Abstract
The serine/threonine kinase AKT plays a pivotal role in signal transduction events involved in malignant transformation and chemoresistance, and is an attractive target for the development of cancer therapeutics. Fragment-based lead discovery combined with structure-based drug design have recently identified AT7867 as a novel and potent inhibitor of both AKT and the downstream kinase p70S6K and also of PKA. This ATP-competitive small molecule potently inhibits both AKT and p70S6K activity at the cellular level, as measured by inhibition of GSK3β and S6RP phosphorylation, and also causes growth inhibition in a range of human cancer cell lines as a single agent. Induction of apoptosis was detected by multiple methods in tumor cells following AT7867 treatment. Oral or intraperitoneal administration of AT7867 (90mg/kg p.o. or 20mg/kg i.p.) to athymic mice implanted with the PTEN-deficient U87MG human glioblastoma xenograft model caused inhibition of phosphorylation of downstream substrates of both AKT and p70S6K, as well as induction of apoptosis, confirming the observations made in vitro. These doses of AT7867 also resulted in inhibition of human tumor growth in PTEN-deficient xenograft models. These data suggest that the novel strategy of AKT and p70S6K blockade may have therapeutic value and supports further evaluation of AT7867 as a single agent anticancer strategy.
Keywords: AT7867, AKT, p70 S6 kinase, cancer, therapeutic
Introduction
AKT (also known as protein kinase B; PKB) is a serine/threonine kinase that lies downstream of phosphatidylinositol 3-kinase (PI3K) and plays a key role in a range of cellular functions, including cell growth, proliferation, metabolism and survival (1, 2). Three closely related isoforms of AKT with overlapping cellular functions have been identified, termed AKT1, 2 and 3 (3).
AKT activation requires the association of its N-terminal pleckstrin homology (PH) domain with cell membrane phosphatidylinositol (3,4,5)-trisphosphate (PIP3) produced by PI3K (4). This facilitates phosphorylation of AKT at threonine 308 and serine 473, both of which are necessary for full activation of the protein. Phosphorylation of threonine 308 requires phosphoinositide-dependent kinase 1 (PDK1) (5, 6). A number of kinases have been reported to phosphorylate serine 473, the most prominent being the mammalian target of rapamycin complex 2 (mTORC2) (7-10). The activation of AKT is antagonized by the tumor suppressor PTEN (phosphatase and tensin homolog on chromosome 10) through the dephosphorylation of PIP3 (11).
There are numerous proteins downstream of AKT which, when phosphorylated, participate in the regulation of critical cellular processes including growth, proliferation, metabolism and survival. Those involved in cell growth include p70 S6 kinase (p70S6K), S6 ribosomal protein (S6RP), and the mammalian target of rapamycin complex 1 (mTORC1) (12), while cell proliferation and metabolism are regulated through glycogen synthase kinase-3β (GSK3β) phosphorylation (13). A number of pro-apoptotic proteins including BAD (14), caspase 9 (15) and the forkhead family of transcription factors (16), enable AKT to regulate cell survival. Importantly, negative feedback loops have been described, which link proteins downstream of AKT with those upstream, or with AKT itself (17).
Aberrations along the PI3K/AKT pathway have been shown to drive a range of malignancies through mechanisms including activation of upstream receptor tyrosine kinases, PIK3CA mutations, PTEN mutations, AKT amplifications and mutations, as well as over-expression and hyperactivation of AKT proteins themselves (2, 18-21). Thus, the pharmacological ablation of AKT activity represents a rational approach to anticancer therapy. Moreover, PI3K/AKT pathway activation is a frequent hallmark of tumors resistant to treatment with chemotherapy or targeted therapies such as growth factor inhibitors (22-24). Therefore, AKT inhibition in these tumor types may also have therapeutic value either as monotherapy or in rational combinations with other antitumor agents (25).
Small molecules have been described which target various vital components of the PI3K/AKT pathway by blocking activation of AKT or its downstream targets (2). These include the PI3K inhibitors LY294002 and wortmannin, and more recently, isoform-specific PI3K inhibitors with differing biological profiles (2, 26, 27). These agents, and also drugs such as rapamycin and its analogs that inhibit mTOR, are currently progressing through clinical trials in a number of cancer types (28). The latter compounds provide proof of principle that the PI3K–AKT pathway can be successfully targeted for clinical benefit in cancer (2).
A number of compounds which block the activation of AKT through a range of different mechanisms have recently been described, emphasizing the validity and current interest in AKT as an antitumor drug target (2). The inhibition of both AKT and p70S6K with a single agent has not been previously described. Targeting these two key components of the PI3K-AKT pathway through specific vertical inhibition may have therapeutic value.
We have utilized high-throughput X-ray crystallography and fragment-based lead discovery technologies to identify fragment hits against AKT. These fragments were validated by structural studies, and rapidly transformed into potent lead compounds using structure-based design to increase the efficiency of the medicinal chemistry. This research was recently described in detail and has identified potent, low molecular weight inhibitors of AKT that exhibit drug-like properties (29-32).
In this paper, we describe the detailed pharmacological profile of one of these compounds, AT7867, and also identify this agent as a potent inhibitor of p70S6K in cells. We demonstrate that this orally bioavailable small molecule causes appropriate biomarker modulation and apoptosis both in vitro and in vivo, and exhibits antitumor efficacy in human tumor xenograft models.
Methods
Cell Culture and Reagents
All cell lines were purchased from the American Type Culture Collection (Rockville, MD, USA) and grown in their recommended culture medium, which was supplemented with 10% fetal bovine serum (FBS) at 37°C in an atmosphere of 5% CO2 and passaged for less than six months. All reagents were purchased from Sigma (Poole, Dorset, UK) unless otherwise stated. AT7867 (31) and LY294002 (Calbiochem, Merck Biosciences, Nottingham, UK) were dissolved to a 10mM stock in dimethyl sulphoxide (DMSO) while okadaic acid (Calbiochem, Merck Biosciences, Nottingham, UK) was dissolved to a 50(M stock in DMSO. The recombinant purified AKT2 enzyme used was the PH-truncated AKT2-Piftide as previously described (29), which was kindly provided by Professor David Barford (The Institute of Cancer Research, London, UK). GSK3β, Histone H1, protein kinase A (PKA) and p70S6K enzymes, phospho-glycogen synthase peptide-2, PKA and p70S6K substrates were purchased from Upstate Biotechnology (Dundee, UK), while protein kinase C (PKC) and glycogen synthase derived peptide GS1-8 were from Calbiochem. CDK2/cyclinA was prepared in-house at Astex Therapeutics.
