | USA | 50% | Pace et al, 200529 | Uganda | 19% |
| Van Stuijvenberg et al, 199853 | The Netherlands | 50% | |||
| Kodish et al, 200442 | USA | 50% | |||
| Howard et al, 198163 | USA | 43% | |||
| Pope et al, 200346 | Canada | 39% | |||
| Benson et al, 198560 | USA | 33% of depression study, 16% of schizophrenia study | |||
| Hietanen et al, 200051 | Finland | 23% | |||
| Schats et al, 200347 | The Netherlands | 22% | |||
Arranged from highest to lowest %.
When broken down into those who had received a written information sheet at least a week before consent, 15% of those who had received the sheet understood that there was a placebo group versus 4% of those who had not received prior written information.
Table 6. Voluntariness: knew could withdraw or refuse*.
| Developed country studies | Developing country studies | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Author | Country | Knew could withdraw | Author | Country | Knew could withdraw |
| Joffe et al, 200149 | USA | 90%; 99% knew could refuse | Ellis et al, 201018 | Mali | 96% of adults 93% of parents |
| Ellis et al, 201018 | USA | 98% | Karim et al, 199834 | South Africa | 93% of evaluation study group, 88% of sensitisation control group† |
| Ravina et al, 201036 | USA | 98% felt ‘free to refuse to participate’ | Pitisuttithum et al, 199735 | Thailand | 88% knew could refuse |
| Marshall et al, 200619 | USA | 97% | Moodley et al, 200527 | South Africa | 87% |
| Criscione et al, 200344 | USA | 96% | Pace et al, 200528 | Thailand | 71% |
| Benson et al, 198560 | USA | Depression study: 95%; 75% knew could refuse. | Marshall et al, 200619 | Nigeria | 67% |
| Schizophrenia study: 83%; 75% knew could refuse | Minnies et al, 200824 | South Africa | 65% | ||
| Bergenmar et al, 200837 | Sweden | 93%; 100% knew could refuse | Pace et al, 200529 | Uganda | 65%; 41% knew could refuse |
| Franck et al, 200740 | UK | 91% | Kaewpoonsri et al, 200625 | Thailand | 53.1% |
| Van Stuijvenberg et al, 199853 | The Netherlands | 91% | Lynöe et al, 200132 | Bangladesh | 48%: 65% knew could refuse |
| Lynöe et al, 200443 | Sweden | 90% | Sarkar et al, 200921 | India | 50% |
| Simon et al, 200348 | USA | 90% of majority English speakers | Ekouevi et al, 200430 | Côte d'Ivoire | 27% |
| 78% of minority English speakers | Joubert et al, 200331 | South Africa | 24% (but 92% said care would no longer be good if they quit) | ||
| 60% of minority non-English speakers | Oduro et al, 200822 | Ghana | 21% | ||
| Montgomery et al, 199852 | UK | 83% | Krosin et al, 200626 | Mali | 10% |
| Penman et al, 198461 | USA | 80% | |||
| Riecken et al, 198262 | USA | 80%; 95% knew could refuse | |||
| ACHRE, 199654 | USA | 78% | |||
| Bergler et al, 198064 | USA | 77% (at start), 61% (3 months later) | |||
| Harth et al, 199556 | Australia | 45% (but 32% said would not be allowed) | |||
| Schats et al, 200347 | The Netherlands | 25%; 59% knew could refuse‡ | |||
Arranged from highest to lowest %.
However, 98% of the evaluation study group and 100% of the sensitisation control group said hospital would not allow them.
Knew participation was not obligatory.
ACHRE, Advisory Committee on Human Radiation Experiments.
Results
Study characteristics
Eighteen studies conducted in 11 different developing countries examined the consent of participants in clinical research on vaccines, nutritional supplements, HIV treatments, immune correlates in children, diarrhoeal disease in children, anti-malarial drugs and genetics (table 1). Sample sizes ranged from 33 to 700 research participants. Seven studies interviewed a parent of a participating child,18,21,22,24,26,29,33 and of those seven, three interviewed only the mothers.22,24,33 Thirteen studies19–21,23,26–33,35 used structured or semi-structured interviews, while five used questionnaires.18,22,24,25,34 In nine studies, participants were interviewed close to the time of consent18,23,24,26,28,29,33–35 and in eight others interviews were conducted 1–14 months or longer after the participant gave consent.19–22,25,27,30,31 In one study, the timing was not specified.32
Thirty-one studies, conducted in eight developed countries, examined the consent of participants involved in oncology, cardiology, gynaecology, HIV, analgesics/anaesthesia, neurological, antidepressant, antipsychotic, emergency management, arthritis, paediatric asthma, paediatric febrile convulsion, diabetes, malaria and genetics research (table 2). Sample sizes ranged from 21 to 570 research participants. Six studies surveyed the parents of children in paediatric trials.40,42,45,48,54,57 Sixteen studies used structured interviews,19,38,39,42,45,47,48,50,54,57,58,60–64 nine used mailed surveys,37,41,43,46,49,51–53,59 and six used questionnaires.18,36,40,44,55,56 In eight studies, questions were asked close in time to when consent was given, in each case within 48 h of consent18,39,40,42,48,55,60,64; the remaining 23 studies surveyed participants weeks to months after consent.19,36–38,41,43–47,49–54,56–59,61–63
Comprehension and recall of trial information
Participant understanding of research purpose, risks/side effects and design varied substantially across informed consent studies from both developing and developed countries. Across studies, comprehension of trial purpose or nature appeared to be better than comprehension of trial design and randomisation.
