Abstract
Importance
Preservation of visual acuity in patients with diabetes is critical to preserve VQOL. Interventions to improve glycemic control through early intensive treatment of diabetes reduce rates of severe retinopathy and preserve VA.
Objective
To assess the effect of prior intensive treatment and risk factors on visual quality of life (VQOL) in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) cohort.
Design
Randomized controlled clinical trial followed by an observational follow-up study.
Setting
28 institutions across the United States and Canada.
Participants
1184 DCCT/EDIC participants with type 1 diabetes completed the National Eye Institute (NEI) Visual Functioning Questionnaire (VFQ) during EDIC years 17-20, up to thirty years after the start of the DCCT.
Main Outcome Measures
The 25-item NEI-VFQ was administered. Visual acuity (VA) was measured by the Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol and the presence and severity of retinopathy and macular edema were detected by masked grading of stereoscopic color fundus photographs using the ETDRS retinopathy severity scheme. The composite VQOL and subscales were scored on a scale of 0 to 100 corresponding to poor to excellent function, respectively. Quantile regression was used to assess the treatment/risk factor effect on median QOL score, owing to the ordinal scoring and a skewed distribution.
Results
The overall average VQOL for DCCT/EDIC participants with a 30 year duration of diabetes was high (median 91.7, interquartile (IQR), 89.7-96.9). After adjustment for gender, age, HbA1c, and retinopathy level at DCCT baseline, the former intensive (INT) treatment group had a significant, albeit modest, improvement in overall VQOL compared to the former conventional (CONV) diabetes treatment group (median difference −1.0 [−1.7, −0.3], p=0.0058). This beneficial treatment effect was fully attributed to the prior glycemic control in DCCT (explained treatment effect: 100%). Those with VA worse than 20/100 reported the lowest VQOL score.
Conclusions and Relevance
In the DCCT/EDIC cohort, VQOL remains high in both treatment groups. Intensive diabetes therapy modestly improved VQOL 30 years after the start of the DCCT. VA had the greatest impact on VQOL from among all risk factors.
The National Eye Institute Visual Function Questionnaire (NEI-VFQ) has been used to assess the relationship of diabetic retinopathy severity and visual acuity with visual quality of life (VQOL).1-12 Data from previous studies have shown that severe retinopathy and poorer visual acuity adversely impact VQOL and that interventions which improve visual acuity, such as vitrectomy and laser photocoagulation, have a beneficial impact on VQOL as measured by the NEI-VFQ. The long-term impact of intensive glycemic control on the VQOL in a controlled clinical trial in type 1 diabetes mellitus has not been examined. In the Diabetes Control and Complications Trial (DCCT), intensive (INT) treatment of type 1 diabetes reduced the risk of development and progression of diabetic retinopathy compared to conventional (CONV) diabetes treatment. The salutary effects of INT vs. CONV were maintained during the Epidemiology of Diabetes Interventions and Complications (EDIC) observational follow-up of the DCCT cohort. 13, 14 The purpose of this report is to assess the long-term effects of prior INT treatment and risk factors on VQOL, using the NEI-VFQ, 30 years after the start of the DCCT.
METHODS
The DCCT/EDIC has been described in detail in previous reports. 15, 16 In brief, between 1983 and 1989, 1441 participants with type 1 diabetes, ages 13-39 years, were enrolled in the DCCT, a multicenter clinical trial comparing the effects of INT, aimed at lowering glycemia as close to the non-diabetic range as safely possible, with those of CONV. INT, which aimed for HbA1c levels <6.05%, used three or more daily insulin injections or treatment with insulin pumps, with dose selection guided by frequent self-monitoring of blood glucose. CONV had no numeric blood glucose targets, but aimed for the absence of symptoms of hyperglycemia and hypoglycemia with one or two daily injections of insulin, the standard of therapy at the time. The trial included two cohorts. The primary prevention cohort had 1 to 5 years of diabetes duration, albumin excretion rate (AER) < 40 mg/24 hr, and no retinopathy. The secondary intervention cohort had diabetes for 1 to 15 years, very mild to moderate non-proliferative retinopathy, and AER ≤ 200 mg/24 hr. After study end, the conventionally treated participants were instructed in INT and all patients were encouraged to implement and instructed in the use of intensive treatment. All participants were then referred to their health care providers for ongoing diabetes care.16 In 1994, 1375 (96%) of the 1428 surviving cohort agreed to participate in the EDIC follow-up study which included annual examinations and periodic evaluation of diabetic complications. To assess the long-term effect of prior intensive treatment and risk factors on visual quality of life (VQOL) in this cohort, 1184 EDIC participants completed the National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) during EDIC years 17-20, a maximum of 30 years after the start of the DCCT.
VQOL Assessment
Beginning in 2004, EDIC administered the NEI-VFQ-25 questionnaire among one quarter of the cohort every year. The NEI-VFQ-25 consists of a base set of 25 vision-targeted questions representing 11 vision-related domains including general vision, ocular pain, near vision, distance vision, limitation on social functioning, mental health symptoms due to vision, role difficulties, dependency on others, driving difficulty, limitation with color vision and peripheral vision, plus an additional single-item general health domain question. Subscale scores ranging from 0 to 100 (with 100 indicating highest function) were generated for each of the 12 domains. The main outcome in our analysis is the composite VQOL, which is an average of the 11 vision-related subscale scores. A composite QOL score was also examined which is comprised of all of the 12 subscales including general health.