In Vitro Kinase Assays
Kinase assays for AKT2, PKA, p70S6K and CDK2/cyclinA were all carried out in a radiometric filter binding format. Assay reactions were set up in the presence of compound. For AKT2, the AKT2 enzyme and 25μM AKTide-2T peptide (HARKRERTYSFGHHA) were incubated in 20mM MOPS, pH 7.2, 25mM β-glycerophosphate, 5mM EDTA, 15mM MgCl2, 1mM sodium orthovanadate, 1mM DTT, 10μg/ml BSA and 30μM ATP (1.16Ci/mmol) for 4 hours. For PKA, the PKA enzyme and 50μM peptide (GRTGRRNSI) were incubated in 2 mM MOPS, pH 7.2, 25mM β-glycerophosphate, 5mM EDTA, 15mM MgCl2, 1mM orthovanadate, 1mM DTT and 40μM ATP (0.88Ci/mmol) for 20 minutes. For p70S6K, the p70S6K enzyme and 25μM peptide substrate (AKRRRLSSLRA) were incubated in 10mM MOPS, pH 7, 0.2mM EDTA, 1mM MgCl2, 0.01% β-mercaptoethanol, 0.1mg/ml BSA, 0.001% Brij-35, 0.5% glycerol and 15μM ATP (2.3Ci/mmol) for 60 minutes. For CDK2, the CDK2/cyclinA enzyme and 0.12μg/ml Histone H1 were incubated in 20mM MOPS, pH 7.2, 25mM β-glycerophosphate, 5mM EDTA, 15mM MgCl2, 1mM sodium orthovanadate, 1mM DTT, 0.1mg/ml BSA and 45μM ATP (0.78Ci/mmol) for 4 hours. Assay reactions were stopped by adding an excess of orthophosphoric acid and the stopped reaction mixture was then transferred to Millipore MAPH filter plates and filtered. The plates were then washed, scintillant added and radioactivity measured by scintillation counting on a Packard TopCount. IC50 values were calculated from replicate curves using GraphPad Prism software. AKT1 and 3 enzyme assays were carried out at Invitrogen Ltd (Paisley, UK), while all other enzyme assays were performed at Upstate Biotechnology (Dundee, UK).
Alamar Blue Cell Proliferation Assay
Cells were plated in 96-well microplates at 5000 cells per well in medium supplemented with 10% FBS, and grown for 24 hours before treatment with AT7867. Inhibitor or vehicle control was added to the cells for 72 hours. Following this, Alamar Blue solution (BioSource, Nivelle Belgium) was added as stated in the manufacturer's instructions. The IC50 value for each inhibitor was calculated in GraphPad Prism (San Diego, USA) using non-linear regression analysis and a sigmoidal dose-response (variable slope) equation.
Phospho-GSK3β (Serine 9) Cellular ELISA Assay
This assay was based on a previously described protocol (33). Cells were plated in 96-well microplates at 16,000 cells per well in medium supplemented with 10% FBS, and grown for 24 hours before treatment with AT7867. AT7867 or vehicle control were added to the cells for 1 hour. Following this, cells were fixed with 3% paraformaldehyde, 0.25% glutaraldehyde, 0.25% Triton-X100, washed and blocked with 5% milk in tris-buffered saline with 0.1% Tween-20 (TBST) prior to overnight incubation with a phospho-GSK3β (serine 9) antibody (Cell Signaling Technology, Boston, MA, USA). The plates were then washed, secondary antibody added, and enhancement of the signal performed using DELFIA reagents (Perkin Elmer, Beaconsfield, Bucks, UK) as stated in the manufacturer's instructions. Europium counts were normalized to the protein concentration, and the IC50 value for each inhibitor was calculated in GraphPad Prism using non-linear regression analysis and a sigmoidal dose-response (variable slope) equation.
Protein Immunoblotting and Immunoassay
Floating and adherent cells were collected and washed with ice-cold phosphate buffered saline (PBS), harvested, lysates prepared and protein estimations performed as described previously (34). Samples were separated by SDS-PAGE and transferred to 0.45 μm Immobilon-P transfer membrane (Millipore, Watford, Herts, UK). The membranes were blocked for 1 hour in blocking solution (5% milk in TBST) before incubating at 4°C, shaking overnight in the following antibodies diluted in blocking solution: AKT, phospho-AKT (Ser473), phospho-GSK3β (Ser9), phospho-S6RP (Ser240/244), FKHR, cleaved PARP (Asp214) all at 1:1000, S6 ribosomal protein at 1:2000, phospho-S6RP (Ser235/236) at 1:10,000, phospho-FKHR (Thr24)/FKHRL1 (Thr32) at 1:500 (all purchased from Cell Signaling Technology), GSK3β at 1:1000 (BD Biosciences, Oxford, UK), cyclin D1 Ab-1 (DCS-6) at 1:1000 (NeoMarkers, Lab Vision, Freemont, CA, USA) and GAPDH at 1:2×106 (Chemicon International, Millipore, Watford, Herts, UK). The membranes were washed before incubation in goat anti-rabbit (1:1000 or 1:5000) or anti-mouse (1:10,000) HRP-conjugated secondary antibody for 1 hour at room temperature. Bands were visualized with ECL Western Blotting Detection Reagents and developed using Hyperfilm (GE Healthcare, Bucks, UK).
