Abstract
Enteropathogenic E. coli (EPEC) cause diarrhea and are the major cause of mortality in developing countries. EPEC use a type III secretion system to deliver effector proteins into the host epithelial cells. To understand the functions of these effectors, majority of studies on EPEC pathogenesis have relied on infections of animals or cell lines with wild type strains of EPEC or mutant strains deficient in one or more effectors. While these studies have provided valuable data, it can be difficult to assess functions of an individual effector in the presence of other EPEC effectors. Recent studies have reported the use of transient transfections with plasmids encoding various EPEC effectors into different cell lines. However, variable transfection efficiencies and expression levels of the effector proteins coupled with their expression for relatively short periods of time pose a problem if the long term effects of these effectors need to be examined. We have generated a MDCK cell line with constitutive expression of the EPEC effector Map (Mitochondrial associated protein) for efficient stable expression of EGFP-tagged Map. We observed that the constitutive expression of Map increased the permeability of charged and non-charged molecules. We also generated polyclonal antibodies against Map and checked for their specificity in MDCK-Map expressing cells. Map has been reported to contribute to the onset of diarrhea but the underlying mechanism is yet to be identified. The MDCK-Map cell line and the anti-Map antibodies generated by us can be used for in vitro studies to examine the role of Map in EPEC pathogenesis.
Keywords: enteropathogenic E coli, mitochondrial associated protein, MDCK, tight junctions
Abbreviations
- CBB
coomassie brilliant blue
- EGFP
enhanced green fluorescent protein
- EPEC
Enteropathogenic E. coli
- Esp
EPEC secreted protein
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- IB
immuno-blot
- IL-8
interleukin-8
- IPTG
Isopropyl β-D-1-thiogalactopyranoside
- LEE
locus of enterocyte effacement
- Map
mitochondrial associated protein
- MDCK
Madin-Darby Canine Kidney
- NF-kB
nuclear factor-kB
- PCR
polymerase chain reaction
- SDS
Sodium dodecyl sulfate
- PVDF
polyvinylidene fluoride
- TER
transepithelial electrical resistance
Introduction
EPEC are one of the leading causes of infantile diarrhea in the developing world.1 EPEC, a gram negative pathogen, intimately adheres to the intestinal epithelium causing attaching and effacing lesions (A/E lesions) at the sites of attachment.2 EPEC uses a type III secretion system to translocate at least 25 effector proteins into the host intestinal epithelial cells.2-4 These effectors are targeted to different cellular locations including the mitochondria, nucleolus, cytoskeleton and the plasma membrane and regulate multiple signaling pathways. Among the EPEC effectors, EspF, EspG, NleA and Map have been reported to contribute to the onset of diarrhea by loosening the intercellular tight junctions that regulate the passage of water and electrolytes through the paracellular space between cells.5-7 We are investigating the effect of the EPEC effector Map on epithelial cell functions. Map is a 203 amino acid protein encoded by a pathogenicity island called the locus of enterocyte effacement (LEE).8 Map induces transient filopodia and causes mitochondrial dysfunction.9,10 Map has also been reported to disrupt intestinal tight junctions leading to the onset of diarrhea, however, the underlying mechanisms have not been elucidated.11 In order to examine the effect of Map on epithelial tight junctions, we have generated a MDCK (Madin-Darby Canine Kidney) cell line stably expressing EGFP-tagged Map for use in functional analysis. Although EPEC infects intestinal epithelial cells, this tissue specificity is lost in cells cultured in vitro. Therefore, we used the MDCK cell line which is derived from the normal canine kidney, to generate the EGFP-Map cell line. The MDCK cell line is a prototypical polarized epithelial cell line widely used to examine the regulation of intercellular junctions.12,13 The MDCK-Map cell line was found to efficiently express Map tagged at the N-terminus with EGFP. The expression of EGFP-Map was confirmed by western blotting using anti-GFP body. EGFP-Map expression was found to increase the passage of charged and non-charged solutes through the tight junctions. Additionally, we generated polyclonal antibodies against recombinant Map and confirmed the specificity of anti-Map antibody with the MDCK-Map cell line.