Trial purpose and nature
Available data show no substantial difference between participants in developing countries and those in developed countries with respect to their understanding of trial purpose, defined as the goal of a given clinical trial (table 3). In the developed country studies that measured it, understanding of trial purpose ranged from 10% of US males who understood the purpose of a variety of trials they were participating in62 to 100% of Canadian participants who understood the purpose of a neurooncology trial.45 Understanding of trial purpose in developing country studies also varied, ranging from 26% of Malian parents who understood the purpose of a malaria trial for their children26 to 90% of mothers with children in a paediatric influenza trial in The Gambia.33 Similarly, reported understanding of trial nature, assessed by participants' understanding that they were participating in research and of the investigational and experimental nature of research interventions, varied from 31% of participants in a US phase 1 oncology trial50 to almost 100% of participants in both a Swedish and a Finnish trial,51,59 and from 47% of women in a Bangladeshi nutritional trial for iron supplements32 to 100% of women in an HIV trial in Côte d'Ivoire.30
Risks/side effects
The percentage of participants who could recognise or name trial side effects and risks also ranged widely among the studies reviewed (table 4). Reported understanding of side effects varied depending on how the questions were framed—more participants were able to recognise side effects from a list than were able to name or explain them in response to open-ended questions. For example, 86% of participants in a US analgesic trial recognised at least one side effect from a list, but only 48% were able to name at least one without the help of a list.58 In a US rheumatoid arthritis trial, 30% responded that they knew the trial drugs were not completely safe, but were not asked to recognise or name the specific risks of the drugs.44
Table 4. Understanding of risks and side effects*.
| Developed country studies | Developing country studies | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Author | Country | Understood risks or side effects | Author | Country | Understood risks or side effects |
| Daugherty et al, 200050 | USA | 100% named >1 side effect | Pace et al, 200528 | Thailand | 98% recognised side effects |
| Harrison et al, 199555 | USA | 89% recognised side effects | Pitisuttithum et al, 199735 | Thailand | 97% recognised side effects |
| Knifed et al, 200838 | Canada | 71% knew at least one general risk† | Minnies et al, 200824 | South Africa | 79.2% knew risks |
| Benson et al, 198560 | USA | Depression study: 62% knew risks | Leach et al, 199933 | The Gambia | 53% named ≥1 side effect |
| Schizophrenia study: 42% knew risks | |||||
| Howard et al, 198163 | USA | 61% could name 1 side effect | Oduro et al, 200822 | Ghana | 20% knew direct risks |
| Miller et al, 199458 | USA | 48% named and 86% recognised >1 risk | Pace et al, 200529 | Uganda | 18% named 1 or more side effects |
| Ravina et al, 201036 | USA | 47% knew which drugs had highest risks, 93% knew PD could get better, worse or not change | Krosin et al, 200626 | Mali | 7% said there were side effects‡ |
| Estey et al, 199457 | Canada | 41% named >1 risk | Kaewpoonsri et al, 200625 | Thailand | 6.6% recalled being told of risks |
| Van Stuijvenberg et al, 199853 | The Netherlands | 40% knew side effects | |||
| Joffe et al, 200149 | USA | 37% knew research risks | |||
| Penman et al, 198461 | USA | 31% named >3 of 11 risks | |||
| Criscione et al, 200344 | USA | 30% knew there were risks§ | |||
| Bergler et al, 198064 | USA | 28% (at start), 3% (3 months later) | |||
| Bergenmar et al, 200837 | Sweden | 18% knew research risks | |||
| Schats et al, 200347 | The Netherlands | 6% knew side effects | |||
Arranged from highest to lowest.
29% did not recall ANY risks of the trial drug, the rest of the participants could name general risks, and at most participants could name up to four specific risks or side effects.