For the electrochemiluminescent immunoassay, the Meso Scale Discovery (MSD®) platform (Gaithersburg, Maryland, USA) was utilized. Cell lysates were prepared as described and probed for phosphorylation of Ser473 AKT, Ser9 GSK3β, Ser235/236 S6RP, and total forms of AKT, GSK3β, and S6RP according to the manufacturer's instructions. Analysis was completed as recommended by the manufacturer (33, 35, 36).
Annexin V Staining
U87MG cells were plated in 6-well plates at 2×105 cells per well in 2ml growth media and grown for 24 hours before treatment. Inhibitor or DMSO was added at the specified concentrations. After the respective treatment times, adherent and floating cells were collected and washed in PBS by centrifugation. Cells were then washed in annexin-binding buffer (10mM HEPES, 140mM NaCl, 2.5mM CaCl2), resuspended in 10μg/ml annexin V solution and incubated at room temperature for 15 minutes. Immediately prior to reading on a FACS Calibur flow cytometer, 10μg/ml propidium iodide was added to the cell mix.
Pharmacokinetic Studies
Male athymic BALB/c mice (nu/nu) were obtained from Harlan (Harlan UK, Bicester, UK) and allowed access to food and water ad libitum. The care and treatment of experimental animals were in accordance with the United Kingdom Coordinating Committee for Cancer Research (UKCCCR) guidelines (37).
A single dose of AT7867 was administered to BALB/c mice at 5mg/kg intravenously (i.v.) and 20mg/kg per os (p.o.) Compound was formulated in a vehicle containing 10% DMSO, 20% water and 70% hydroxypropyl-β-cyclodextrin (25% aqueous, w/v). Plasma samples were collected from duplicate animals at each of the following time points; 0.083, 0.167, 0.33, 0.67, 1, 2, 4, 6, 16 and 24 hours following i.v. dosing and at 0.25, 0.5, 1, 2, 4, 6 and 24 hours following p.o. dosing. Mice were bled by cardiac puncture and all blood samples were centrifuged to obtain plasma, which was then frozen at −20°C until analysis. For bioanalysis, all plasma samples were prepared by protein precipitation with acetonitrile containing internal standard. Quantification of sample extracts was by comparison with a standard calibration line constructed with AT7867 and using an inhibitor specific liquid chromatography tandem mass spectrometry (LC-MS/MS) method. Pharmacokinetic parameters were determined using WinNonLin® software.
In vivo Tumor Studies
Human MES-SA uterine sarcoma cells or U87MG human glioblastoma cells were injected subcutaneous (s.c.) in the right flank of each animal. Animals were randomized and treatment was started with vehicle or AT7867 when established tumors were ~100mm3 in mean volume. Control mice received vehicle only (10% DMSO, 90% saline) and treated mice received 20mg/kg AT7867 intraperitoneally (i.p.) or 90mg/kg AT7867 p.o. once every 3 days. Tumor size and body weight were monitored three times a week. Tumor size was evaluated by measurement with digital calipers and changes in tumour volume in treated mice (T) versus control mice mice (C) are given as T divided by C (T/C ratios).
To assess the pharmacokinetic (PK) and pharmacodynamic (PD) profile, a single dose of AT7867 (90mg/kg p.o. or 20mg/kg i.p.) was given to mice bearing MES-SA tumor xenografts. Compound was formulated in vehicle containing 0.1% Tween-20. Plasma and tumor samples were harvested at 2, 6 and 24 hours following dosing. Mice were bled by cardiac puncture and plasma samples were collected and frozen at −20°C until analysis. Tumors were dissected and divided into two approximately equal pieces. One piece was frozen at −20°C until PK analysis whilst the other was snap frozen in liquid nitrogen until PD analysis. For PK bioanalysis, all tissue samples were first homogenized in 5 volumes (w/v) of acetonitrile/water (50/50). AT7867 was extracted from plasma and tissue homogenates and quantified as described above. For PD studies, tumors were ground to a powder under liquid nitrogen, lysed and centrifuged to remove debris. Protein content was analyzed using BCA reagent and samples run on western blots as described above.
Results
Identification of a potent ATP-competitive inhibitor of AKT
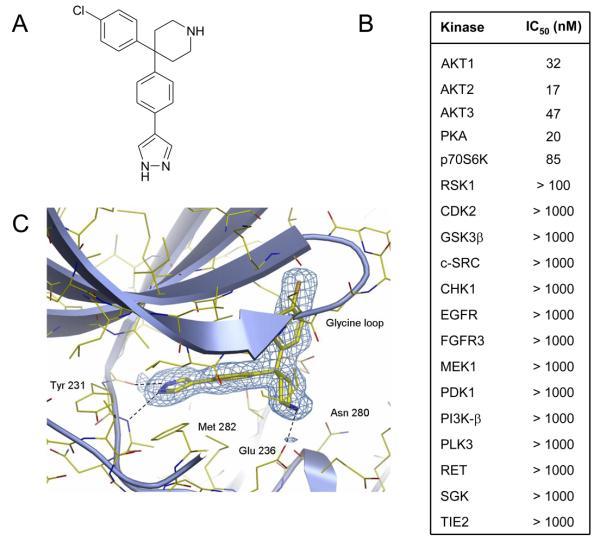
AT7867 was discovered using fragment-based screening combined with structure-based design, and was previously referred to as compound 8a by Saxty et al (31). The molecule is a pyrazole, linked via the 4-position to a geminally substituted 4,4-biaryl piperidine, with the terminal aromatic group incorporating a para-chloro substituent (Figure 1A). The compound is further distinguished by its relatively low molecular weight of 337 Da. For the first time, we report here the detailed biological activity of AT7867. This compound exhibited nanomolar potency towards all 3 AKT isoforms using isolated enzyme assays (Figure 1B). AT7867 also displayed potent activity against the structurally related AGC kinases p70S6K and PKA, but showed a clear window of selectivity against kinases from other kinase sub-families (Figure 1B).
Figure 1.
Structure and kinase inhibitory activity of AT7867. A, chemical structure of AT7867. B, activity of AT7867 against selected kinases as determined by in vitro kinase assays. C, X-ray structure of AT7867 bound to the ATP binding site of AKT2.