Results
Cloning of the map gene into pEGFP-C1 mammalian expression vector
The gene encoding map was amplified from the genomic DNA of EPEC strain E2348/69 by PCR using specific primers (Fig. 1A). The resulting PCR fragment, which contained sites for EcoRI at the 5′end and SalI at the 3′end, was ligated with the pEGFP-C1 vector digested with the same enzymes. The positive colonies were confirmed by releasing the map insert by digestion with EcoRI and SalI.(Fig. 1B)
Figure 1.

PCR amplification and cloning of the map gene. (A).The PCR amplified map gene was checked on a 1% agarose gel and the expected band of ~630 bp was observed (arrow). PCR reaction was set up with map primers alone as a negative control (PCR -ve) (B) The map fragment was inserted into the pEGFP-C1 vector and the positive colonies were confirmed by restriction digest with EcoRI and SalI.
Generation of EGFP-Map stable cell line
MDCK II cells were transiently transfected with pEGFP-Map and the total cell lysates of transfected cells were analyzed by protein gel blotting with anti-GFP antibody to confirm the expression of EGFP-Map. A band of ~50 kDa was observed corresponding to the molecular weight of EGFP-Map (Fig. 2). The plasmid pEGFP-Map was then used to generate the stable cell line for constitutive expression of N-terminal EGFP-tagged Map. For this, pEGFP-Map was transfected into MDCK II cells using the calcium phosphate method.14 Several clones were screened for the presence of EGFP-Map by anti-GFP antibody and finally 11 positive clones were isolated (Fig. 3A). We selected clones #1 and #2 which exhibited similar levels of expression, as shown in Figure 3A, for use in future experiments. EGFP-Map localized to the cytoplasm as well as the plasma membrane in these cells.(Fig. 3B)
Figure 2.

Transient transfection of pEGFP-Map in MDCK cells. The EGFP-Map expression was checked by transient transfection of pEGFP-Map into MDCK cells. (A) The total cell lysates of pEGFP-Map transfected cells were analyzed by western blotting with anti-GFP antibody. Untransfected MDCK cell lysates and lysates from MDCK cells transfected with pEGFP-C1 vector only were loaded as negative controls. (B) The same PVDF membrane was stained with Amido Black to visualize the amount of total proteins in each lane.
Figure 3.
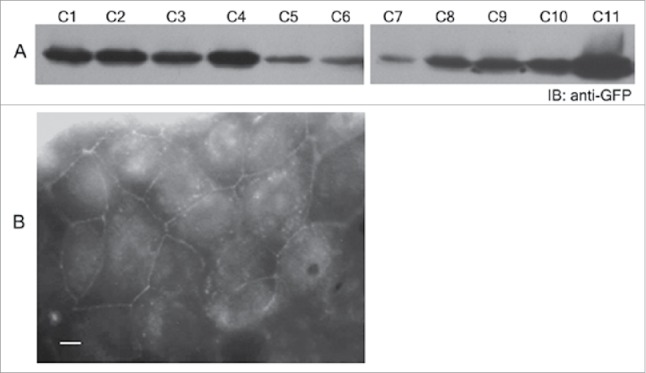
Generation of EGFP-Map stable cell line. (A) Cell lines with stable expression of EGFP-map were generated and checked for Map expression with anti-GFP antibody. A total of 11 positive clones (C1–11) were obtained. (B) The cellular localization of EGFP-map was examined by immunofluorescence. Map expression was seen in the cytoplasm and the plasma membrane. Scale bar: 10 μm.
Expression of recombinant GST-Map in bacteria
The map PCR product, described above, was ligated with the pGEX-4T-3 vector linearized with the same restriction enzymes (EcoRI and SalI) to generate recombinant GST-tagged Map for expression in bacteria. BL21(DE3)pLysS cells, transformed with the pGEX-4T-3-Map construct, were induced with IPTG and the expression of GST-Map was confirmed by western blotting with anti-GST antibody (Fig. 4A) and coomassie brilliant blue staining.(Fig. 4B)
Figure 4.

Generation of recombinant GST-Map. (A) BL21(DE3)pLysS cell lysates expressing GST-Map were bound to Glutathione sepharose beads, separated on 12% gels and blotted on PVDF membrane for probing with anti-GST antibody. Cells transformed with the pGEX-4T-3 vector only and untransformed BL21(DE3)pLysS cell lysates were taken as negative controls. (B) Expression of GST-tagged Map was also confirmed in BL21(DE3)pLysS cells by coomassie brilliant blue (CBB) staining.