Visual Acuity
Visual acuity (VA) measurement was administered by certified EDIC VA examiners every 4 years in EDIC based on the Early Treatment Diabetic Retinopathy Study (ETDRS) charts and procedures. VA was tested first at the 4-meter distance. If the number of letters read correctly at 4 meters was less than twenty, the test was repeated at 1 meter. If the number of letters read correctly at 1 meter was zero, then the patient's ability to count fingers, detect hand motion, or have light perception was evaluated. For each eye, the best-corrected VA was recorded as the number of letters read correctly from 0-2 (Worse than 20/800), to 98-100 (20/10).17
Retinopathy and Ocular Surgeries
During EDIC, retinopathy was assessed by standardized seven-field fundus photography in one quarter of the cohort each year and in the entire cohort at EDIC years 4 and 10. All photographs were graded centrally, with graders masked to the former DCCT therapy assignment, using the final ETDRS grading scale and DCCT methods. 13, 18 Retinopathy level was classified as no retinopathy (10/10), microaneurysms only (20/< 20), mild non-proliferative diabetic retinopathy (NPDR) (35/< 35), moderate NPDR (43/<43-47/47), severe NPDR (53/< 53), and proliferative diabetic retinopathy (PDR) (61/<60 or greater).
Clinically significant macular edema was based on the detailed grading of fundus photos and was defined as the presence of any one of the following: retinal thickening at or within 500 μm of the center of the macula; and/or hard exudates at or within 500 μm of the center of the macula if associated with thickening of the adjacent retina; and/or a zone or zones of retinal thickening 1 disc area in size, at least part of which was within 1 disc diameter of the center.19
Pan-retinal and focal photocoagulation was assessed by patient annual report and confirmed by grading of photocoagulation scars in fundus photographs. Ocular surgeries, including cataract extraction, vitrectomy, glaucoma-related surgeries, corneal-related surgeries, capsulotomy, and eye enucleation, were reported annually in DCCT and EDIC.
Biomedical and Clinical Evaluations
Demographics, marital, education, unemployment status, and history of smoking were assessed by annual questionnaires. Blood pressure and hemoglobin A1c (HbA1c) were measured quarterly during DCCT and annually during EDIC.20 AER and plasma lipid concentrations were measured yearly during the DCCT and every 2 years during the EDIC study.21 Serum creatinine was measured annually in DCCT/EDIC. Estimated GFR (eGFR) was calculated from serum creatinine, age, sex and race using the CKD-EPI equation. 22
Nephropathy outcomes reported in the current analysis are a single or sustained eGFR < 60mL/min/1.73m2, AER>300 mg/24 hr, or a sustained AER>40 mg/24 on two consecutive visits. 22 Clinical neurologic assessment, nerve conduction study, and cardiac autonomic neuropathy testing were conducted at EDIC years 13 and 14. 23, 24 Cardiac autonomic neuropathy testing was repeated at EDIC years 16 and 17. Confirmed clinical neuropathy was defined as the presence of both definite clinical neuropathy (the presence of signs and symptoms consistent with distal symmetrical polyneuropathy based on examination by a board-certified neurologist) and confirmed by abnormal nerve conduction (one or more abnormal attributes in at least two anatomically distinct nerves among the sural, peroneal or median nerves). 24 Cardiac autonomic neuropathy was defined as either a R-R variation <15 or an R-R variation between 15 and 19.9 in combination with a Valsalva ratio ≤ 1.5 or a decrease of >10 mm Hg in diastolic blood pressure upon postural testing.23
Diabetes QOL
The diabetes QOL (DQOL) was administered annually in DCCT and at every other year during EDIC. DQOL is a self-administered multiple-choice 46-item questionnaire assessing different aspects of QOL including satisfaction, impact, diabetes worry and social/vocational worry. 25
Psychiatric Events
Psychiatric history was reported annually during EDIC. Presence of a psychiatric event was defined as at least one occurrence of nervousness or anxiety, affective disorder, or suicide attempt, with inpatient or outpatient treatment for the event during the year in which it was reported.
Statistical Analysis
Clinical characteristics were compared using Wilcoxon rank-sum test for quantitative or ordinal variables and Chi-square test for categorical variables. The composite VQOL or QOL scale used in the analyses was a weighted average of the subscales with an equal weight assigned to each of the 11 (excluding general health) or 12 (including general health) subscales rather than to each of the 25 questions. Internal consistency reliability among the VQOL subscales was assessed with Cronbach's alpha.26 Spearman correlation was employed to evaluate the strength of the association among the VQOL subscales, and of VQOL with each risk factor.
Between-group comparisons in composite and subscales were conducted by Wilcoxon rank-sum test. For subscale comparisons, to adjust for multiple tests, the Benjamini and Hochberg method was employed to control the false discovery rate at the 0.05 level.27 Owing to the ordinal scoring and a skewed distribution of the VQOL scores, quantile regression was employed to assess the effect of former DCCT treatment groups and risk factors on median VQOL composite score.28, 29 Robust confidence intervals and p-values were generated with Huber sandwich estimates to incorporate any not identically or independently distributed data.30 The proportion of the treatment group effect explained by each covariate was calculated as the percent reduction in the magnitude of the t value for the treatment group effect before and after adjustment for the covariate.