The inhibition of AKT2 by AT7867 was shown to be ATP-competitive with a Ki of 18nM. Binding at the ATP site was confirmed by determining the three-dimensional structure of the AT7867-AKT2 complex using X-ray crystallography (Figure 1C). The structure revealed that AT7867 fulfills a 3-point pharmacophore required for potent binding to AKT2, forming hydrogen bonding interactions with the kinase hinge region, electrostatic interactions with the ribose site, as well as hydrophobic contacts with a lipophilic pocket in the glycine-rich loop. Specifically, the pyrazole makes a bi-dentate interaction with the hinge, forming hydrogen bonds with the backbone carbonyl of Glu 230, and the amide nitrogen of Ala 232. The central phenyl ring is slightly twisted with respect to the pyrazole, and is sandwiched between the hydrophobic side-chains of Val 166 and Met 282, which form the top and bottom of the ATP-cleft. The ribose binding site is occupied by the piperidine ring, where the basic nitrogen forms electrostatic and hydrogen bonding interactions with the side-chain of Glu 236, and the backbone carbonyl of Glu 279. The final element of the AKT-pharmacophore is fulfilled by the chlorophenyl, which is directed towards a lipophillic pocket formed by the side-chains of Lys 181, Leu 183, and the face of the glycine-rich loop in the region of Gly 161 and Gly 164.
AT7867 inhibits AKT activity in cells
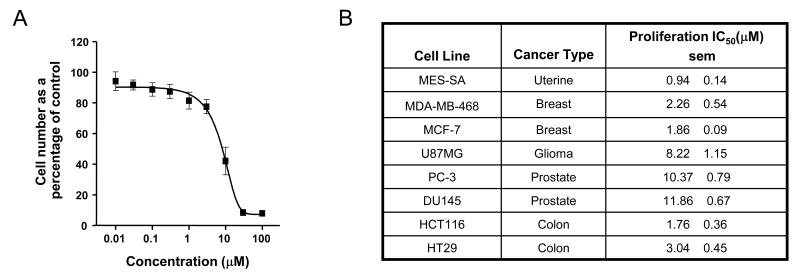
In vitro growth inhibition studies showed that AT7867 blocked proliferation in a number of human cancer cell lines (Figures 2A and 2B). These cell lines represent common cancers that have been reported to exhibit deregulation of the PI3K/AKT pathway by mechanisms such as PTEN or PIK3CA mutations. AT7867 appeared to be most potent at inhibiting proliferation in MES-SA uterine, MDA-MB-468 and MCF-7 breast, and HCT116 and HT29 colon lines (IC50 values range from 0.9-3 μM), and least effective in the two prostate lines tested (IC50 values range from 10-12 μM), as indicated in the figure.
Figure 2.
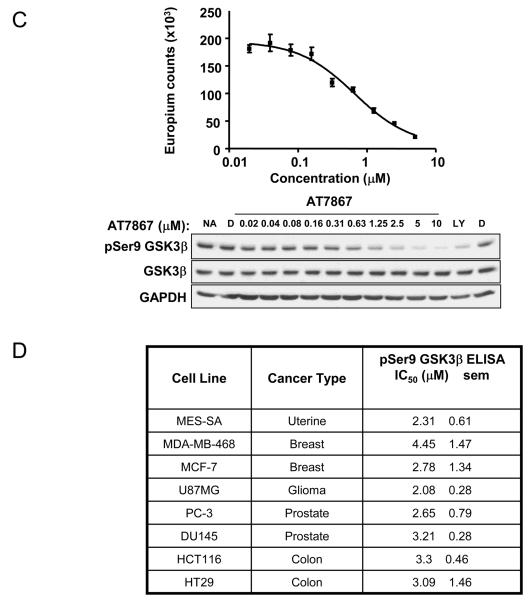
AT7867 inhibits proliferation of human cancer cell lines and reduces phosphorylation of GSK3β. A, PTEN-negative U87MG human glioblastoma cells were treated with a range of concentrations of AT7867 for 72 hours. Alamar blue cell viability assays were performed to measure cell growth inhibition. B, inhibition of cell growth across a panel of human cell lines from various tumor types. IC50 values are shown as the mean (n=3) with the standard error of the mean (sem). C, Cells were incubated with a range of concentrations of AT7867 for 1 hour and the in-cell phospho-GSK3β ELISA was performed in parallel with western blot of cell lysates in U87MG cells. No addition (NA) and DMSO (D) vehicle were used as negative controls, and 20μM LY294002 (LY) was used as a positive control. D, a panel of human tumor cell lines were exposed to AT7867, the in-cell GSK3β ELISA performed and the IC50 for phospho-Ser9 GSK3β signal determined.
The U87MG glioblastoma cell line is PTEN-deficient and exhibits a high level of phosphorylation of serine 473 on AKT (38), suggesting an overactive AKT pathway. This cell line has previously been used to investigate the cellular properties of several PI3K pathway inhibitors (35, 36, 39-41). For these reasons, further detailed investigations were performed using U87MG cells.
The ability of AT7867 to inhibit AKT activity in human tumor cells was measured by investigating the phosphorylation state of the direct downstream substrate GSK3β following a 1 hour incubation with compound (Figure 2C). For accurate quantification of AKT inhibition activity in cells, a 96-well plate-based ELISA was established, which allowed quantification of the level of phosphorylation of GSK3β at serine 9 in intact cells. Figure 2C shows western blot data and the corresponding IC50 data generated using the cell-based ELISA. The assay was transferable between cell lines, thus allowing the quantification of AT7867 activity in multiple lines. These experiments showed that AT7867 was equipotent at inhibiting phosphorylation of GSK3β across all cancer cell lines tested with IC50 values in the range 2 – 4μM (Figure 2D).
