Abstract
A school-based campaign in Knox County, TN immunized 41% and 48% of school children with live attenuated influenza vaccine in 2005 and 2006, respectively. We computed the relative risk (RR) of a positive rapid influenza test in Knox compared to Knox-surrounding county children (controls) during 5 baseline and 2 campaign seasons. Among children aged <5 years, the RR for a positive test remained relatively constant throughout the study seasons. Among children aged 5-17 years (target population), the RR was 1 during baseline and declined by 21% (RR: 0.79, 95%CI: 0.59-1.05) and 27% (RR: 0.73, 95%CI: 0.60-0.87) during the first and second campaign season, respectively. These findings suggest a direct beneficial effect of the immunization campaign.
Keywords: Live Attenuated Influenza Vaccine, School-based Immunization, Influenza, Rapid Influenza tests
1. Introduction
Influenza epidemics impose a major burden of morbidity and mortality worldwide. Children are affected early during influenza epidemics and they serve as vectors introducing influenza into the communities [1, 2]. Schools, where children interact in close contact, provide a mean for transmission of influenza and subsequent spread in the community [2]. Therefore, school-based immunization provides opportunities for interruption of influenza transmission [2-4].
Needleless live attenuated influenza vaccine (LAIV) is particularly attractive for massive school-based immunization. LAIV has shown higher efficacy than inactivated vaccines in young children [5] and in asthmatic children aged 6-17 years [6]. However, similar comparison data in healthy school-aged children are limited. Furthermore, it was previously demonstrated that in children, LAIV increased nasal IgA levels and reduced virus shedding during influenza episodes, compared to inactivated vaccines. Thus, LAIV also has the potential to reduce the transmission of influenza and prevent disease even in unvaccinated subjects (herd protection) [2, 5, 7, 8].
In 2008, the Advisory Committee on Immunization Practices (ACIP) recommended routine influenza immunization for all US children aged 6 months or older [7]. Mathematical models previously suggested that massive immunization of children could substantially mitigate the impact of an influenza epidemic in a community [9]. Recent studies suggested that the direct effect of immunization of school aged children with LAIV, reduced influenza-confirmed disease, clinical symptoms and absenteeism [10-12]. Moreover, previous evidence from observational studies[13, 14] and from clinical trials performed primarily outside the US [15], indicated beneficial population effects of immunization of school-aged children. In the present study, we used data from influenza rapid antigen detection tests to assess the effect of a large county-wide school-based immunization campaign with LAIV implemented in Knox County, Tennessee.
2. Patients and Methods
In 2005, the Knox County Health Department of Tennessee initiated a county-wide school-based influenza immunization campaign targeting public school children aged ≥5 years. Knox County has a single public school system and according to the Census Bureau, the 2005 estimated County population was 409,116 with 6% aged <5 years and 19% aged 5-19 years [16]. Approximately 88% of the population was white. From October-December 2005, LAIV was offered through weekday in-school and weekend clinics. Consent for immunization was obtained by sending a form to parents through their school children. Children presenting signed forms and without known contraindications for LAIV immunization were considered eligible. LAIV for the campaign was donated by MedImmune and costs associated with education and campaign information were assumed by the Knox Health Department [17].
Prior to the 2005-2006 influenza season, 24,198 (45%) children were immunized with LAIV among 53,420 public school students. Approximately 58% of 5,099 children aged <9 years received a second dose. During the second campaign year, LAIV as well as additional resources were donated by MedImmune to strengthen immunization efforts and to extend the campaign to private schools. The reported immunization coverage was 56% among Knox children attending kindergarten or elementary schools (typical age 5-11 years) and the coverage was approximately 45% and 30% among children attending middle and high school (typical age 12-18) [17]. Prior to the 2006-2007 season, Knox County immunized 47% of 54,786 public school children and 61% of 5,998 private school children. Approximately 53% of children <9 years received a second dose. The estimated immunization coverage was 60% among Knox children attending kindergarten and elementary schools and the coverage was approximately 45% and 26% among children attending middle and high school, respectively (John Lott, personal communication). We estimated that overall, approximately 41% and 48% of Knox school children aged ≥5 years received at least one dose of LAIV during the first and second campaign years, respectively.