The question was complex and multi-choice. The correct answer was the only one that included a mention of side effects. However, it also included information about the potential benefits of the medicine (eg, that it could prevent malaria and correct other health problems).
But were not asked to name or identify them.
PD, Parkinson's disease.
In consent studies of developing country trials, 79% of participants in a South African vaccine trial knew the risks involved22 and 97% of Thai participants recognised possible side effects of an experimental HIV vaccine,35 yet only 7% of Malian parents recognised that the investigational vaccine being given to their child might have side effects.26
Randomisation and placebo trial design
Understanding of randomisation also varied among participants in both developing and developed country trials, but across all studies, understanding of randomisation was low compared to understanding of other aspects of a trial (table 5). In developed country studies, understanding of randomisation appeared to vary according to how close to actual consent it was measured. For example, 68% of parents understood randomisation when asked within 48 h of consent in US paediatric oncology trials,48 and as many as 79% understood randomisation in an HIV vaccine trial when assessed immediately after disclosure.55 Yet fewer than half of the participants were reported to comprehend randomisation in six developed country studies in which understanding was assessed months or years after consent.44,46,47,51,53,63
Five developing country studies measured understanding of randomisation (table 5); four of those five measured it within 1 week of consent.18,20,26,28,29 Comprehension of randomisation ranged from as high as 90% of parents whose children were enrolled in a malaria vaccine trial in Mali18 to as low as 19% of parents whose children were enrolled in a malaria treatment trial in Uganda.29
Between 64% and 88% of participants understood the study design in six developed country trials,41,44,46,53,63,64 yet only 39% of the participants in a set of Canadian trials recalled their own chance of receiving placebo, and 29% of them “thought that the doctor [had known] what kind of medication they were taking”.46 Knowledge of placebo was measured in three developing country studies: 10% of mothers enrolling children in a Gambian trial understood the placebo control design,33 13% of Ghanaian trial participants knew that not all trial capsules were the same,23 and 49% of South African participants knew they had a 50% chance of receiving placebo.27
Although measured infrequently, individuals' understanding of research design diverges from their understanding of how it specifically applies to them. In one Thai HIV treatment trial, 31% correctly responded that half the participants would get the investigational drug, yet 48% said they had a 50/50 chance of receiving it.28 In a Ugandan malaria trial, 19% of parents knew that not all children would receive the same treatment, even though 84% recalled being told about treatment assignment.29 Similarly, in a US rheumatoid arthritis trial, 87% of participants said that some people in the trial would get placebo, but only 50% thought they personally could receive placebo.44
Voluntariness
Data on voluntariness is organised into two categories: (1) participants' perceptions of pressure (not reported in a table); and (2) participants' knowledge of the right to refuse or withdraw from participation (table 6).
Pressure
Questions assessing perceptions of pressure differed across informed consent studies—some focused on whether or not participants knew or felt that participation was voluntary, while others asked more specific questions about the source and amount of pressure felt by participants.
Most (90%–99%) participants in a US hypertension trial, a Canadian neuro-oncology trial and UK paediatric trials reported no pressure to participate19,38,40 or reported that participation was voluntary.52 At the same time, 31% of US oncology and cardiology trial participants said that they felt that they had little other choice than to participate,54 25% of parents in a Netherlands paediatric oncology trial indicated that they felt obliged to participate53 and 18% of Danish participants in an acute myocardial infarction trial reported feeling ‘under pressure’, although 70% said the decision was ‘fully theirs’.41
Five developing country informed consent studies measured general perceptions of pressure and voluntariness. Most mothers (95%) in a Ghanaian paediatric trial21 and most participants (99%) in a South African influenza vaccine trial27 said participation was voluntary. Similarly, most parents in an Indian paediatric trial (98%) reported that they joined the study freely without any pressure or compulsion.24 In contrast, in another South African trial, 84% of the evaluation group and 93% of the sensitisation group reported feeling that participation was compulsory.34
In consent studies that distinguished sources of pressure, more trial participants reported feeling pressure from their disease or circumstances than from other people. Although 29% of US phase I and phase II oncology trial participants said that their physician did not actively want them to make their own decision,61 only 14% in a Swedish gynaecology trial,59 7% in another US oncology trial62 and 6% in a set of varied US trials reported feeling pressure from a clinician.39 In the same oncology study in which 7% reported pressure from a clinician and 9% from their families, a full 75% reported pressure due to their progressive cancer.39 In another US paediatric oncology trial, 70% of the parents cited high levels of distress and ‘feeling overwhelmed’ during the consent process.45 Few participants in developed country trials reported pressure from anticipated consequences of withdrawing: 98% of UK anaesthesia trials participants,52 86% of Canadian neuro-oncology trial participants38 and 85% of Danish cardiology trial participants41 knew that refusal to participate would not compromise their care.