Generation of polyclonal antibody against Map in mice
Recombinant GST-Map, purified by immobilizing on glutathione agarose beads, was used to immunize mice following which antiserum was collected. The specificity of the antiserum was checked both with recombinant GST-Map (Fig. 5A) and total cell lysates of MDCK-Map expressing cells (Fig. 5B). Anti-Map antibody was found to detect a band of ˜ 50kDa in both samples.
Figure 5.

Production of anti-map antibody. (A) BL21(DE3)pLysS cell lysates expressing GST-Map were separated on 12% gel and probed with either the pre-immune serum (negative control) or anti-map antibody. A major band of ~50kDa was detected with anti-map antibody although some non-specific bands were also seen. Pre-immune serum did not detect any bands. (B) MDCK-Map cell lysates derived from 2 separate clones were probed with anti-map antibody and a single band of ~50kDa was detected. No band was detected in MDCK cell lysates.
Effect of EGFP-Map on host cell tight junctions
We next tested the functionality of the MDCK-Map cell line. Previous studies have shown that Map is involved in the onset of diarrhea,5,11 but the mechanism is unknown. Diarrheal disease is characterized by excessive loss of water and electrolytes from the body leading to dehydration and death. Since the intercellular tight junction complex regulates the paracellular flux of solutes in intestinal epithelial cells,15 we examined the effect of EGFP-Map expression on tight junctions. MDCK-Map cells were grown on permeable filter supports and the paracellular flux of charged molecules through the tight junctions was evaluated by measuring transepithelial electrical resistance (TER). Expression of EGFP-Map was found to decrease the TER to 0.73 fold as compared to wild type MDCK cells (p value < 0.05; Fig. 6A). Since TER is a measure of tight junction integrity, lower TER values imply that Map compromises junctional integrity by loosening the tight junction complex. We next assessed the passage of non-charged molecules through the tight junctions in MDCK-Map cells. After maximum values of TER were achieved, the confluent monolayers of wild type MDCK cells and MDCK-Map cells were incubated with FITC-labeled 4 kDa Dextran and Rhodamine B-labeled 70 kDa Dextran for 4 hours. Subsequently, the medium was taken from the basal side and the fluorescent intensity was measured on a Fluorometer. MDCK-Map was found to increase the passage of 4 kDa Dextran by 1.57 fold as compared to wild type cells (Fig. 6B). The permeability of 70 kDa Dextran was found to be increased by 1.21 fold (Fig. 6C). These data confirm that Map is functionally active in MDCK cells and increases the permeability of both the charged and non-charged molecules through the tight junctions.
Figure 6.

Map expression affects tight junctions. (A) The rate of passage of charged molecules through tight junctions in MDCK-Map cells was assessed by measuring TER. Map expression decreased the TER by 0.73 fold (B) The rate of passage of the 4 kDa FITC-dextran tracer and 70 kDa dextran-tracer (C) was measured. Map expression increased the flux of the 4 kDa dextran by 1.57 fold and that of the 70 kDa dextran by 1.21 fold as compared to wild type MDCK cells. Error bars indicate mean ± SD.