We examined the effects of the Knox County school-based influenza immunization campaign using results of rapid influenza tests performed at the East Tennessee Children's Hospital (ETCH) in Knoxville (Directigen® Flu A+B, sensitivity (range: 75%-100%) and specificity (88%-99%) as compared with viral culture, used during years 2000-2002; and QuickVue®, sensitivity (77%-93%) and specificity (83%-94%) as compared with viral culture, used during years 2003-2007) [18]. An influenza season began on the week when the cumulative proportion of positive rapid influenza tests observed during Fall/Winter attained 2.5%. The season ended on the week when the cumulative proportion reached 97.5%. This approach yielded similar results to strategies previously described [1] and each season contained at least 95% of positive influenza tests.
ETCH is the regional hospital that provides care for children from Knox and Knox-surrounding counties. We analyzed data for the five pre-campaign (baseline) influenza seasons and for the two campaign seasons, and classified rapid test information according to patient's County of residence: Knox county and Knox-surrounding counties (eight counties surrounding Knox, where no immunization campaign was implemented). According to the Census Bureau, the 2005 estimated population of Knox-surrounding counties combined was 451,203 with 6% aged <5 years and 18.5% aged 5-19 years. Approximately 96% of the population was white. [16]
We calculated the ratio of the proportion of positive test results between Knox and Knox-surrounding counties during seven consecutive influenza seasons. A ratio (as an approximation to the relative risk, RR) of 1 indicated similar influenza activity in Knox and Knox-surrounding counties and 95% confidence intervals were calculated using the delta method. Confidence intervals encompassing 1 indicated no significant difference in risk between study populations. Children aged 5-17 years (target population of the campaign) were the primary study group. Sub-group analyses were done for children aged 5-11 and 12-17 years, and data for children aged <5 years, who did not participate in the campaign, were also analyzed to assess potential herd protection. The study protocol was approved by the Institutional Review Boards of Vanderbilt and ETCH.
3. Results
During the seven influenza seasons, 10,425 and 6,670 children aged <18 years were tested with rapid influenza tests in Knox and Knox-surrounding counties, respectively. The number of tested children in Knox ranged from 431 during the 2000-2001 season to 2,808 during the 2003-2004 season. Similarly, the number of tested children in Knox-surrounding counties ranged from 250 during the 2000-2001 season to 1,871 during the 2003-2004 season.
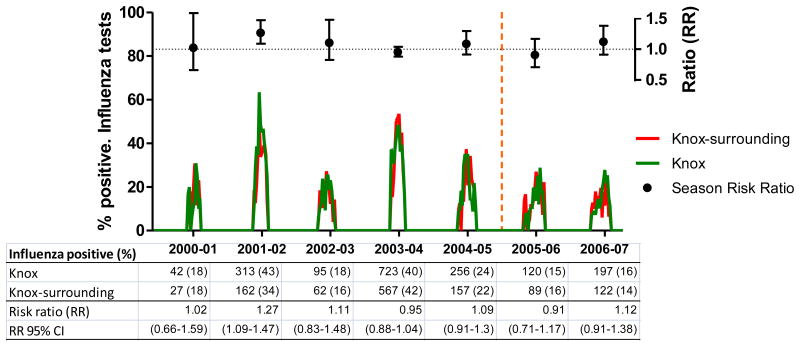
3.1. Children aged <5 years
In this age group, the mean age was 1.53 years (95% CI: 1.49-1.56) and 1.48 (95% CI: 1.45-1.52) years for children from Knox and Knox-surrounding counties (p=0.089), respectively. Mean ages were similar throughout the study seasons, except for season 2004-2005 when mean age of Knox children was significantly higher (p=0.045). Overall, 27.0% of 6,460 Knox County tested children were positive for influenza, whereas 26.3% of 4,517 Knox-surrounding counties tested children yielded positive results (RR: 1.03, 95% CI: 0.97-1.10). Ratios of positive rapid tests were close to 1 and remained relatively constant throughout the study seasons (Figure 1).
Figure 1.