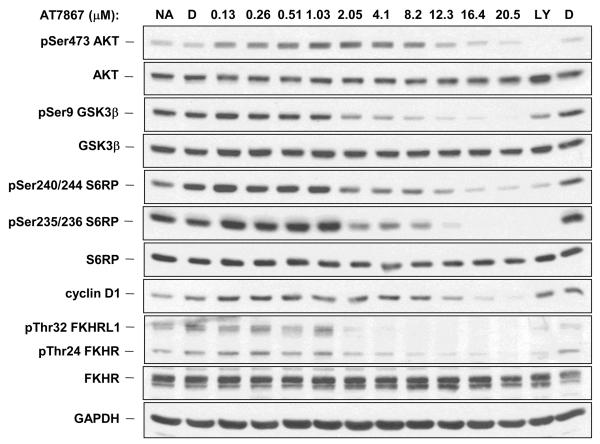
Investigation of additional AKT pathway components demonstrated that a 1 hour treatment of the U87MG cell line with AT7867 induced effects on the levels of a number of cellular proteins in a concentration-dependent fashion (Figure 3A). This included the phosphorylation of the following AKT direct substrates: GSK3β, pro-apoptotic transcription factors FKHR (FoxO1a) and FKHRL1 (FoxO3a) and the downstream target S6RP. Expression of the cell cycle protein cyclin D1 was also measured. In addition to the decrease in phosphorylation of downstream substrates and a decrease in expression of cyclin D1, there was an induction of AKT phosphorylation at serine 473. However, at higher concentrations of compound, phosphorylation on AKT itself was also inhibited.
Figure 3.
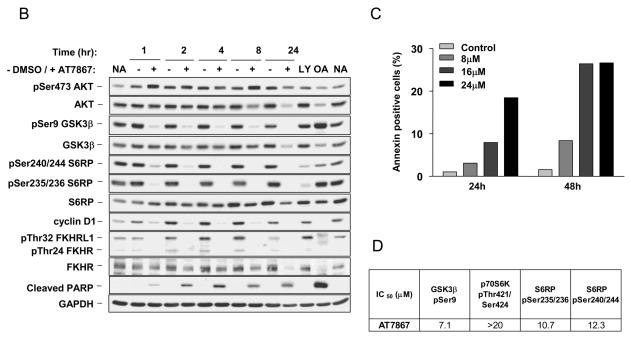
AT7867 suppresses AKT signalling in U87MG human glioblastoma cells in a concentration-dependent and time-dependent manner and induces apoptosis. A, PTEN-negative U87MG human glioblastoma cells were incubated with AT7867 for 1 hour and western blot analyses performed to assess the phosphorylation and expression of AKT pathway proteins. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. No addition (NA) and DMSO (D) vehicle were used as negative controls, and 20μM LY294002 (LY) was used as a positive control. B, U87MG cells were treated with 16 μM AT7867 for, 1, 2, 4, 8 and 24 hours, and western blot analyses performed for AKT pathway proteins as indicated in the figure and the appearance of cleaved PARP. GAPDH was used as a loading control. No addition (NA) and DMSO (D) vehicle were used as negative controls, 20μM LY294002 (LY) was used as a positive control. 100 nM Okadaic acid for 24 hours (OA) was used as a positive control for apoptosis. C annexin V staining of U87MG cells following treatment with 8, 16 or 24μM AT7867 or DMSO vehicle (Control) for 24 or 48 hours. D, U87MG cells were treated with 0.1-20μM AT7867 for 1 hour. Lysates were prepared and analysis of AKT pathway proteins was performed by electrochemiluminescent immunoassay (MSD®) as indicated in the figure.
An additional study investigating the cellular consequences over time of AKT inhibition was also carried out. This confirmed the loss of phosphorylation on the direct AKT substrates GSK3β, FKHR and FKHRL, as well as on the downstream target S6RP (Figure 3B). Between 1-8 hours, there was a substantial loss of all phosphorylation signals under investigation. This inhibition was sustained together with loss of total proteins at 24 hours in the presence of AT7867. Loss of cyclin D1 expression was seen at one hour post-treatment and was maintained over the 24-hour period of the experiment, at which time the amount of AKT pathway proteins was reduced to below control levels. Since loss of phosphorylation on the pro-apoptotic proteins FKHR and FKHRL allows their entry into the nucleus to facilitate gene transcription leading to activation of apoptosis, the effect of AT7867 on apoptosis was studied (16). Cleavage of the protein poly(ADP-ribose) polymerase (PARP) is an event that occurs early in the apoptotic process and as such, cleaved PARP serves as a marker of cells undergoing apoptosis (42). The appearance of cleaved PARP was observed in cells exposed to AT7867 from one hour onwards for the duration of the study and correlated with the reduction in protein levels described above for the pathway components (Figure 3B). Apoptosis was further investigated by Annexin V staining, which detects phosphatidylserine on the external surface of intact cells, another hallmark of apoptosis (43). A concentration- and time-dependent increase in the amount of Annexin V staining was observed (Figure 3C).
In view of the effects of AT7867 on AKT and p70S6K, the MSD® electrochemiluminescent immunoassay platform was used to quantify the inhibitory effects of this compound on the phosphorylation of GSK3β, p70S6K and S6RP following AT7867 treatment in U87MG glioblastoma cells (Figure 3D). At one hour post-treatment, the IC50 for pSer9 GSK3β was 7.1μM. An IC50 value could not, however, be obtained for pThr421/424 of p70S6K, as it was greater than the maximal concentration of AT7867 used in the assay (20μM). In contrast, IC50 values of 10.7 μM and 12.3μM were obtained for pSer235/236 and pSer240/244 of S6RP.
In vivo activity of AT7867
Having shown concentration-dependent and time-dependent effects of AT7867 in vitro, the properties of the compound were evaluated in vivo in mice. The plasma clearance of AT7867 following 5mg/kg i.v. administration was moderate and the compound was still detectable in plasma at 24 hours post administration (Figure 4A). Following oral administration at 20mg/kg, the elimination of AT7867 from plasma appeared to be similar to that observed after i.v. administration (Figure 4A). Plasma levels of AT7867 remained above 0.5μM for at least 6 hours following an oral dose of 20mg/kg. Assuming linear pharmacokinetics following i.v. administration, the bioavailability by the oral route was calculated to be 44% (Figure 4B).
Figure 4.