In developing country studies, reported pressure from others was also generally low, ranging from 6% of participants reporting pressure from spouses, family or the research team in a Ugandan paediatric malaria treatment trial29 to 26% reporting pressure from village elders in a Malian paediatric vaccine trial.26 Reported pressure came from various sources, for example, from village elders (26%), the research team (12%) and a spouse (7%) in the aforementioned Malian study,26 and from a close friend (15%), a family member (7%) or their doctor (2%) in an HIV treatment trial in Thailand.28 Similarly, in a Ugandan paediatric trial, 15% of parents reported feeling pressure from others, including spouses (6%), family or friends (6%) or the research team (6%), but 58% reported pressure because of their child's illness.29 However, in one Gambian trial, 9% of mothers offered spontaneously and 36% agreed when directly questioned that it would have been hard to refuse participation–some reported feeling group pressure after watching other mothers agree to participate.33
Participants in developing countries reported pressure from fear of the consequences of withdrawing. Although in one South African trial, 88% said their usual care would not be affected if they refused,27 87% of participants in a Bangladeshi trial felt that the trial offered such advantages that they couldn't refuse.32 Similarly, 32% of the evaluation study group and 23% of the sensitisation group in a South African perinatal HIV transmission trial thought that care would be compromised if they did not participate,34 and 44% of parents in a paediatric malaria vaccine trial in Mali said they would lose healthcare access if they withdrew.26
Knew they could refuse or withdraw
The clearest differences between respondents in developed and developing country informed consent studies were related to knowledge of the right to refuse to participate in research or to withdraw (table 6). In 15 of 18 developed country studies that measured this, more than 75% of trial participants knew they could withdraw or refuse,18,19,36,37,40,43,44,49,52–54,60–62,64 and in 10 of these studies, 90% or more said they could withdraw from research.18,19,36,37,40,43,44,49,53,60 In one US paediatric oncology trial, 90% of the majority race English speaking parents, 78% of the minority race English speaking parents and 60% of the minority race non-English speaking parents knew they had a right to withdraw their children from the trial.48
In contrast, in five of 15 developing country studies that measured it, less than half of respondents knew they could withdraw from research.22,26,30–32 As few as 10% of mothers in Mali knew they could withdraw their child from a malaria vaccine trial at any time,26 and 27% of participants in an HIV trial in Côte d'Ivoire knew they could withdraw at any time.30 However, in some developing country trials a higher percentage of participants knew they could withdraw or refuse, for example 50% of parents in a paediatric diarrhoeal trial21 knew they could leave the trial at any time, >90% of adults and parents of children in a Malian malaria vaccine trial18 knew they could withdraw from the trial and 88% of Thai vaccine participants knew they could ‘refuse to participate at any time’.35 One study of a South African HIV trial17 reported that 93% of the women knew they had the right to quit, but 98% said they believed the hospital would not allow them to quit.34
Discussion
This is the first comparison of quantitative studies of the quality of informed consent from individuals participating in clinical trials in both developed and developing countries. Our review shows that the assertion that research informed consent is worse in developing countries than in developed countries is an oversimplification of a complex picture of the quality of consent. The quality of informed consent depends on the type and amount of information disclosed, adequate comprehension of trial information, and a voluntary decision to enrol. The existing data, which use comprehension and voluntary decision-making as measures of the quality of consent, do not support a categorical difference between the quality of consent from individuals in developed countries and the quality of consent from individuals in developing countries.
A paucity of data, especially from participants from developing countries, as well as variations in trial type, study methodology, sample size, measures used and timing of data collection relative to obtaining consent, limits comparison and statistical aggregation. Nonetheless, these data suggest certain important trends and point to the need for further research.
Our review highlights the following: (1) comprehension of study information varies among trial participants in both developed and developing countries, and comprehension of randomisation and placebo controlled designs is generally lower than comprehension of other aspects of a trial; (2) research participants report different sources of pressure to enrol, and those in developing countries are less likely than those in developed countries to say they can refuse or withdraw from participation, and more likely to worry about the consequences of refusal or withdrawal.
Data show a range of understanding of trial information in both developed and developing country trials. Individuals across studies tended to know that they were involved in research and often responded correctly to questions about the nature and purpose of the research, yet participants everywhere had more difficulty understanding information about trial design, randomisation and placebo controls. Not only are these methods and concepts unfamiliar to many people, but such methods may be contrary to their expectations or hope for therapeutic benefit, making them more difficult to comprehend. Notably, some studies reveal discrepancies between participants' understanding of what will happen in a trial and how this information will affect them directly. Knowledge of facts and appreciation of those facts are different aspects of understanding, both of which are important to informed consent.65 This discrepancy is a challenge for informed consent everywhere, and although few studies attempted to measure it, the present data do not suggest a difference in appreciation between developed and developing country participants.