Changes in the ratio of positive influenza rapid tests among children aged <5 years. East Tennessee Children's Hospital, influenza seasons 2000-01 through 2006-07
Footnote: Influenza seasons data are from East Tennessee Children Hospital (ETCH). Ratios (RR, relative risk) indicate the proportion of influenza positive tests among Knox residents divided by the proportion of influenza positive tests among residents of Knox-surrounding counties (controls).
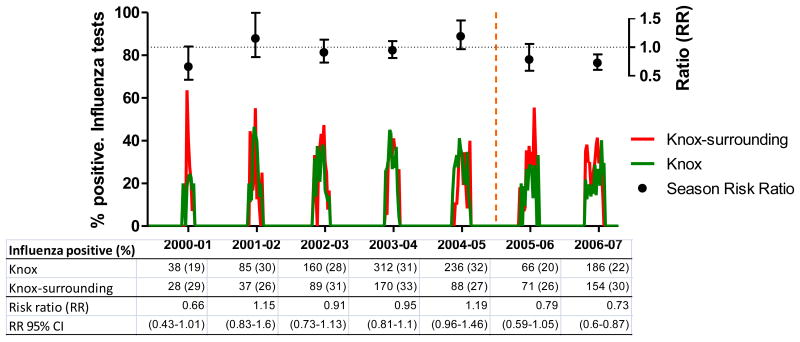
3.2. Children aged 5-17 years
The mean age among tested children from Knox County in this age group was 9.43 years (95% CI: 9.32-9.55) and 8.54 years (95% CI: 8.39-8.68) among children from Knox-surrounding counties (p<0.001). The mean age of Knox County children was consistently higher than the mean age of children from Knox-surrounding counties during all seasons except for the 2005-2006 season when means were similar (p=0.082). During all study seasons, 27.3% of 3,965 Knox County children tested positive for influenza, whereas 29.6% of 2,153 Knox-surrounding counties children tested positive (RR: 0.92, 95% CI: 0.85-1.00).
During the five pre-campaign seasons, the ratios of positive rapid influenza tests remained relatively constant. During the first campaign season, we observed a non-significant decline in the ratio (RR: 0.79, 95% CI: 0.59-1.05) followed by a significant decline (RR: 0.73, 95%CI: 0.60-0.87) during the second campaign season (2006-2007). A stratified analysis of children within the 5-17 year age group yielded similar results but was based on a small number of observations and had wide confidence intervals. For instance, the analysis of children aged 5-11 years showed RRs of 0.86 (95% CI: 0.6-1.24) and 0.76 (95% CI: 0.62-0.94) for the first and second campaign seasons, respectively. Similarly, the respective RRs were 0.61 (95% CI: 0.37-1.02) and 0.63 (95% CI: 0.42-0.96) for children aged 12-17 years. Moreover, the visual inspection of the distribution of weekly influenza activity suggested a shift among Knox children during the second campaign season (e.g. among children aged 5-17 years, the peak of activity was delayed by three weeks compared with Knox-surrounding counties and most pre-campaign seasons (Figure 2)).
Figure 2.
Changes in the ratio of positive influenza rapid tests among children aged 5-17 years. East Tennessee Children's Hospital, influenza seasons 2000-01 through 2006-07
Footnote: Influenza seasons data are from East Tennessee Children Hospital (ETCH). Ratios (RR, relative risk) indicate the proportion of influenza positive tests among Knox residents divided by the proportion of influenza positive tests among residents of Knox-surrounding counties (controls).
4. Discussion
Our study suggests that immunization of a large proportion of school-aged children in Knox County reduced laboratory-diagnosed influenza associated health care visits in the community. The risk of a positive rapid influenza test was reduced after the implementation of the immunization campaign compared to five pre-campaign seasons. The risk of a positive influenza rapid test was significantly reduced by 27% during the second campaign season.
Our main analysis indicated a significant direct protection among Knox children aged 5-17 years old, the main target of the campaign. Furthermore, despite differences in immunization coverage, a subgroup analysis indicated significant protection in both children aged 5-11 and 12-17 years. However, the precision of these estimates was low due to a limited number of observations. These findings are consistent with a similar evaluation based on active surveillance of laboratory confirmed-influenza conducted in Knox and Davidson County, TN. That study suggested a protective effect of the immunization campaign for Knox children aged 5-12 years.[19]
Similar to the study comparing children in Knox and Davidson Counties, our results do not support indirect or “herd” effects for children aged <5 years, who were not part of the campaign.[19] However, influenza vaccine was recommended for children aged 6-23 months starting in 2004 and those aged 6 months to 4 years starting in 2006. The proportion of Tennessee children aged 6-23 months fully immunized against influenza was 15.5% and 21.2% for the 2005-2006 and 2006-2007 seasons, respectively [20, 21]. Thus, there was likely modest vaccination of children aged <5 years in both the campaign and control counties, which limited our ability to detect herd effects if they occurred.