The plasma pharmacokinetic (PK) profile of AT7867 in vivo and comparison of expression of AKT pathway proteins in the MES-SA human uterine versus U87MG glioblastoma and MCF-7 and MDA-MB-468 breast cell lines. A and B, plasma PK in mice following administration of AT7867 at 20mg/kg p.o. and 5mg/kg i.v. C, western blot analysis of AKT pathway proteins in lysates prepared from MES-SA, U87MG, MCF7 and the MDA-MB-468 cell lines. GAPDH was used as a loading control.
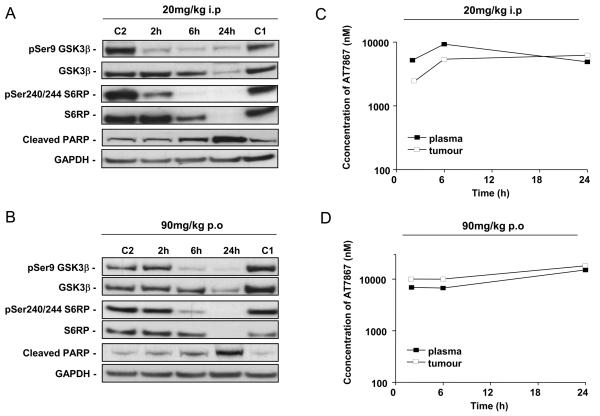
From in vitro cell growth studies, the MES-SA uterine cell line appeared to be the most sensitive to growth inhibition by AT7867. This cell line does not express PTEN and contains high levels of phosphorylated AKT, indicating an overactive pathway (Figure 4C). In vivo PD biomarker studies were therefore performed with this model. Following pharmacokinetic (see above) and tolerability studies, doses of AT7867 (90mg/kg p.o. or 20mg/kg i.p.) were administered to athymic mice bearing MES-SA tumors and the phosphorylation status of GSK3β and S6RP in tumors was monitored over time. Clear inhibition of phosphorylation of the two markers of pathway activity was seen at 2 and 6 hours following treatment with AT7867 (Figures 5A and 5B). By 24 hours, total levels of both GSK3β and S6RP were greatly reduced, as also seen in the in vitro studies. For both routes of administration and at each time point investigated, the level of compound in the plasma and tumor exceeded the cellular IC50, which was consistent with the prolonged mechanistic biomarker effect (Figures 5C and 5D). As seen in studies in vitro, sustained compound exposure induced an increase in cleaved PARP, indicating that AT7867 also induces apoptosis in the xenograft tumor cells (Figures 5A and B).
Figure 5.
The pharmacodynamic (PD) and pharmacokinetic (PK) profile of AT7867. Athymic BALB/c mice bearing MES-SA human uterine xenograft tumors were treated with a single dose of AT7867 at 20mg/kg i.p. and 90mg/kg p.o.. Plasma and tumors were harvested at 2, 6 or 24 hours after dosing. A and B, phospho and total GSK3β, S6RP, cleaved PARP and GAPDH were assessed from tumor protein lysates by western blotting at each dose as indicated in the figure. C1 (control 1), C2 (control 2). C and D, plasma and tumor concentrations of AT7867 were assessed by LC-MS/MS at each dose as indicated in the figure.
Next we determined the effect of AT7867 on the growth of MES-SA tumor xenografts. Marked inhibition of tumor growth, as assessed by changes in volume, were seen with this model when dosed at the same levels and routes utilized for the PD studies described above. Specifically, a T/C of 0.37 was observed at 20mg/kg i.p. (measured at the termination of the experiment on day 12; Figure 6A) and of 0.38 at 90mg/kg p.o. (measured on day 10; Figure 6B). Additional in vivo xenograft studies were carried out using the U87MG cell line which was sensitive to the effects of AT7867 in vitro, although less so than the MES-SA cell line. Figure 6C shows a T/C of 0.51 (measured on day 13), when 20mg/kg AT7867 was dosed on this schedule via the i.p. route of administration.
Figure 6.
AT7867 suppresses MES-SA human uterine and U87MG human glioblastoma xenograft tumor growth. A, athymic BALB/c mice bearing MES-SA xenograft tumors were administered AT7867 20mg/kg i.p. once every 3 days for 16 days. B, athymic BALB/c mice bearing MES-SA xenograft tumors were administered AT7867 at 90mg/kg p.o. once every 3 days for 12 days. C, athymic BALB/c mice bearing U87MG xenograft tumors were administered AT7867 at 20mg/kg i.p. once every 3 days for 13 days. Tumor size was monitored three times a week and relative tumour volumes calculated.
Discussion
AKT activity has been implicated in the regulation of tumor cell growth, proliferation, metastasis and apoptosis (1). Recently the PI3K/AKT signal transduction pathway has become the focus of intense interest as a critical regulator of tumor cell survival, and a number of AKT pathway inhibitors have been disclosed with a wide variety of potencies and specificities (2, 44, 45). In this report, we describe the in vitro and in vivo effects of AT7867, a recently developed inhibitor of AKT and p70S6K, which has been identified using fragment-based lead discovery and structure-based design technologies (29-32).
AT7867 is a potent inhibitor of AKT and p70S6K that blocks the cellular phosphorylation of the AKT substrate GSK3β and the p70S6K substrate S6RP and inhibits the proliferation of a range of human tumor cell lines. It is interesting to note that while the IC50 for growth inhibition varied 12-fold (1-12μM), the IC50 for GSK3β phosphorylation was much more consistent (2-4.5μM) across the same cell line panel. This discrepancy is perhaps not surprising since the response of different cell lines to the same level of pathway inhibition may be dependent on factors other than genetic alterations on the PI3K/AKT pathway. Further detailed studies in the PTEN-deficient human glioblastoma U87MG cell line demonstrated that AT7867 caused both a concentration-dependent and time-dependent reduction in the phosphorylation of proteins downstream of AKT, including the AKT substrate GSK3β and p70S6K target S6RP. Cyclin D1 expression and thus cell cycle progression is regulated at the post-translational level by GSK3β and also by protein translation through p70S6K via mTOR (12). The demonstration that AT7867 suppresses the expression of cyclin D1 is consistent with growth inhibition being mediated via these kinases. Induction of phosphorylation on AKT itself at serine 473 was also noted, consistent with the compensatory but futile feedback regulation of AKT previously described with other inhibitors of this pathway (46-48).