Discussion
EPEC causes life threatening diarrhea in developing countries but the underlying molecular mechanisms are poorly understood. EPEC infects the epithelial cells of the intestine by transferring several effector proteins into the host cells. EPEC infection is characterized by the onset of watery diarrhea causing severe loss of water and electrolytes from the body and is fatal in infants. Intestinal epithelial cells possess specialized cell adhesion complexes called tight junctions which selectively regulate the passage of charged and non-charged solutes.15-17 The tight junction complex, located at the apical surface of epithelial cells, seals the paracellular space between adjacent cells.16 Tight junctions are the first point of contact for several intestinal pathogens which have devised sophisticated strategies to loosen this barrier.18 Several effector proteins of EPEC have been reported to disrupt tight junctions. The EPEC effector Map is implicated in having a major role in the loosening of the tight junction barrier,5,11 through as yet unidentified mechanisms. Map induces the formation of transient filopodia,9 and contains a mitochondrial targeting signal which localizes it to the mitochondria where it alters the mitochondrial functions.10 Its effect on tight junctions is not dependent on mitochondrial targeting.5 We are investigating the role of Map in the onset of diarrhea caused due to leakage of solutes through the tight junctions. We have developed a MDCK cell line for stable expression of Map in which Map was tagged with EGFP at the N-terminus to block mitochondrial targeting. In transient and stable expression analyses, the expected size of ~50 kDa was observed by protein gel blotting. We obtained several EGFP-Map expressing clones from which we selected 2 clones which exhibited similar levels of expression for further work. The expression of Map in these clones was confirmed by immunofluorescence. EGFP-Map was observed in several cellular locations including the plasma membrane where the tight junctions are located (Fig. 3B). Distinct punctate structures were also seen in the cytoplasm. We are in the process of identifying the other cellular organelles to which EGFP-Map is targeted. Our cell line will be a useful tool to identify the cellular sites to which Map localizes other than the mitochondria. In order to develop Map-specific resources to study its function, we also generated polyclonal antibody against GST-tagged Map. This antibody was very specific as it recognized Map in MDCK-Map cell lysates but did not show any reactivity in wild type MDCK cell lysates (Fig. 5B). The anti-map antibody can be used in cellular in vitro localization studies. Finally, we tested the effect of Map on tight junctions. EGFP-Map expression was found to decrease the transepithelial electrical resistance and increase the paracellular flux of non-charged tracers indicating that Map can loosen the tight junctions in the absence of other EPEC effectors. These data indicate that the MDCK-Map cell line can be used as an effective in vitro model to study the mechanism of EPEC-mediated barrier alterations during pathogenesis. Despite the advances in the knowledge of EPEC effectors, very little is known about the mechanisms that regulate EPEC-induced diarrhea. One major hurdle has been the absence of a suitable model that can mimic the infection of EPEC in the human intestine. In the absence of such a model, related pathogens that naturally infect animals such as rabbits (REPEC) and mice (C. rodentium) have been widely used to study the host immune response to EPEC infection and functions of effector proteins. These models have provided valuable data,19,20 but whether they are also relevant for human infections is difficult to determine. Other studies have reported the use of tissue culture cells as in vitro models to study the interactions of various effectors with the host proteins.19,20 Non-polarized cell lines such as HeLa, infected with EPEC, have proved to be an excellent model to study the mechanisms involved in actin pedestal formation, functions of EPEC effectors and host targets.21,22 However, non-polarized cells are quite distinct from polarized intestinal epithelial cells and cannot be used as models to study the barrier properties of epithelia and the role of EPEC effectors involved in barrier dysfunction. To study these processes, several laboratories have used polarized cells such as Caco-2, T84, and HT29.19,23 Among these polarized cell lines, the intestinal epithelial cell line T84 has been used to examine the effect of EPEC on tight junction components and the barrier functions. For example, T84 cells, infected with EPEC, were reported to cause a reduction in TER which coincided with the dephosphorylation and displacement of occludin from the tight junction complex.24 Further elegant studies on the T84 cell line model identified the important role of the EPEC effector EspF in the dissociation of occludin from the tight junctions as well as break-down of the tight junction barrier.25 T84 cells can also serve as a model to study the host immune response as EPEC infection of T84 cells has been reported to activate the transcription factor NF-kB leading to increased IL-8 production.26 More recently, a new cell line (TC-7) that is derived from the Caco-2 cell line and closely mimics the in vivo differentiated enterocytes has been proposed as another in vitro model system to study microvillus effacement, barrier function and interactions of EPEC with host proteins.27
A common feature in all these studies has been the prior infection of tissue culture cells with EPEC to examine the effects on various host cell functions. This method can potentially mask the contributions of individual effectors that are translocated into the host cells. In this respect, the MDCK-Map model described in this paper does not require infection with EPEC. Taking advantage of the loss of tissue tropism of EPEC in cells cultured in vitro, we have used the robust, prototype epithelial MDCK cell line to constitutively express EGFP-tagged Map. Using this model, we have conclusively shown that Map is independently capable of breaking the tight junction barrier making it more permeable to both charged molecules and non-charged tracers. Identification of Map-induced effects on tight junctions is important to dissect its precise role in tight junction disruption. We are further characterizing this cell line to study the effect of Map expression on the localization and functions of tight junction proteins as well as its interacting partners.