Second, the data on refusal and withdrawal indicate a troubling trend. Finding it difficult to refuse participation in or withdraw from a trial, feeling pressure to join or stay enrolled in a trial, or worrying about the consequences of withdrawing all relate to the voluntariness of an enrolment decision. Studies which used these measures of voluntariness show that a disquieting number of participants, and more in developing country trials than developed country trials, do not know or do not believe that they can refuse to participate or can withdraw from research. Few studies probed these responses further to explain why participants felt they could not refuse or withdraw. Possible explanations include deference to authority, cultural norms, or a founded or unfounded fear of not being able to access needed care.
Lastly, while investigations of the impact of pressure on voluntariness were limited, overall few research participants report feeling pressured to participate in research, and those that did often felt pressure from their circumstances–such as worsening illness or fear that care would be withdrawn—more than from other people. Participants in developing countries were more likely to report pressure from fear of the consequences of withdrawing, including decreased access to healthcare. These issues merit further study.
Recommendations for future research
These data reveal that there is much to be done to improve the quality of informed consent in both developed and developing countries and that additional research would facilitate definitive conclusions about the quality of informed consent around the world. Currently available evidence regarding the effectiveness of strategies to improve consent is limited.66–68 Variation in methodology, trial types and populations across studies reviewed raised challenges about how to accurately understand and measure the quality of informed consent. Design and implementation of improvement measures depends on careful attention to, and rigorous delineation of, what the quality of consent entails.
Studies of the quality of informed consent would be greatly enhanced by a core set of validated questions that measure the comprehension and voluntariness of participants at the time of decision-making, and by comparison of participants from similar medically defined groups participating in similar types of research. Studying the quality of consent in multi-national trials, such as was done in one multi-site hypertension study we reviewed,19 would allow for useful comparisons between developed and developing countries. Additionally, more detailed and comprehensive studies of voluntariness are needed, including investigation of sources of pressure to participate and fears about withdrawal or refusal. Future studies should include detailed investigation of associations between cultural norms and attitudes, and socio-demographic characteristics such as education, literacy and socioeconomic status to better understand the impact of these factors on informed consent in both developed and developing countries. Innovative strategies and rigorous studies are sorely needed to facilitate improvement in informed consent to better satisfy one of the fundamental requirements of ethical research.
Acknowledgments
Funding This research was supported by the Department of Bioethics of the NIH Clinical Center.
Footnotes
Contributors AM, CP and BC were responsible for acquisition of data, analysis and interpretation of data, drafting of the manuscript and critical revisions of the manuscript. EE was responsible for the original conception and design of the study and critical revisions of the manuscript. CG was responsible for conception, design and implementation of the study, acquisition of data, analysis and interpretation of data, critical revisions of the manuscript, supervision and final approval.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Angell M. Investigators' responsibilities for human participants in developing countries. N Engl J Med. 2000;342:967–9. doi: 10.1056/NEJM200003303421309. [DOI] [PubMed] [Google Scholar]
- 2.Annas GJ, Grodin MA. Human rights and maternal-fetal HIV transmission prevention trials in Africa. Am J Public Health. 1998;88:560–3. doi: 10.2105/ajph.88.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christakis NA. The ethical design of an AIDS vaccine trial in Africa. Hastings Center Rep. 1988;18:31–7. [PubMed] [Google Scholar]