RESULTS
Clinical Characteristics
The characteristics of the 1184 DCCT/EDIC participants who completed the NEI-VFQ-25 questionnaire in EDIC years 17-20 are described in Table 1, by original DCCT treatment group. At DCCT entry, there was a marginally significant difference between treatment groups in age. During the DCCT and by study design, the INT group had a significantly lower mean HbA1c level than the CONV group (7.2% vs. 9.0%, p<0.0001). During EDIC, the mean HbA1c for both INT and CONV converged (approximately 8.0% both groups, not significant). The overall DCCT/EDIC updated mean HbA1c remained statistically lower in the INT group (7.8% vs. 8.2%, p<0.0001).
Table 1.
Clinical Characteristics of the 1184 Participants with Visual QOL Evaluation during EDIC Years 17-20
| DCCT Baseline (1983-1989) | EDIC Years 17-20 (2011-2014) | ||||
|---|---|---|---|---|---|
| INT(n=605) | CONV(n=579) | INT(n=605) | CONV(n=579) | P value | |
| Age (yr) | 27.5 (7.1) | 26.7 (7.0) | 52.8 (6.9) | 51.8 (6.9) | 0.0236 |
| Female (%) | 49.8 | 46.1 | 49.8 | 46.1 | 0.2104 |
| Primary Prevention Cohort (%) | 47.4 | 50.6 | 47.4 | 50.6 | 0.2759 |
| Duration of Diabetes (yr) | 6.1 (4.3) | 5.7 (4.1) | 30.5 (5.0) | 29.9 (5.0) | 0.0725 |
| Unemployed/Retired (%) | 1.3 | 0.5 | 14.4 | 11.6 | 0.1510 |
| Married (%) | 49.3 | 51.1 | 72.7 | 73.1 | 0.8985 |
| College or Above Education (%) | 73.6 | 73.1 | 90.1 | 90.2 | 0.9665 |
| Smoking (%) | 19.2 | 18.7 | 10.9 | 11.2 | 0.8619 |
| Mean Arterial Pressure (mm Hg) * | 86.0 (8.8) | 86.8 (8.7) | 87.6 (9.9) | 87.1 (9.3) | 0.3567 |
| Hypertension (%) † | 3.3 | 3.1 | 65.0 | 69.1 | 0.1313 |
| Diabetes QOL ‡ | 1.9 (0.3) | 1.9 (0.3) | 75.1 (10.8) | 74.5 (10.4) | 0.2333 |
| Depression or Psychiatric Event (%) § | - | - | 28.2 | 27.6 | 0.8444 |
| Eye Complications: | |||||
| Retinopathy (%) | <0.0001 | ||||
| No Retinopathy (10/10) | 47.5 | 50.6 | 10.7 | 5.2 | |
| MA Only (20/(<)20) | 35.8 | 29.5 | 38.7 | 26.1 | |
| Mild NPDR (35/(<)35) | 12.3 | 14.9 | 22.0 | 24.2 | |
| Moderate or Severe NPDR (43/<44 +) | 4.5 | 5.0 | 17.9 | 19.7 | |
| PDR or Worse (61/<61 +) | 0 | 0 | 10.7 | 24.9 | |
| CSME (%) | 0 | 0 | 16.4 | 25.2 | 0.0002 |
| Visual Acuity in the Worse Eye (%) | 0.0483 | ||||
| ≤20/20 | 85.1 | 85.2 | 60.3 | 53.9 | |
| >20/20 - < 20/40 | 14.9 | 14.9 | 34.7 | 38.5 | |
| 20/40 - < 20/100 | 0 | 0 | 3.0 | 5.5 | |
| ≥20/100 | 0 | 0 | 2.0 | 2.1 | |
| Visual Acuity in the Better Eye (%) | 0.0490 | ||||
| ≤20/20 | 95.9 | 96.7 | 81.3 | 76.2 | |
| >20/20 - < 20/40 | 4.1 | 3.3 | 17.9 | 21.9 | |
| 20/40 - < 20/100 | 0 | 0 | 0.5 | 1.7 | |
| ≥20/100 | 0 | 0 | 0.3 | 0.2 | |
| Any Prior Ocular Surgeries in DCCT/EDIC (%) ∥ | 0 | 0 | 8.6 | 14.9 | 0.0008 |
| Renal Complications: | |||||
| Any AER>300 ug/24hr or Sustained CKD GFR<60 mL/min/1.73 m (%) | 0 | 0 | 8.4 | 16.6 | <0.0001 |
| Any Sustained AER>30 ug/24hr or CKD GFR<60 mL/min/1.73 m (%) | 5.3 | 3.5 | 27.3 | 36.8 | 0.0004 |
| Neuropathy Complications: | |||||
| Abnormal Autonomic Response (%) | 3.8 | 5.4 | 35.3 | 40.2 | 0.0872 |
| Confirmed Clinical Neuropathy (%) | 7.0 | 5.4 | 23.6 | 32.8 | 0.0004 |
| Glycemic Control: | |||||
| HbA1c (%) | 9.0 (1.6) | 8.9 (1.6) | 8.0 (1.2) | 7.9 (1.2) | 0.3345 |
| DCCT Mean HbA1c (%) ** | - | - | 7.2 (0.8) | 9.0 (1.2) | <0.0001 |
| EDIC Mean HbA1c (%) ** | - | - | 8.0 (1.0) | 8.0 (1.0) | 0.5891 |
Data are means (standard deviation) or percent. There were not significant treatment group differences (p<0.05) at DCCT baseline with the exception of a marginally significant difference in age.