In conclusion, we have generated a MDCK cell line for stable expression of EGFP-Map and confirmed that Map is functionally active in these cells and disrupts the tight junction barrier functions. We have also generated polyclonal antibodies against Map and both these resources will greatly help in our understanding of the mechanism of EPEC pathogenesis.
Materials and Methods
PCR amplification of the map gene
The following primers were used to amplify the map gene:
Map 1: 5′-AAAAATCTAGAGTCGACCAGCCGAGTATCCTGCACATTGT-3′
Map 2: 5′-AAAAAGAATTCCCTTAAGATGGTTAGTCCAACGGCAATGGTA-3′
Briefly, a 50 μl PCR reaction was set up containing 100 ng of genomic DNA from EPEC strain E2348/69 (obtained from the Health Protection Agency, United Kingdom), 200 μM of dNTPs, 0.5 μM of each primer and 1 unit of Phusion DNA polymerase (New England BioLabs). PCR was performed for 30 cycles using the standard protocol. The resulting map PCR product contained restriction sites for EcoRI at the 5′-end and SalI at the 3′-end.
Directional cloning of map
The EcoRI - SalI digested map PCR fragment was ligated with the pEGFP-C1 vector digested with the same restriction enzymes for expression in MDCK cells. The pGEX-4T-3 vector was used for expression of map in bacteria using the same method. Ligations were set with 1:3 molar ratios for the vector and insert respectively using T4 DNA ligase (New England BioLabs).
Transient transfection of MDCK cells
For transient transfections, MDCK II cells were grown in 24 well plates in DMEM supplemented with 10% FBS but without penicillin-streptomycin solution. After 24 hours, 0.5 to 1μg of pEGFP-Map plasmid DNA mixed with 4 μl of Lipofectamine 2000 reagent (Life Technologies) was added to the cells in each well. After 18 hours, the transfected cells were mixed with 1X SDS gel loading buffer and analyzed by western blotting using anti-GFP antibody (Sigma Aldrich).
Stable cell line generation
The EGFP-Map stable cell line was generated using the calcium phosphate method.14 Briefly, MDCK II cells were seeded in a 100 mm plate. When the cells were 50% confluent, approximately 5 µg of purified pEGFP-Map DNA was mixed with 0.5 ml of 2X HEPES buffer and 0.5ml of 0.25M CaCl2 solution was added drop-wise to the mixture. After 30 minutes incubation at room temperature, the mixture was added to the MDCK cells and the cells were incubated for 24 hours. The next day 12.5% glycerol solution was added for 2 minutes following which normal medium was added to the cells. After 24 hours, the selection medium containing 500 µg/ml G418 was added to the cells. The cells were allowed to grow for 2–3 weeks until positives clones appeared. Clones were picked up and confirmed by protein gel blotting with anti-GFP antibody and immunofluorescence.
Expression of GST-tagged Map
The pGEX-4T-3 construct containing GST-tagged Map was used to transform BL21(DE3)pLysS cells. Cultures were induced with 0.5mM IPTG for 4 hours at 37°C after which the cells were pelleted by centrifugation. The cell pellet was resuspended in 1X SDS gel loading buffer, heated at 95°C for 2 minutes and subjected to electrophoresis on a 12% SDS gel. The gel was either stained with coomassie brilliant blue (CBB) or transferred to PVDF membrane and probed with anti-GST or anti-map antibodies.
Generation of polyclonal anti-map antibody in mice
For raising polyclonal antibodies, 100 μg of GST-Map expressing cell lysates were bound with 100 μl of Glutathione agarose slurry (Sigma Aldrich) for 2 hours at 4°C and electrophoresed on a 12% polyacrylamide gel. The band corresponding to GST-Map was excised and homogenized. Approximately 20 μg of purified GST-Map protein was mixed with Freund's complete adjuvant and injected into 5 mice. Three booster doses of 15 μg of purified GST-Map protein mixed with Freund's incomplete adjuvant were given at intervals of 1 week and antiserum was obtained prior to each booster dose. The specificity of anti-map antibody was checked on MDCK-Map cell lysates and BL21(DE3)pLysS cell lysates.
Immunofluorescence
MDCK-Map cells were grown on glass cover slips for 24 hours following which the coverslips were picked up and mounted on glass slides using ProLong Gold antifade mountant (Life Technologies). Samples were viewed through an ApoTome microscope (Zeiss) at 63X magnification.