- 4.Gostin L. Ethical principles for the conduct of human subject research: population-based research and ethics. Law Med Health Care. 1991;19:191–201. doi: 10.1111/j.1748-720x.1991.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 5.LaFraniere S, Flaherty MP, Stephens J. The dilemma: submit or suffer. The Washington Post. 2000:A1. [Google Scholar]
- 6.Levine C. Placebos and HIV: lessons learned. Hastings Center Rep. 1998;28:43–8. [PubMed] [Google Scholar]
- 7.Resnick DB. The ethics of HIV research in developing nations. Bioethics. 1998;12:286–306. doi: 10.1111/1467-8519.00118. [DOI] [PubMed] [Google Scholar]
- 8.Rothman DJ. The Shame of Medical Research. New York Rev Books. 2000;47:60–4. [PubMed] [Google Scholar]
- 9.White MT. Guidelines for IRB review of international collaborative medical research: a proposal. J Law Med Ethics. 1999;27:87–94. doi: 10.1111/j.1748-720x.1999.tb01440.x. [DOI] [PubMed] [Google Scholar]
- 10.Krogstad DJ, Diop S, Diallo A, et al. Informed consent in international research: the Rationale for different approaches. Am J Trop Med Hyg. 2000;83:743–7. doi: 10.4269/ajtmh.2010.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lema VM, Mbondo M, Kamau EM. Informed consent for clinical trials: a review. East Afr Med J. 2009;85:133–42. doi: 10.4314/eamj.v86i3.54968. [DOI] [PubMed] [Google Scholar]
- 12.Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines for Biomedical Research Involving Human Participants. Geneva: CIOMS; 1993. [Google Scholar]
- 13.National Bioethics Advisory Commission (NBAC) Ethical and Policy Issues in International Research: Clinical Trials in Developing Countries. Bethesda: NBAC; 2001. [PubMed] [Google Scholar]
- 14.French H. AIDS research in Africa: juggling risks and hopes. The New York Times. 1997:A1. [PubMed] [Google Scholar]
- 15.Sugarman J, McCrory DC, Powell D, et al. Empirical research on informed consent: an annotated bibliography. Hastings Center Rep. 1999;29:S1–42. [PubMed] [Google Scholar]
- 16.Edwards SJ, Lilford RF, Thornton J, et al. Informed consent for clinical trials: in search of the “best” method. Soc Sci Med. 1998;47:1825–40. doi: 10.1016/s0277-9536(98)00235-4. [DOI] [PubMed] [Google Scholar]
- 17.Verheggen FW, van Wijmen FC. Informed consent in clinical trials. Health Policy. 1996;36:131–53. doi: 10.1016/0168-8510(95)00805-5. [DOI] [PubMed] [Google Scholar]
- 18.Ellis RD, Sagara I, Durbin A, et al. Comparing the understanding of subjects receiving a Candidate malaria vaccine in the United States and Mali. Am J Trop Med. 2010;83:868–72. doi: 10.4269/ajtmh.2010.10-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall PA, Adebamowo CA, Adeyemo AA, et al. Voluntary participation and informed consent to international genetic research. Am J Public Health. 2006;96:1989–95. doi: 10.2105/AJPH.2005.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallely A, Lees S, Shagi C, et al. How informed is consent in vulnerable populations? Experience using a continuous consent process during the MDP301 vaginal microbicide trial in Mwanza, Tanzania. [accessed 3 Jan 2011];BMC Med Ethics. 2010 11:10. doi: 10.1186/1472-6939-11-10. http://www.biomedcentral.com/1472-6939/11/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar R, Grandin EW, Gladstone BP, et al. Comprehension and recall of informed consent among participating families in a Birth Cohort Study on diarrhoeal disease. Public Health Ethics. 2009;2:37–44. [Google Scholar]
- 22.Oduro AR, Aborigo RA, Amugsi D, et al. Understanding and retention of the informed consent process among parents in rural northern Ghana. [accessed 23 Nov 2010];BMC Med Ethics. 2008 9:12. doi: 10.1186/1472-6939-9-12. http://www.biomedcentral.com/1472-6939/9/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill Z, Tawiah-Agyemang C, Odei-Danso S, et al. Informed consent in Ghana: what do participants really understand? J Med Ethics. 2008;34:48–53. doi: 10.1136/jme.2006.019059. [DOI] [PubMed] [Google Scholar]
- 24.Minnies D, Hawkridge T, Hanekom W, et al. Evaluation of the quality of informed consent in a vaccine field trial in a developing country setting. [accessed 23 Nov 2010];BMC Med Ethics. 2008 9:15. doi: 10.1186/1472-6939-9-15. http://www.biomedcentral.com/1472-6939/9/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaewpoonsri N, Okanurak K, Kitayaporn D, et al. Factors related to volunteer comprehension of informed consent for a clinical trial. Southeast Asian J Trop Med Public Health. 2006;37:996–1004. [PubMed] [Google Scholar]
- 26.Krosin MT, Klitzman R, Levin B, et al. Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clin Trials. 2006;3:306–13. doi: 10.1191/1740774506cn150oa. [DOI] [PubMed] [Google Scholar]