Mean arterial pressure was defined as 1/3 systolic blood pressure + 2/3 diastolic blood pressure.
Hypertension was defined by a systolic blood pressure of at least 140 mmHg, a diastolic blood pressure of at least 90 mmHg, documented hypertension, or the use of antihypertensive agents. Medication data was not collected during the DCCT.
Diabetes QOL is a self-administered multiple-choice 46-item assessment assessing different aspects of quality of life employed by DCCT.
Depression or psychiatric event is defined as at least one occurrence of nervousness or anxiety, affective disorder, suicide attempt in EDIC accompanied by hospitalization or out-patient treatment including tranquilizers.
Other ocular surgery includes glaucoma-related surgeries, corneal-related surgeries, YAG capsulotomy, and eye enucleation.
DCCT or EDIC mean hemoglobin HbA1c values are time-averaged throughout the DCCT or EDIC.
- Data not available or not applicable.
By EDIC years 17-20, the original DCCT INT group had significantly less overall retinopathy severity (p<0.0001), a lower prevalence of CSME (INT: 16.4% vs. CONV: 25.2%, p=0.0002), better visual acuity level in the better and worse eye (p=0.05), and a decreased incidence of ocular surgeries (8.6% vs. 14.9%, p=0.0008), compared to the CONV group. (31) The INT group also demonstrated a significantly lower incidence of renal complications in DCCT/EDIC including AER>300 mg/24 hr or sustained eGFR<60 (8.4% vs. 16.6%, p<06.0001) and sustained AER>30 mg/24 hr or single eGFR<60 (27.3% vs 36.8%, p=0.0004), as well as a significantly lower prevalence of confirmed clinical neuropathy (23.6% vs. 32.8%, p=0.0004). Notably, after 30 years, the diabetes-related QOL and the number of psychiatric events were similar between the two treatment groups.
Comparing clinical characteristics from DCCT baseline, those who did not complete the NEIVFQ-25 part of the examination (n=257, including 99 who were deceased) were more likely to be smokers, have poor visual acuity, and worse glycemic control than those who did participate (eTable 1 in the Supplement). Participants and nonparticipants in the NEI-VFQ-25 did not differ in retinopathy status.
Effect of Intensive Diabetes Management on VQOL
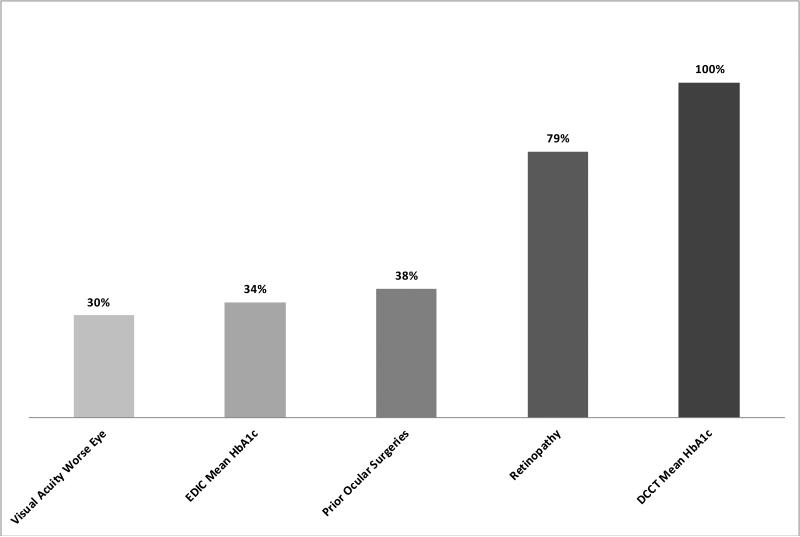
The distributions of the NEI-VFQ-25 and its subscales are presented in Table 2. The overall VQOL in both treatment groups was high (INT: median 94.7, IQR, 91.0-97.2; CONV: 94.0, IQR, 88.4-96.9, p=0.0060). Few participants had scale scores at or near 0, while a sizable proportion had scale scores of 100. Subscale scores for general health and general vision were lowest (i.e., median score ≤ 80). The INT group had significantly higher subscale scores in the visual health domains of difficulty with distance activities, mental health symptoms due to vision, and driving difficulty (p<0.01). Multivariate analyses of treatment group effect on VQOL (not including general health) after adjustment for age, gender, HbA1c at DCCT screening, and retinopathy level at DCCT baseline, demonstrated a modest, yet statistically significant, lower VQOL in the CONV group compared to the INT group [Table 3, −1.0 (−1.7, −0.3, p=0.0058]. These differences, while statistically significant, were not in the range usually considered clinically meaningful.32-35 The treatment group effect on VQOL was largely attributed to the higher DCCT mean HbA1c and more rapid progression of retinopathy in CONV (Figure 1, explained treatment group effect 100% and 79%, respectively).