Measurement of transepithelial electrical resistance
Approximately 1 × 105 MDCK-Map cells were cultured for one week on 12 mm polycarbonate Transwell filters with a pore size of 0.4 μm (Corning). Four filters were used for each clone and the experiments were performed 3 times. TER was measured using a Millicell ERS-2 Voltohm meter (Merck Millipore) daily until maximum values were obtained. Readings were normalized against wild type MDCK cells. Data were expressed as mean ± SD.
Paracellular Flux Assay
Cells, plated on the permeable transwell filters, were allowed to grow for 24 hours after the completion of the TER measurements and then used to measure paracellular permeability of the 4 kDa FITC labeled and 70 kDa Rhodamine B labeled dextrans (Sigma Aldrich). The tracers were dissolved in DMEM medium and used at a final concentration of 1 mg/ml. Briefly, 500 μl of each tracer solution was added to the apical chamber of each filter. The basal chamber was filled with 1.5 ml of DMEM medium. The filters were kept in the CO2 incubator at 37°C for 4–5 hours to allow fluorescent tracer molecules to pass from the apical chamber to the basal chamber. Fluorescence was measured by taking aliquots of medium from the basal chamber. The measurement of fluorescence for FITC (490–525 nm) and Rhodamine B (523–595 nm) was carried out on a Fluorometer.
Statistical Analyses
All experiments were performed at least 3 times using 2 separate clones stably expressing MDCK-Map. Differences between wild type and Map-expressing MDCK cells were analyzed using the Student's t-test and p values <0.05 were considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants to SA from the Department of Biotechnology and Jawaharlal Nehru University (UPE II scheme). APS received the junior and senior research fellowship from the University Grants Commission, India.
References
- 1.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev 1998; 11:142-201; PMID:9457432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol 2004; 2:123-40; PMID:15040260; http://dx.doi.org/ 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- 3.Dean P, Kenny B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol 2009; 12:101-9; PMID:19144561; http://dx.doi.org/ 10.1016/j.mib.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallance BA, Finlay BB. Exploitation of host cells by enteropathogenic Escherichia coli. Proc Natl Acad Sci U S A 2000; 97:8799-806; PMID:10922038; http://dx.doi.org/ 10.1073/pnas.97.16.8799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean P, Kenny B. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol Microbiol 2004; 54:665-75; PMID:15491358; http://dx.doi.org/ 10.1111/j.1365-2958.2004.04308.x [DOI] [PubMed] [Google Scholar]
- 6.Glotfelty LG, Hecht GA. Enteropathogenic E. coli effectors EspG1/G2 disrupt tight junctions: new roles and mechanisms. Ann N Y Acad Sci 2012; 1258:149-58; PMID:22731728; http://dx.doi.org/ 10.1111/j.1749-6632.2012.06563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thanabalasuriar A, Koutsouris A, Weflen A, Mimee M, Hecht G, Gruenheid S. The bacterial virulence factor NleA is required for the disruption of intestinal tight junctions by enteropathogenic Escherichia coli. Cell Microbiol 2010; 12:31-41; PMID:19712078; http://dx.doi.org/ 10.1111/j.1462-5822.2009.01376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iguchi A, Thomson NR, Ogura Y, Saunders D, Ooka T, Henderson IR, Harris D, Asadulghani M, Kurokawa K, Dean P, et al. Complete genome sequence and comparative genome analysis of enteropathogenic Escherichia coli O127:H6 strain E2348/69. J Bacteriol 2009; 191(1):347-54; PMID:18952797; http://dx.doi.org/ 10.1128/JB.01238-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alto NM, Shao F, Lazar CS, Brost RL, Chua G, Mattoo S, McMahon SA, Ghosh P, Hughes TR, Boone C, et al. Identification of a bacterial type III effector family with G protein mimicry functions. Cell 2006; 124:133-45; PMID:16413487; http://dx.doi.org/ 10.1016/j.cell.2005.10.031 [DOI] [PubMed] [Google Scholar]
