Abstract
Control of influenza A virus (IAV) in pigs is done by vaccination of females to provide maternally-derived antibodies (MDA) through colostrum. Our aim was to evaluate if MDA interfere with IAV infection, clinical disease, and transmission in non-vaccinated piglets. In a first study, naïve sows were vaccinated with H1N2-δ1 whole inactivated virus (WIV) vaccine. In a follow-up study seropositive sows to 2009 pandemic H1N1 (H1N1pdm09) were boosted with H1N1pdm09 WIV or secondary experimental infection (EXP). MDA-positive pigs were challenged with homologous or heterologous virus, and MDA-negative control groups were included. WIV-MDA piglets were protected from homologous infection. However, piglets with WIV-derived MDA subsequently challenged with heterologous virus developed vaccine associated enhanced respiratory disease (VAERD), regardless of history of natural exposure in the sows. Our data indicates that although high titers of vaccine-derived MDA reduced homologous virus infection, transmission, and disease, MDA alone was sufficient to induce VAERD upon heterologous infection.
Keywords: swine, influenza, vaccine associated enhanced respiratory disease, maternally-derived antibodies
Introduction
Influenza A virus (IAV) is endemic in pigs in North America and greatly impacts the swine industry due to health related losses. In addition, there are public health concerns in regards to the zoonotic potential of swine-adapted lineages of IAV (Myers et al., 2007). IAV evolves rapidly in pigs and many distinct strains currently co-circulate in North American swine populations. Endemic swine viruses can be classified by their hemagglutinin (HA) gene into different phylogenetic clusters within the H3 (IV-A, -B, -C, -D, -E, -F) and H1 (α, β, γ, δ1, δ2 and H1N1pdm09) subtypes (Lorusso et al., 2013; Vincent et al., 2008b). Different genetic clusters are often antigenically distinct, and limited serological cross-reactivity can be detected even within a cluster, such as the δ clusters (Lewis et al., 2014; Lorusso et al., 2011; Vincent et al., 2008b). This limited cross-reactivity represents an obstacle to efficacious vaccine development. Vaccination of breeding females against IAV to stimulate passive antibody transfer via colostrum is a common practice in the U.S. swine industry and is typically done using multivalent whole inactivated virus (WIV) vaccines (Vincent et al., 2008b). In homologous infections, in which vaccine and challenge viruses are similar or matched, MDA acquired via colostrum are correlated with protection of piglets from clinical disease, but without a reduction of upper respiratory tract viral shedding (Kitikoon et al., 2006; Loeffen et al., 2003). However, significant levels of MDA were associated with inhibition of the active IgA, IgM, IgG, and hemagglutination inhibition (HI) responses, as well as the proliferative T-cell response upon primary or secondary exposure to the virus (Loeffen et al., 2003; Loving et al., 2014; Loving et al., 2013; Sandbulte et al., 2014; Vincent et al., 2012).
Non-neutralizing, cross-reacting immunity elicited following administration of adjuvanted, inactivated vaccines not only fails to protect against homosubtypic heterologous viruses, but can lead to severe bronchointerstitial pneumonia with necrotizing bronchiolitis, a phenomenon known as vaccine-associated enhanced respiratory disease (VAERD) (Gauger et al., 2012; Gauger et al., 2011; Vincent et al., 2008a). Exacerbated pneumonia was reported in unvaccinated piglets with MDA from sows vaccinated with a commercial WIV (Pyo et al., 2015). Yet, in that same study and others (Loving et al., 2012; Vincent et al., 2007) live-attenuated influenza virus (LAIV) vaccines induced mucosal immune responses and provided improved cross-protection to heterologous IAV challenge in pigs, even in the presence of MDA (Pyo et al., 2015; Vincent et al., 2012), thus presenting an alternative to improve vaccine efficacy in piglets.
Though currently available commercial inactivated products do not provide optimal protection, vaccination of dams with WIV can be beneficial in case of homologous exposure of litters, reducing clinical signs and shedding, and is still frequently used as a control measure against IAV infection. Here, we investigated if the presence of passive MDA at the time of heterologous challenge would result in enhanced disease. Our study used two scenarios: one in which seronegative sows were vaccinated with WIV as a proof of concept and the other a scenario likely to occur in the field in which seropositive sows previously naturally exposed to IAV were vaccinated with the same virus strain and their litters were challenged with the homologous or heterologous virus. Our findings show that although high titers of vaccine-derived MDA reduced homologous virus infection, transmission, and disease, MDA alone was sufficient to induce VAERD upon heterologous infection.