Table 2.
Visual Quality of Life (NEI-VFQ 25) during EDIC Years 17-20
| Domain | Subscale | INT(n=605) | CONV(n=579) | P value * | ||
|---|---|---|---|---|---|---|
| Mean (std) | Median (Q1,Q3) | Mean (std) | Median (Q1,Q3) | |||
| 1 | General Health | 62.5 (22.3) | 75.0 (50.0, 75.0) | 60.4 (23.4) | 50.0 (50.0, 75.0) | 0.2161 |
| 2 | General Vision | 79.7 (13.9) | 80.0 (80.0, 80.0) | 78.0 (15.0) | 80.0 (80.0, 80.0) | 0.0531 |
| 3 | Ocular Pain | 92.6 (12.4) | 100.0 (87.5, 100.0) | 92.4 (12.3) | 100.0 (87.5, 100.0) | 0.6140 |
| 4 | Difficulty with Near Activities | 86.7 (14.3) | 91.7 (83.3, 100.0) | 85.1 (16.0) | 91.7 (75.0, 100.0) | 0.2286 |
| 5 | Difficulty with Distance Activities | 92.6 (11.3) | 100.0 (91.7, 100.0) | 89.5 (14.9) | 91.7 (83.3, 100.0) | 0.0013† |
| 6 | Limitation on Social Functioning | 98.6 (5.8) | 100.0 (100.0, 100.0) | 97.6 (9.0) | 100.0 (100.0, 100.0) | 0.0252‡ |
| 7 | Mental Health Symptoms due to Vision | 90.1 (12.2) | 93.8 (87.5, 100.0) | 88.0 (13.6) | 93.8 (87.5, 93.8) | 0.0007† |
| 8 | Role Difficulties due to Vision | 94.0 (14.0) | 100.0 (100.0, 100.0) | 92.7 (15.0) | 100.0 (87.5, 100.0) | 0.0410‡ |
| 9 | Dependency on Others due to Vision | 98.0 (8.1) | 100.0 (100.0, 100.0) | 97.1 (11.1) | 100.0 (100.0, 100.0) | 0.3089 |
| 10 | Driving Difficulty | 91.7 (11.3) | 91.7 (83.3, 100.0) | 89.1 (13.3) | 91.7 (83.3, 100.0) | 0.0004† |
| 11 | Limitation with Color Vision | 98.2 (7.2) | 100.0 (100.0, 100.0) | 97.3 (9.8) | 100.0 (100.0, 100.0) | 0.1019 |
| 12 | Limitation with Peripheral Vision | 95.3 (12.7) | 100.0 (100.0, 100.0) | 93.6 (16.4) | 100.0 (100.0, 100.0) | 0.2184 |
| Composite I | Overall QOL (Subscale 1-12) | 90.0 (8.2) | 92.1 (87.7, 94.8) | 88.4 (10.1) | 91.0 (85.6, 94.5) | 0.0052 |
| Composite II | Overall Visual QOL (Subscale 2-12) | 92.5 (7.9) | 94.7 (91.0, 97.2) | 90.9 (10.0) | 94.0 (88.4, 96.9) | 0.0060 |
Data are means (standard deviations).
P values are from between-group comparisons using Wilcoxon Rank-Sum test are reported. For subscales comparisons, Benjamini and Hochberg (1995)'s false discovery rate (FDR) alpha adjustment for multiple tests is also conducted to control the overall FDR at the 0.05 level.
Subscales 5, 7, and 10 remain significant after Benjamini and Hochberg's FDR alpha adjustment, P values are 0.0018 and 0.0056 and 0.0012.
Subscale 6 and 8 lost significance after Benjamini and Hochberg's FDR alpha adjustment, P>0.05
Table 3.
DCCT Treatment Effect on Overall Visual QOL using Quantile Regression
| Estimated Difference In Median | t value | P-value | |
|---|---|---|---|
| Treatment: Conventional vs. Intensive | −1.0 (−1.7, −0.3) | −2.76 | 0.0058 |
| Gender: Female vs. Male | −1.3 (−2.0, −0.6) | −3.57 | 0.0004 |
| Age at DCCT baseline: Per 10 year increase | −0.9 (−1.3, −0.4) | −3.49 | 0.0005 |
| HbA1c at DCCT Eligibility Per 10% increase | −0.3 (−0.5, −0.1) | −2.97 | 0.0030 |
| Retinopathy at DCCT baseline: | |||
| ETDRS 20/(<)20 vs. 10/10 | −0.2 (−1.1, 0.6) | −0.65 | 0.5834 |
| ETDRS 35/(<)35 vs. 10/10 | −0.3 (−1.4, 0.8) | −0.85 | 0.5710 |
| ETDRS 43/(<)43 vs. 10/10 | −2.4 (−5.6, 0.7) | −1.46 | 0.1260 |
Figure 1.
Proportion of the effect of DCCT treatment assignment on VQOL attributed to various risk factors at EDIC Years 17-20. Proportion is calculated as the percent reduction in the magnitude of the t value for treatment group (Table 3) before and after adjustment for each risk factor separately. Risk factors explaining ≥30% of the treatment group effect are presented.