- 27.Moodley K, Pather M, Myer L. Informed consent and participant perceptions of influenza vaccine trials in South Africa. J Med Ethics. 2005;3:727–32. doi: 10.1136/jme.2004.009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pace C, Emanuel EJ, Chuenyam T, et al. The quality of informed consent in a clinical research study in Thailand. IRB. 2005;27:9–17. [PubMed] [Google Scholar]
- 29.Pace C, Talisuna A, Wendler D, et al. Quality of parental consent in a Ugandan malaria study. Am J Public Health. 2005;95:1184–9. doi: 10.2105/AJPH.2004.053082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekouevi KD, Becquet R, Viho I, et al. Obtaining informed consent from HIV-infected pregnant women, Abidjan, Cote d'Ivoire. AIDS. 2004;18:1486–8. doi: 10.1097/01.aids.0000131349.22032.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joubert G, Steinberg H, van der Ryst E, et al. Consent for participation in the Bloemfontein vitamin A trial: how informed and voluntary? Am J Public Health. 2003;93:582–4. doi: 10.2105/ajph.93.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynöe N, Hyder Z, Chowdhury M, et al. Obtaining informed consent in Bangladesh. N Engl J Med. 2001;344:460–1. doi: 10.1056/NEJM200102083440617. [DOI] [PubMed] [Google Scholar]
- 33.Leach A, Hilton S, Greenwood BM, et al. An evaluation of the informed consent procedure used during a trial of a Haemophilus influenzae type B conjugate vaccine undertaken in The Gambia, West Africa. Soc Sci Med. 1999;48:139–48. doi: 10.1016/s0277-9536(98)00317-7. [DOI] [PubMed] [Google Scholar]
- 34.Karim QA, Karim SS, Coovadia HM, et al. Informed consent for HIV testing in a South African hospital: is it truly informed and truly voluntary? Am J Pub Health. 1998;488:637–40. doi: 10.2105/ajph.88.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitisuttithum P, Migasena S, Laothai A, et al. Risk behaviours and comprehension among intravenous drug users volunteered for HIV vaccine trial. J Med Assoc Thai. 1997;80:47–50. [PubMed] [Google Scholar]
- 36.Ravina B, Swearingen C, Elm J, et al. Long term understanding of study information in research participants with Parkinson's disease. Parkinsonism Relat Disord. 2010;16:60–3. doi: 10.1016/j.parkreldis.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergenmar M, Molin C, Wilking N, et al. Knowledge and understanding among cancer patients consenting to participate in clinical trials. Eur J Cancer. 2008;44:2627–33. doi: 10.1016/j.ejca.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Knifed E, Lipsman N, Mason W, et al. Patients' perception of the informed consent process for neurooncology clinical trials. Neuro Oncol. 2008;10:348–54. doi: 10.1215/15228517-2008-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal M, Grady C, Fairclough DL, et al. Patients' decision-making process regarding participation in phase 1 oncology research. J Clin Oncol. 2006;24:4479–84. doi: 10.1200/JCO.2006.06.0269. [DOI] [PubMed] [Google Scholar]
- 40.Franck LS, Winter I, Oulton K. The quality of parental consent for research with children: a prospective repeated measure self-report survey. Int J Nurs Stud. 2007;44:525–33. doi: 10.1016/j.ijnurstu.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Gammelgaard A, Mortensen OS, Rossel P. Patients' perceptions of informed consent in acute myocardial infarction research: a questionnaire based survey of the consent process in the DANAMI-2 trial. Heart. 2004;90:1124–8. doi: 10.1136/hrt.2003.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kodish E, Eder M, Noll RB, et al. Communication of randomization in childhood leukemia trials. JAMA. 2004;294:470–5. doi: 10.1001/jama.291.4.470. [DOI] [PubMed] [Google Scholar]
- 43.Lynöe N, Näsström B, Sandlund M. Study of the quality of information given to patients participating in a clinical trial regarding chronic hemodialysis. Scand J Urol Nephrol. 2004;38:517–20. doi: 10.1080/00365590410033362. [DOI] [PubMed] [Google Scholar]
- 44.Criscione LG, Sugarman J, Sanders L, et al. Informed consent in a clinical trial of a novel treatment for rheumatoid arthritis. Arthritis Rheum. 2003;49:361–7. doi: 10.1002/art.11057. [DOI] [PubMed] [Google Scholar]
- 45.Kupst MJ, Patenaude AF, Walco GA, et al. Clinical trials in pediatric cancer: parental Perspectives on informed consent. J Pediatr Hematol Oncol. 2003;25:787–90. doi: 10.1097/00043426-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Pope JE, Tingey DP, Arnold JMO, et al. Are subjects satisfied with the informed consent process? A survey of research participants. J Rheumatol. 2003;30:815–24. [PubMed] [Google Scholar]