Using our data we calculated crude estimates of vaccine efficacy for children aged 5-17 years. During the first campaign season, approximately 41% of all Knox school aged children were immunized. Given the observed relative risk of 0.79 and assuming that the risk of influenza was the same for unvaccinated children from Knox and Knox-surrounding counties, the estimated protection provided by LAIV would be 51.2% (RR: 0.79=[1-(0.41*Vaccine Efficacy)]/1). Similarly, during the second campaign season, approximately 48% of all Knox school aged children were immunized. Given the observed relative risk of 0.73, the estimated protection provided by LAIV would be 56.3% (RR: 0.73=[1-(0.48*Vaccine Efficacy)]/1). These estimates assume no immunization of school-aged children in Knox-surrounding counties. Accounting for any influenza immunization of these children would increase our vaccine efficacy estimates.
Several limitations in our study must be discussed. First, although ETCH is the primary children's hospital in the Knox County region, it is a single hospital and our results are not population-based. The lack of a defined population receiving care at ETCH precluded the calculation of incidence rates. Second, we do not have information on the children's disease severity. Although the application of rapid tests provides a specific measurement of influenza activity, the effects of the campaign on clinical outcomes (i.e. hospitalizations or outpatient visits) are unknown. Third, our estimates could be affected if Knox children infected with influenza were less likely to be tested at ETCH during campaign seasons. Fourth, in spite of the high specificity of influenza rapid tests, they are imperfect and their systematic application could result in non-differential misclassification of cases, making campaign effects harder to ascertain. Furthermore, during the campaign seasons (2005-2007), influenza activity was mild and the vaccine match was suboptimal (http://www.cdc.gov/flu/weekly/fluactivity.htm). Finally, although we assessed effects of the immunization campaign on the target age group, the effects among older unvaccinated groups remain unknown. However, a comparison of adults aged ≥50 years in Knox and Davidson counties during the second campaign season found no difference in influenza hospitalization rates.[22]
In conclusion, our findings suggest that a large school-based influenza immunization campaign with LAIV significantly reduced healthcare visits for influenza among Knox children aged 5-17 years, the main target of the campaign. The declines in the probability of laboratory-diagnosed influenza infections, in years with suboptimal vaccine match, suggest substantial direct vaccine effectiveness.
Acknowledgments
This study was supported by MedImmune, the manufacturer of the live attenuated influenza vaccine. MedImmune had no role in the design, analysis or writing of this report. Dr. Grijalva is supported by a Career Development Award (K01 IP000163) from the Centers for Disease Control and Prevention. We are indebted to Caroline Graber RN, Jacque Van Audenhove MS and Lori Patterson, MD for the collection of influenza rapid tests information from East Tennessee Children Hospital.
Footnotes
Conflict of interest statement: Drs. Grijalva and Griffin received grant support from MedImmune. Drs. Grijalva and Griffin received speaker honoraria from Wyeth.