All multi-item subscales demonstrated a moderately high internal consistency (Cronbach's alpha, 0.62-0.87) (eTable 2 in the Supplement), similar to those reported in other studies.1, 7, 8 The Spearman correlation among the 11 visual-related subscales ranges from 0.13 (between general health and limitation with color vision) to 0.58 (between difficulty with distance and driving difficult) (eTable 3 in the Supplement).
Risk Factors Impacting VQOL
Among participants in both treatment groups combined, univariate analysis revealed that the overall VQOL score was most strongly associated with the following risk factors (eTable 4 in the Supplement): DQOL(r=0.43), AER (−0.41), VA in the worse eye (−0.31), VA in the better eye (−0.28), DCCT/EDIC HbA1c (−0.26), severity of retinopathy (−0.24), EDIC mean HbA1c (−0.24), and ocular surgeries (−0.23) (each p<0.0001). Particularly, those with VA worse than 20/100 reported the lowest VQOL, further supporting the validity of the measure. VQOL fell to a median of 81 when VA was poorer than 20/100 in the worse eye and further declined to 49 if the better eye was similarly impaired.
In multivariate risk factor analyses (Table 4), gender, depression or psychiatric events in EDIC, CSME, reduced VA, prior ocular surgery, and higher mean HbA1c in DCCT/EDIC were associated with significantly lower VQOL scores, when adjusted for all other risk factors (p<0.05). Those with VA poorer than 20/100 in the worse eye had a 21 point lower median VQOL score compared to those with VA 20/20 or better.
Table 4.
Multivariate Risk Factor Effect on Visual QOL using Quantile Regression
| Risk Factor at EDIC Years 17-20 | Es timated Difference In Median | t value | P-value |
|---|---|---|---|
| Age at DCCT baseline: per 10 year increase | −0.4 (−1.0, 0.1) | −1.94 | 0.0527 |
| Female vs. Male | −0.9 (−1.6, −0.3) | −2.84 | 0.0046 |
| Unemployed or Retired vs. Employed | −0.1 (−1.5, 1.2) | −0.21 | 0.8300 |
| Married vs. Not Married | 0.01 (−0.7, 0.7) | 0.03 | 0.9760 |
| College or Above vs. Secondary School or Below | 0.2 (−0.6, 1.0) | 0.48 | 0.6302 |
| Hypertension: yes vs. no | −0.3 (−0.9, 0.4) | −0.83 | 0.4080 |
| Depression or Psychiatric Events in EDIC: yes vs. no | −1.3 (−2.0, −0.6) | −3.76 | 0.0002 |
| Retinopathy: ETDRS 43/(<)43 or above vs. below 43/(<)43 | 0.4 (−0.3, 1.1) | 1.19 | 0.2362 |
| CSME: yes vs. no | −1.1 (−2.2, −0.1) | −1.98 | 0.0474 |
| Visual Acuity in the Worse Eye: | |||
| >20/20 - < 20/40 vs. ≤20/20 | −1.9 (−2.8, −1.1) | −4.64 | <0.0001 |
| 20/40 - < 20/100 vs. ≤20/20 | −4.1 (−6.2, −2.0) | −3.85 | 0.0001 |
| ≥20/100 vs. ≤20/20 | −21.0 (−40.5, −1.6) | −2.13 | 0.0336 |
| Prior Ocular Surgeries vs. No Ocular Surgeries | −2.1 (−3.1, −1.1) | −4.19 | <0.0001 |
| Any AER>300 or Sustained CKD GFR<60 vs. Never | −1.1 (−3.0, 0.7) | −1.21 | 0.2266 |
| Cardiac Autonomic Neuropathy: yes vs. no | −0.6 (−1.5, 0.2) | −1.40 | 0.1631 |
| Confirmed Clinical Neuropathy at EDIC Years 13/14: yes vs. no | −0.9 (−1.9, 0.1) | −1.87 | 0.0616 |
| HbA1c at DCCT Eligibility: per 10% increase | 0.04 (−0.2, 0.2) | 0.42 | 0.6740 |
| DCCT-EDIC Weighted Mean HbA1c: per 10% increase | −0.7 (−1.0, −0.4) | −4.66 | <0.0001 |
DISCUSSION
The NEI-VFQ-25 has been shown to be a reliable and valid questionnaire for patients with five chronic eye conditions or low vision from any cause.36 The data presented herein extend these findings to the DCCT/EDIC cohort of persons with long-term type 1 diabetes. Remarkably, after an average duration of diabetes of 30 years, the overall VQOL among all questionnaire participants is very high, with a median composite score of 91.7 at EDIC years 17-20, almost certainly reflecting the modest degree of eye disease in the DCCT/EDIC cohort. Notably, although both former treatment groups reported relatively high VQOL levels, intensive management of diabetes during the DCCT still resulted in a statistically significant higher VQOL composite score, up to 30 years after the start of the DCCT. This is the first report of a difference (albeit only ~1.0 point on average on a median score of ~92, Table 3) in VQOL (not including general health) in conventional compared with intensive diabetes management.