Growing evidence has challenged the view of a linear PI3K/AKT/mTOR pathway, with the identification of crosstalk and feedback regulation within the pathway (8, 18). Despite induction of pSer473 AKT, the phosphorylation of downstream AKT targets was markedly decreased in the presence of AT7867 both in cancer cell lines in vitro and human tumor xenografts in immunosuppressed mice. Recent papers have suggested that dual inhibition of PI3K and mTOR could overcome these feedback mechanisms through vertical blockade of key components of the pathway (35, 41, 49). AT7867 has little activity outside the AGC kinase family members but is a potent inhibitor of the structurally related AGC kinases PKA, and p70S6K, as well as AKT. In order to dissect the respective contributions of AKT and p70S6K inhibition along this pathway to AT7867-mediated effects, the relative modulation in substrate phosphorylation signals were quantified in U87MG cells. Whilst the IC50 for pSer9 GSK3β was 7.1μM, an IC50 value could not be generated for p70S6K as it was greater than the highest concentration of 20μM used in this assay. This suggests a dilution of the inhibition of the pathway signal, as p70S6K is more distal than GSK3β from the AKT target. In contrast, IC50 values were obtained for both pSer235/236 and pSer241/244 of S6RP of 10.7μM and 12.3μM respectively confirming the direct inhibitory effects of AT7867 against p70S6K. It is an intriguing possibility that the combined effects of targeting p70S6K and AKT with this compound may serve to improve its therapeutic potential by delivering a double vertical blockade to the pathway.
It should be noted that, based on kinase profiling in in vitro biochemical assays, it is possible that inhibition of PKA may contribute to the cellular effects of AT7867. However, as there are no suitable methods or reagents that can accurately dissect the specific effects of PKA on its target substrates in the cell lines used, this possibility could not be pursued at this time. Further studies are also needed to profile the potential effects of AT7867 on other signaling pathways and gene expression profiling studies using cDNA microarray are now underway to address this in an unbiased fashion.
A major function of AKT in cancer cells is to prevent apoptosis by the phosphorylation and inactivation of pro-apoptotic targets, such as the forkhead transcription factors and BAD (50). We have shown that AT7867 prevents phosphorylation of FKHR and FKHRL1 and inhibits tumor cell growth, an effect that may in part be attributed to the induction of apoptosis caused by the compound. The pro-apoptotic effect of AT7867 is supported by the induction of cleaved PARP and annexin V staining in treated cells, which correlated with the reduction in protein levels for PI3K/AKT pathway components at later time points (Figure 3B).
Although the PI3K/AKT pathway has often been associated with survival signaling and apoptosis (1), there has been equivocal evidence in support of this hypothesis from studies involving the pharmacological ablation of key components of this key signaling cascade. We have previously shown that the potent and selective class I PI3K and mTOR inhibitor PI-103 does not cause significant apoptosis in a wide range of tumor cell lines tested (35, 41). In contrast, we have shown here that AT7867 results in extensive apoptosis, both in vitro and in vivo, suggesting a difference in the simultaneous effects of inhibiting both AKT and p70S6K versus PI3K and mTOR. These data suggest that there may be unknown mechanisms resulting in this vital difference in survival signaling, with the possibility of crosstalk or other undiscovered pathway proteins playing a key role. Also, the results suggest that combined inhibitors of AKT and p70S6K may potentially be efficacious as monotherapy.
Pharmacokinetic studies showed that potentially active concentrations of AT7867 could be achieved in plasma and tumor after 20mg/kg i.p. or 90 mg/kg p.o. reflecting the relatively high oral bioavailability of this compound. The in vitro PD biomarker effects of AT7867 described above were also observed in tumor models in vivo. Administration of AT7867 p.o. or i.p. to mice bearing human tumor xenografts caused suppression of phosphorylation of the AKT and p70S6K molecular biomarkers GSK3β and S6RP and a concomitant increase in the levels of cleaved PARP. These PD effects were achieved at plasma and tumor drug concentrations similar to those required to suppress the same markers in vitro and at doses that cause inhibition of human tumor xenograft growth (Figures 6A-C). Therefore, these data are consistent with inhibition of AKT and p70S6K as the mechanism of tumor growth suppression for AT7867 in vivo.
In conclusion, we have shown that AT7867 suppresses tumor cell proliferation, induces apoptosis and exhibits oral antitumor activity. Importantly, we show that AT7867 suppresses the phosphorylation of downstream markers of AKT and p70S6K in tumor tissue at doses that also inhibit human xenograft tumor growth. The therapeutic response to AKT and p70S6K pathway inhibition observed in the current study makes this approach attractive for clinical application, where such a novel inhibitor of both AKT and p70S6K may have the potential to cause antitumor activity as a single agent or in combination with other agents.
Acknowledgements
This work was supported by Cancer Research UK grant number C309/A8274, through the Experimental Cancer Medicine Centre (ECMC) network initiative, CR-UK grant number C51/A7401, The Institute of Cancer Research and was carried out as part of a funded research collaboration with Astex Therapeutics. We acknowledge NHS funding to the NIHR Biomedical Research Centre.
Financial support:
Grant support was provided to Lisa-Jane K. Hunter, Timothy A Yap, Mike I. Walton, Paul Workman and Michelle D. Garrett by Cancer Research UK (CR-UK) grant number C309/A8274 and to Simon Heaton through the Experimental Cancer Medicine Centre (ECMC) network initiative, CR-UK grant number C51/A7401. Additional support was provided to Michelle D. Garrett by The Institute of Cancer Research and to Lisa-Jane K. Hunter by Astex Therapeutics.