- 10.Papatheodorou P, Domańska G, Öxle M, Mathieu J, Selchow O, Kenny B, Rassow J. The enteropathogenic Escherichia coli (EPEC) Map effector is imported into the mitochondrial matrix by the TOM/Hsp70 system and alters organelle morphology. Cell Microbiol 2006; 8:677-89; PMID:16548893; http://dx.doi.org/ 10.1111/j.1462-5822.2005.00660.x [DOI] [PubMed] [Google Scholar]
- 11.Dean P, Maresca M, Schuller S, Phillips AD, Kenny B. Potent diarrheagenic mechanism mediated by the cooperative action of three enteropathogenic Escherichia coli-injected effector proteins. Proc Natl Acad Sci USA 2006; 103:1876-81; PMID:16446436; http://dx.doi.org/ 10.1073/pnas.0509451103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaush CR, Hard WL, Smith TF. Characterization of an established line of canine kidney cells (MDCK) Proc Soc Exp Biol Med 1966; 122(3):931-5; PMID:5918973; http://dx.doi.org/ 10.3181/00379727-122-31293 [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK). J Membr Biol 1985; 86:113-25; PMID:4032460; http://dx.doi.org/ 10.1007/BF01870778 [DOI] [PubMed] [Google Scholar]
- 14.Wigler M, Pellicer A, Silverstein S, Axel R, Urlaub G, Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A 1979; 76(3):1373-6; PMID:286319; http://dx.doi.org/ 10.1073/pnas.76.3.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balda MS, Matter K. Transmembrane proteins of tight junctions. Sem Cell Dev Biol 2000; 11:281-9; http://dx.doi.org/ 10.1006/scdb.2000.0177 [DOI] [PubMed] [Google Scholar]
- 16.Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol 2006; 248:261-98; PMID:16487793; http://dx.doi.org/ 10.1016/S0074-7696(06)48005-0 [DOI] [PubMed] [Google Scholar]
- 17.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142-9; PMID:20061152; http://dx.doi.org/ 10.1016/j.tcb.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 18.Sousa S, Lecuit M, Cossart P. Microbial strategies to target, cross or disrupt epithelia. Curr Opin Cell Biol 2005; 17:489-98; PMID:16102958; ; http://dx.doi.org/ 10.1016/j.ceb.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 19.Law RJ, Gur-Arie L, Rosenshine I, Finlay BB. In vitro and in vivo model systems for studying enteropathogenic Escherichia coli infections. Cold Spring Harb Perspect Med 2013; 3:a009977; PMID:23457294; http://dx.doi.org/ 10.1101/cshperspect.a009977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallance BA, Finlay BB. Exploitation of host cells by enteropathogenic Escherichia coli. Proc Natl Acad Sci U S A 2000; 97:8799-806; PMID:10922038; http://dx.doi.org/ 10.1073/pnas.97.16.8799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenshine I, Ruschkowski S, Stein M, Reinscheid DJ, Mills SD, Finlay BB. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J 1996; 15:2613-24; PMID:8654358 [PMC free article] [PubMed] [Google Scholar]
- 22.Frankel G, Phillips AD. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: Getting off the pedestal. Cell Microbiol 2008; 10:549-56; PMID:18053003; http://dx.doi.org/ 10.1111/j.1462-5822.2007.01103.x [DOI] [PubMed] [Google Scholar]
- 23.Sears CL. Molecular physiology and pathophysiology of tight junctions V. assault of the tight junction by enteric pathogens. Am J Physiol Gastrointest Liver Physiol 2000; 279:G1129-34; PMID:11093933 [DOI] [PubMed] [Google Scholar]
- 24.Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight Junctions. Cell Microbiol 2000; 2(4):305-15; PMID:11207587; http://dx.doi.org/ 10.1046/j.1462-5822.2000.00055.x [DOI] [PubMed] [Google Scholar]
- 25.McNamara BP, Koutsouris A, O'Connell CB, Nougayréde JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest 2001; 107:621-9; PMID:11238563; http://dx.doi.org/ 10.1172/JCI11138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savkovic SD, Koutsouris A, Hecht G. Activation of NF-kB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol Cell Physiol 1997; 273:C1160-7 [DOI] [PubMed] [Google Scholar]
- 27.Dean P, Young L, Quitard S, Kenny B. Insights into the Pathogenesis of Enteropathogenic E. coli Using an Improved Intestinal Enterocyte Model. PLoS One 2013; 8(1):e55284; PMID:23383137; http://dx.doi.org/ 10.1371/journal.pone.0055284 [DOI] [PMC free article] [PubMed] [Google Scholar]