Despite differences in incidence of ocular and systemic complications between the INT and CONV treatment groups, the difference in the scores for the VQOL, 1.0, is considered not clinically meaningful. A 5-point change in NEI-VFQ score is thought to represent a clinically meaningful change with respect to VA. 32-35 The difference might have been higher had we included the non-participants who had higher HbA1c levels upon entry and throughout the DCCT and more renal and neurological complications related to their diabetes. These were all factors associated with a decline in VQOL based upon our analyses. Together with the tendency for more non-responders to be in the CONV group than INT group (56% vs. 44%) suggests a selection bias to our cross-sectional analysis. This may have influenced our modest treatment group effect and resulted in an underestimation of the beneficial effect of intensive diabetes management on VQOL.
Another explanation for the relatively high VQOL scores in both the CONV and INT groups was the preservation of good visual acuity in both groups (81.3% of INT subjects and 76.2% of CONV subjects had VA of 20/20 or better in the better eye), despite differences in the presence of severe retinopathy between the groups. Projecting forward, the 30-50% increases in severe eye disease and macular edema in the CONV group are likely to progress over time, adversely affect VA, and, thus, more substantially impact the VQOL score in the CONV group. Supporting this premise, we reported the rise in ocular surgeries in the CONV compared to INT subjects, which were principally complication-related surgeries largely performed to improve VA. (31) In the end, the success of these surgeries in restoring VA may also help in sustaining high VQOL scores.
Preserving VA over time in patients with diabetes remains critical. Analysis of the VQOL subscales demonstrates a consistent trend in the CONV group: more difficulty with distance activities, such as driving, was reported by the CONV group. Over time, visual impairment and limitations in driving in the ageing population can induce feelings of depression and anxiety, a subscale also found to be statistically lower in the CONV group compared to the INT group.
Not surprisingly, multivariate analysis demonstrated that other known diabetes-related outcomes, such as the presence of clinically-significant renal or neurologic disease, and diabetes duration were independently correlated to VQOL. It is these latter diabetes-related factors, reflecting longer duration of poor control in the CONV group and impacting DQOL, and not hypoglycemia, that likely mitigate the modest association of low DQOL with VQOL.
A limitation of this study is the lack of baseline VQOL data at DCCT entry. However, given that the DCCT was a well-designed randomized clinical trial, and retinopathy, VA and all the other major risk factors were well-balanced between the two treatment groups at baseline, we believe that the baseline VQOL should also be balanced between the two groups, and therefore should not substantively bias our study conclusions.
In summary, our findings show that in the EDIC cohort the VQOL score remains high in both treatment groups, with only a modest benefit accruing to the INT group. This may reflect, in part, the relatively good VA in this cohort, the factor with greatest impact on VQOL from among all risk factors.
Supplementary Material
ACKNOWLEDGEMENTS
A complete list of participants in the DCCT/EDIC Research Group is presented in the Supplementary Material published online for the article in N Engl J Med 2015;372:1722-33.
Funding/Support: The DCCT/EDIC has been supported by cooperative agreement grants (1982-1993, 2012-2017), and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA.
Footnotes
Authors’ Contributions: Dr. Rose A. Gubitosi-Klug wrote the manuscript. Drs. Wanjie Sun and Barbara H. Braffett researched the data, preformed analysis, and contributed to writing the manuscript. Patricia A. Cleary and Drs. Lloyd Paul Aiello, Arup Das, William Tamborlane, and Ronald Klein researched the data and contributed to writing the manuscript.
Industry Contributions: Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA) , Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN) , and Sanofi-Aventis (Bridgewater NJ).
Trial Registration: clinicaltrials.gov NCT00360815 and NCT00360893.
Access to Data: Dr. Rose A. Gubitosi-Klug had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest and Financial Disclosures: Rose A. Gubitosi-Klug, Wanjie Sun, Patricia A. Cleary, Barbara H. Braffett, Lloyd Paul Aiello, Ronald Klein have no potential conflicts of interest to disclose. Arup Das is a consultant for Regeneron and TEVA, a speaker for Novartis, and has research support from Genentech, National Eye Institute, and the Department of Veteran Affairs. William Tamborlane is a consultant for Novo Nordisk, Sanofi, and Medtronic.