Abbreviations
- GSK3β
glycogen synthase kinase-3β
- mTORC
mammalian target of rapamycin complex
- PDK1
phosphoinositide-dependent kinase 1
- PI3K
phosphatidylinositol 3-kinase
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- PTEN
phosphatase and tensin homolog on chromosome 10
- p70S6K
p70 S6 kinase
- S6RP
S6 ribosomal protein
- TBST
tris-buffered saline with 0.1% Tween-20
References
- 1.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002 Jul;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008 Aug 27; doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 4.Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. The EMBO journal. 1996 Dec 2;15(23):6541–51. [PMC free article] [PubMed] [Google Scholar]
- 5.Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997 Apr 1;7(4):261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 6.Stokoe D, Stephens LR, Copeland T, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997 Jul 25;277(5325):567–70. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005 Feb 18;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 8.Balendran A, Casamayor A, Deak M, et al. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999 Apr 22;9(8):393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- 9.Persad S, Attwell S, Gray V, et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001 Jul 20;276(29):27462–9. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004 Sep 24;279(39):41189–96. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 11.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998 May 29;273(22):13375–8. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 12.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006 Oct 16;25(48):6416–22. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 13.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochemical Journal. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downward J. How BAD phosphorylation is good for survival. Nat Cell Biol. 1999 Jun;1(2):E33–5. doi: 10.1038/10026. [DOI] [PubMed] [Google Scholar]
- 15.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998 Nov 13;282(5392):1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 16.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999 Mar 19;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 17.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008 Sep 18;27(41):5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 18.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007 Jul 26;448(7152):439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 19.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5034–7. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000 Feb 18;100(4):387–90. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 21.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004 Apr 23;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 22.Kim D, Dan HC, Park S, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005 Jan 1;10:975–87. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 23.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001 May 15;61(10):3986–97. [PubMed] [Google Scholar]
- 24.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004 Aug;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Lopiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: Effective combinations and clinical considerations. Drug Resist Updat. 2007 Dec 29; doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006 May 19;125(4):733–47. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Workman P, Clarke P, Raynaud F, van Montfort R. Drugging the PI3 Kinome: From Chemical Tools to Drugs in the Clinic. Cancer Res. 2010 Mar 15;70(6) doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang S, Houghton PJ. Inhibitors of mammalian target of rapamycin as novel antitumor agents: from bench to clinic. Curr Opin Investig Drugs. 2002 Feb;3(2):295–304. [PubMed] [Google Scholar]
- 29.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nature structural biology. 2002 Dec;9(12):940–4. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- 30.Donald A, McHardy T, Rowlands MG, et al. Rapid evolution of 6-phenylpurine inhibitors of protein kinase B through structure-based design. J Med Chem. 2007 May 17;50(10):2289–92. doi: 10.1021/jm0700924. [DOI] [PubMed] [Google Scholar]
- 31.Saxty G, Woodhead SJ, Berdini V, et al. Identification of inhibitors of protein kinase B using fragment-based lead discovery. J Med Chem. 2007 May 17;50(10):2293–6. doi: 10.1021/jm070091b. [DOI] [PubMed] [Google Scholar]
- 32.Davies TG, Verdonk ML, Graham B, et al. A structural comparison of inhibitor binding to PKB, PKA and PKA-PKB chimera. Journal of molecular biology. 2007 Mar 30;367(3):882–94. doi: 10.1016/j.jmb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Gowan SM, Hardcastle A, Hallsworth AE, et al. Application of meso scale technology for the measurement of phosphoproteins in human tumor xenografts. Assay Drug Dev Technol. 2007 Jun;5(3):391–401. doi: 10.1089/adt.2006.044. [DOI] [PubMed] [Google Scholar]
- 34.Fry DW, Bedford DC, Harvey PH, et al. Cell cycle and biochemical effects of PD 0183812. A potent inhibitor of the cyclin D-dependent kinases CDK4 and CDK6. J Biol Chem. 2001 May 18;276(20):16617–23. doi: 10.1074/jbc.M008867200. [DOI] [PubMed] [Google Scholar]
- 35.Guillard S, Clarke PA, Te Poele R, et al. Molecular pharmacology of phosphatidylinositol 3-kinase inhibition in human glioma. Cell cycle (Georgetown, Tex. 2009 Feb 1;8(3):443–53. doi: 10.4161/cc.8.3.7643. [DOI] [PubMed] [Google Scholar]
- 36.Raynaud FI, Eccles SA, Patel S, et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Molecular cancer therapeutics. 2009 Jul;8(7):1725–38. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Workman P, Twentyman P, Balkwill F, et al. United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) Guidelines for the Welfare of Animals in Experimental Neoplasia (Second Edition) Br J Cancer. 1998;77(1):1–10. doi: 10.1038/bjc.1998.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998 Oct 22;8(21):1195–8. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 39.Shingu T, Yamada K, Hara N, et al. Synergistic augmentation of antimicrotubule agent-induced cytotoxicity by a phosphoinositide 3-kinase inhibitor in human malignant glioma cells. Cancer research. 2003 Jul 15;63(14):4044–7. [PubMed] [Google Scholar]
- 40.Yu K, Lucas J, Zhu T, et al. PWT-458, a novel pegylated-17-hydroxywortmannin, inhibits phosphatidylinositol 3-kinase signaling and suppresses growth of solid tumors. Cancer Biol Ther. 2005 May;4(5):538–45. doi: 10.4161/cbt.4.5.1660. [DOI] [PubMed] [Google Scholar]
- 41.Raynaud FI, Eccles S, Clarke PA, et al. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer research. 2007 Jun 15;67(12):5840–50. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 42.Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998 Dec 11;273(50):33533–9. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- 43.Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995 Nov 1;182(5):1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008 Sep 18;27(41):5511–26. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 45.Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Cancer Ther. 2009 Jan;8(1):1–9. doi: 10.1158/1535-7163.MCT-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han EK, Leverson JD, McGonigal T, et al. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007 Aug 16;26(38):5655–61. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 47.Luo Y, Shoemaker AR, Liu X, et al. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005 Jun;4(6):977–86. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 48.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006 Feb 1;66(3):1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006 May;9(5):341–9. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005 Jan-Mar;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]