- 47.Schats R, Brilstra EH, Rinkel GJ, et al. Informed consent in trials for neurological emergencies: the example of subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2003;74:988–91. doi: 10.1136/jnnp.74.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon C, Zyzanski SJ, Eder M, et al. Groups potentially at risk for making poorly informed decisions about entry into clinical trials for childhood cancer. J Clin Oncol. 2003;21:2173–8. doi: 10.1200/JCO.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Joffe S, Cook EF, Cleary PD, et al. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet. 2001;358:1772–7. doi: 10.1016/S0140-6736(01)06805-2. [DOI] [PubMed] [Google Scholar]
- 50.Daugherty CK, Banik DM, Janish L, et al. Quantitative analysis of ethical issues in phase I trials: a survey interview study of 144 advanced cancer patients. IRB. 2000;22:6–14. [PubMed] [Google Scholar]
- 51.Hietanen P, Aro AR, Holli K, et al. Information and communication in the context of a clinical trial. Eur J Cancer. 2000;36:2096–104. doi: 10.1016/s0959-8049(00)00191-x. [DOI] [PubMed] [Google Scholar]
- 52.Montgomery JE, Sneyd JR. Consent to clinical trials in anaesthesia. Anaesthesia. 1998;53:227–30. doi: 10.1046/j.1365-2044.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- 53.Van Stuijvenberg M, Suur MH, deVos, et al. Informed consent, parental awareness and reasons for participating in a randomized controlled study. Arch Dis Child. 1998;79:120–5. doi: 10.1136/adc.79.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Advisory Committee on Human Radiation Experiments (ACHRE) Final Report. New York: Oxford University Press; 1996. [Google Scholar]
- 55.Harrison K, Vlahov D, Jones K, et al. Medical eligibility, comprehension of the consent process, and retention of injection drug users recruited for an HIV trial. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:386–90. [PubMed] [Google Scholar]
- 56.Harth SC, Thong YH. Parental perceptions and attitudes about informed consent in clinical research involving children. Soc Sci Med. 1995;41:1647–51. doi: 10.1016/0277-9536(95)00058-f. [DOI] [PubMed] [Google Scholar]
- 57.Estey E, Wilkin G, Dossetor J. Are research participants able to retain the information they are given during the consent process. Health Law Rev. 1994;3:37–41. [Google Scholar]
- 58.Miller C, Searight HR, Grable D, et al. Comprehension and recall of the informational content of the informed consent document: an evaluation of 168 patients in a controlled clinical trial. J Clin Res Drug Dev. 1994;8:237–48. [Google Scholar]
- 59.Lynöe N, Sandlund M, Dahlqvist G, et al. Informed consent: study of quality of information given to participants in a clinical trial. BMJ. 1991;202:610–13. doi: 10.1136/bmj.303.6803.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benson PR, Roth LH, Winslade WJ. Informed consent in psychiatric research: preliminary findings from an ongoing investigation. Soc Sci Med. 1985;20:1331–41. doi: 10.1016/0277-9536(85)90388-0. [DOI] [PubMed] [Google Scholar]
- 61.Penman DT, Holland JC, Bahna GF, et al. Informed consent for investigational chemotherapy: patients' and physicians' perceptions. J Clin Oncol. 1984;2:849–55. doi: 10.1200/JCO.1984.2.7.849. [DOI] [PubMed] [Google Scholar]
- 62.Riecken HW, Ravich R. Informed consent to biomedical research in veterans administration hospitals. JAMA. 1982;248:344–8. [PubMed] [Google Scholar]
- 63.Howard JM, DeMets D The BHAT Research Group. How informed is informed consent? The BHAT experience. Control Clin Trials. 1981;2:287–303. doi: 10.1016/0197-2456(81)90019-2. [DOI] [PubMed] [Google Scholar]
- 64.Bergler JH, Pennington AC, Metcalfe M, et al. Informed consent: how much does the patient understand? Clin Pharmacol Ther. 1980;27:435–9. doi: 10.1038/clpt.1980.60. [DOI] [PubMed] [Google Scholar]
- 65.Berg JW, Appelbaum PS, Lidz CW, et al. Informed Consent: Legal Theory and Clinical Practice. New York: Oxford University Press; 2001. pp. 101–2. [Google Scholar]
- 66.Flory J, Emanuel EJ. Interventions to improve research participants' understanding in informed consent for research. JAMA. 2006;292:1593–601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez S, Salazar G, Tijero M, et al. Informed consent procedures: responsibilities of researchers in developing countries. Bioethics. 2001;15:398–412. doi: 10.1111/1467-8519.00250. [DOI] [PubMed] [Google Scholar]
- 68.Benitez O, Devaux D, Dausset J. Audiovisual documentation of oral consent: a new method of informed consent for illiterate populations. Lancet. 2002;359:1406–7. doi: 10.1016/S0140-6736(02)08361-7. [DOI] [PubMed] [Google Scholar]