REFERENCES
- 1.Broman AT, Munoz B, Rodriguez J, Sanchez R, Quigley HA, Klein R, et al. The impact of visual impairment and eye disease on vision-related quality of life in a Mexican-American population: proyecto VER. Invest Ophthalmol Vis Sci. 2002;43:3393–8. [PubMed] [Google Scholar]
- 2.Broman AT, Munoz B, West SK, Rodriguez J, Sanchez R, Snyder R, et al. Psychometric properties of the 25-item NEI-VFQ in a Hispanic population: Proyecto VER. Invest Ophthalmol Vis Sci. 2001;42:606–13. [PubMed] [Google Scholar]
- 3.Globe DR, Varma R, Torres M, Wu J, Klein R, Azen SP. Self-reported comorbidities and visual function in a population-based study: the Los Angeles Latino Eye Study. Arch Ophthalmol. 2005;123:815–21. doi: 10.1001/archopht.123.6.815. [DOI] [PubMed] [Google Scholar]
- 4.Hariprasad SM, Mieler WF, Grassi M, Green JL, Jager RD, Miller L. Vision-related quality of life in patients with diabetic macular oedema. Br J Ophthalmol. 2008;92:89–92. doi: 10.1136/bjo.2007.122416. [DOI] [PubMed] [Google Scholar]
- 5.Hirai FE, Tielsch JM, Klein BE, Klein R. Ten-year change in vision-related quality of life in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 2011;118:353–8. doi: 10.1016/j.ophtha.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein R, Moss SE, Klein BE, Gutierrez P, Mangione CM. The NEI-VFQ-25 in people with long-term type 1 diabetes mellitus: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2001;119:733–40. doi: 10.1001/archopht.119.5.733. [DOI] [PubMed] [Google Scholar]
- 7.Mazhar K, Varma R, Choudhury F, McKean-Cowdin R, Shtir CJ, Azen SP. Severity of diabetic retinopathy and health-related quality of life: the Los Angeles Latino Eye Study. Ophthalmology. 2011;118:649–55. doi: 10.1016/j.ophtha.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKean-Cowdin R, Varma R, Hays RD, Wu J, Choudhury F, Azen SP. Longitudinal changes in visual acuity and health-related quality of life: the Los Angeles Latino Eye study. Ophthalmology. 2010;117:1900–7. 7 e1. doi: 10.1016/j.ophtha.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heintz E, Wirehn AB, Peebo BB, Rosenqvist U, Levin LA. QALY weights for diabetic retinopathy--a comparison of health state valuations with HUI-3, EQ-5D, EQ-VAS, and TTO. Value Health. 2012;15:475–84. doi: 10.1016/j.jval.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Cusick M, SanGiovanni JP, Chew EY, Csaky KG, Hall-Shimel K, Reed GF, et al. Central visual function and the NEI-VFQ-25 near and distance activities subscale scores in people with type 1 and 2 diabetes. Am J Ophthalmol. 2005;139:1042–50. doi: 10.1016/j.ajo.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Tranos PG, Topouzis F, Stangos NT, Dimitrakos S, Economidis P, Harris M, et al. Effect of laser photocoagulation treatment for diabetic macular oedema on patient's vision-related quality of life. Curr Eye Res. 2004;29:41–9. doi: 10.1080/02713680490513191. [DOI] [PubMed] [Google Scholar]
- 12.Matza LS, Rousculp MD, Malley K, Boye KS, Oglesby A. The longitudinal link between visual acuity and health-related quality of life in patients with diabetic retinopathy. Health Qual Life Outcomes. 2008;6:95. doi: 10.1186/1477-7525-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetes Control and Complications Trial Research Group Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Ophthalmology. 1995;102:647–61. doi: 10.1016/s0161-6420(95)30973-6. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Control and Complications Trial Research Group The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 15.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 16.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein R, Klein BE, Moss SE. Visual impairment in diabetes. Ophthalmology. 1984;91:1–9. [PubMed] [Google Scholar]
- 18.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 19.Early Treatment Diabetic Retinopathy Study research group Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 20.Steffes M, Cleary P, Goldstein D, Little R, Wiedmeyer HM, Rohlfing C, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem. 2005;51:753–8. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–67. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–76. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009;119:2886–93. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–6. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Influence of intensive diabetes treatment on quality-of-life outcomes in the diabetes control and complications trial. Diabetes Care. 1996;19:195–203. doi: 10.2337/diacare.19.3.195. [DOI] [PubMed] [Google Scholar]
- 26.Cronbach LJ, Warrington WG. Time-limit tests: estimating their reliability and degree of speeding. Psychometrika. 1951;16:167–88. doi: 10.1007/BF02289113. [DOI] [PubMed] [Google Scholar]
- 27.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–8. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 28.Bassett GWaK R. “An Empirical Quantile Function for Linear Modela with iid Errors”. Journal of the American Statistical Association. 1982:401–15. [Google Scholar]
- 29.Koenker RaB GW. Regression Quantiles. Econometrika. 1978:33–50. [Google Scholar]
- 30.Koenker RaM AF. Goodness of Fit and Related Inference Processes for Quantile Regression. Journal of the American Statistical Association. 1999;1999:1296–310. [Google Scholar]
- 31.The DCCT/EDIC Research Group. Aiello LP, Sun W, Das A, Gangaputra S, Kiss S, Klein R, Cleary PA, Lachin JM, Nathan DM. Intensive diabetes therapy and ocular surgery in type 1 diabetes. NEJM. 2015;372(18):1722–33. doi: 10.1056/NEJMoa1409463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hays RD, Brodsky M, Johnston MF, Spritzer KL, Hui KK. Evaluating the statistical significance of health-related quality-of-life change in individual patients. Eval Health Prof. 2005;28:160–71. doi: 10.1177/0163278705275339. [DOI] [PubMed] [Google Scholar]
- 33.Mangione CM, Phillips RS, Seddon JM, Lawrence MG, Cook EF, Dailey R, et al. Development of the ‘Activities of Daily Vision Scale’. A measure of visual functional status. Med Care. 1992;30:1111–26. doi: 10.1097/00005650-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Submacular Surgery Trials Research Group Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ) SST Report Number 19. Ophthalmic Epidemiol. 2007;14:205–15. doi: 10.1080/09286580701502970. [DOI] [PubMed] [Google Scholar]
- 35.Miskala PH, Hawkins BS, Mangione CM, Bass EB, Bressler NM, Dong LM, et al. Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization--SST Report No. 1. Arch Ophthalmol. 2003;121:531–9. doi: 10.1001/archopht.121.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.