References
- 1.Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006 Jul 6;355(1):31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP. Herd protection against influenza. J Clin Virol. 2006 Dec;37(4):237–43. doi: 10.1016/j.jcv.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Halloran ME, Longini IM., Jr Public health. Community studies for vaccinating schoolchildren against influenza. Science. 2006 Feb 3;311(5761):615–6. doi: 10.1126/science.1122143. [DOI] [PubMed] [Google Scholar]
- 4.King JC, Jr, Cummings GE, Stoddard J, Readmond BX, Magder LS, Stong M, et al. A pilot study of the effectiveness of a school-based influenza vaccination program. Pediatrics. 2005 Dec;116(6):e868–73. doi: 10.1542/peds.2005-1301. [DOI] [PubMed] [Google Scholar]
- 5.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007 Feb 15;356(7):685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 6.Fleming DM, Crovari P, Wahn U, Klemola T, Schlesinger Y, Langussis A, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006 Oct;25(10):860–9. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 7.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008 Aug 8;57(RR-7):1–60. [PubMed] [Google Scholar]
- 8.Johnson PR, Feldman S, Thompson JM, Mahoney JD, Wright PF. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J Infect Dis. 1986;154(1):121–7. doi: 10.1093/infdis/154.1.121. [DOI] [PubMed] [Google Scholar]
- 9.Weycker D, Edelsberg J, Halloran ME, Longini IM, Jr, Nizam A, Ciuryla V, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005 Jan 26;23(10):1284–93. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 10.King JC, Jr, Stoddard JJ, Gaglani MJ, Moore KA, Magder L, McClure E, et al. Effectiveness of school-based influenza vaccination. N Engl J Med. 2006 Dec 14;355(24):2523–32. doi: 10.1056/NEJMoa055414. [DOI] [PubMed] [Google Scholar]
- 11.Davis MM, King JC, Jr, Moag L, Cummings G, Magder LS. Countywide School-Based Influenza Immunization: Direct and Indirect Impact on Student Absenteeism. Pediatrics. 2008;(122):e260–e5. doi: 10.1542/peds.2007-2963. [DOI] [PubMed] [Google Scholar]
- 12.Piedra PA, Gaglani MJ, Kozinetz CA, Herschler GB, Fewlass C, Harvey D, et al. Trivalent live attenuated intranasal influenza vaccine administered during the 2003-2004 influenza type A (H3N2) outbreak provided immediate, direct, and indirect protection in children. Pediatrics. 2007 Sep;120(3):e553–64. doi: 10.1542/peds.2006-2836. [DOI] [PubMed] [Google Scholar]
- 13.Monto AS, Davenport FM, Napier JA, Francis T., Jr Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis. 1970 Jul-Aug;122(1):16–25. doi: 10.1093/infdis/122.1-2.16. [DOI] [PubMed] [Google Scholar]
- 14.Piedra PA, Gaglani MJ, Kozinetz CA, Herschler G, Riggs M, Griffith M, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine. 2005 Feb 18;23(13):1540–8. doi: 10.1016/j.vaccine.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008(2):CD004879. doi: 10.1002/14651858.CD004879.pub3. [DOI] [PubMed] [Google Scholar]
- 16.United States Census Bureau. [January 5th 2009];Population Estimates Datasets [internet site] Available from http://www.census.gov/popest/datasets.html.
- 17.Carpenter LR, Lott J, Lawson BM, Hall S, Craig AS, Schaffner W, et al. Mass distribution of free, intranasally administered influenza vaccine in a public school system. Pediatrics. 2007 Jul;120(1):e172–8. doi: 10.1542/peds.2006-2603. [DOI] [PubMed] [Google Scholar]
- 18.Uyeki TM. Influenza diagnosis and treatment in children: a review of studies on clinically useful tests and antiviral treatment for influenza. PediatrInfectDisJ. 2003;22(2):164–77. doi: 10.1097/01.inf.0000050458.35010.b6. [DOI] [PubMed] [Google Scholar]
- 19.Poehling KA, Talbot HK, Williams JV, Zhu Y, Lott J, Patterson L, et al. Impact of a School-based Influenza Immunization Program on Disease Burden: Comparison of Two Tennessee Counties. 2009 doi: 10.1016/j.vaccine.2009.02.043. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Influenza vaccination coverage among children aged 6-23 months--United States, 2005-06 influenza season. MMWR Morb Mortal Wkly Rep. 2007 Sep 21;56(37):959–63. [PubMed] [Google Scholar]
- 21.Influenza vaccination coverage among children aged 6--23 months--United States, 2006-07 influenza season. MMWR Morb Mortal Wkly Rep. 2008 Sep 26;57(38):1039–43. [PubMed] [Google Scholar]
- 22.Talbot HK, Poehling KA, Williams JV, Zhu Y, Chen Q, McNabb P, et al. Influenza in Older Adults: Impact of Vaccination of School Children. 2009 doi: 10.1016/j.vaccine.2009.01.108. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]