Material and Methods
Vaccines and viruses
The antigen for the WIV vaccine in Study 1 was obtained via reverse genetics and contained the HA from A/swine/Minnesota/02011/08 H1N2 δ1 (H1N2-δ1) and the other seven genes from A/turkey/Ohio/313053/2004 H3N2 (here on referred to as H1N2-δ1(1:7)). The H1N1pdm09 antigen used for the WIV vaccine and booster exposure in Studies 2 and 3 was A/New York/18/2009 H1N1. The WIV vaccines were generated by UV inactivation of the viruses, using the “sterilize” setting in a UV cross-linking chamber (GS Gene Linker; Bio-Rad, Hercules, CA). A commercial oil-in-water adjuvant (Emulsigen D, MVP Laboratories, Inc., Ralston, NE) was added at a 1:5 ratio (v/v), and each dose of WIV contained approximately 64 hemagglutination (HA) units. Viruses used for challenge were the H1N2-δ1(1:7) and another reverse genetic-generated virus containing the surface genes from the H1N1pdm09 A/California/04/2009 and the other six genes from A/turkey/Ohio/313053/2004 H3N2 (here on referred to as H1N1pdm09(2:6)). Vaccine and challenge viruses were grown in Madin-Darby canine kidney (MDCK) cells or embryonated chicken eggs.
In vivo Study 1
To investigate if presence of MDA would result in enhanced disease after heterologous infection, we challenged piglets obtained from WIV vaccinated sows. Four naive sows were obtained from a high-health status herd free of porcine reproductive and respiratory syndrome virus (PRRSV) and IAV. Sows were shown to be free of anti-IAV antibodies prior to the start of the study, and were vaccinated intramuscularly with 2 ml of the H1N2-δ1(1:7) WIV. Each sow received 3 doses: 2 weeks prior to breeding; 6 and 4 weeks prior to farrowing. All sows delivered their litters without intervention, and piglets suckled their own dams.
Piglets were bled at 3 days of age for evaluation of IAV-specific MDA transfer by hemagglutination inhibition (HI) assay, and they were weaned at approximately 2 weeks of age. Forty-five two-week-old cross-bred healthy pigs from serologically negative dams were obtained from the same source herd to be used as controls and naïve contacts without IAV-specific MDA. All pigs were treated with ceftiofur crystalline-free acid (Excede®, Zoetis, Florham Park, NJ) and enrofloxacin (Baytril®, Bayer Animal Health, Shawnee Mission, KS) at weaning or arrival to reduce respiratory bacterial contaminants. Pigs were free of IAV by nasal swab sampling, and MDA-negative controls were free of IAV-antibodies prior to start of the study. Pigs were housed in biosafety level 2 (BSL2) containment and cared for in compliance with the Institutional Animal Care and Use Committee of the National Animal Disease Center. At weaning, 20 pigs with MDA and 20 pigs without MDA were divided into 4 groups (Table 1). Four pigs with and 5 pigs without MDA were combined into a non-challenged control (NC) group. At 3 weeks-of-age, pigs in each challenge group (principal pigs) were inoculated simultaneously with 2 ml intratracheally and 1 ml intranasally of 1 × 105 50% tissue culture infective dose (TCID50) per ml of the indicated challenge virus (Table 1). Inoculation was performed under anesthesia following an intramuscular injection of a cocktail of xylazine (4 mg/kg), ketamine (8 mg/kg of body weight), and Telazol (6 mg/kg) (Zoetis Animal Health, Florham Park, NJ). At 2 days post-infection (dpi), 20 MDA-negative pigs (n=5 per group) were placed in separate raised decks in the same room as each inoculated group, to evaluate indirect contact transmission. Principal pigs were observed daily for clinical signs of respiratory disease and rectal temperatures were measured at 0 to 5 dpi. Principal pigs were humanely euthanized with a lethal dose of pentobarbital (Fatal Plus®, Vortech Pharmaceuticals, Dearborn, MI) and necropsied at 5 dpi, when bronchoalveolar lavage fluid (BALF), trachea and right cardiac or affected lung lobe were collected. Indirect contact pigs were bled and humanely euthanized at 15 days post-contact (dpc).
Table 1.
Experimental design and group assignments for all three studies.
| Treatment group | Source of MDA | Challenge strain or vaccine typea | N | Indirect contacts (N) |
|---|---|---|---|---|
| Study 1 | ||||
| No MDA/NC | None | None | 9 | - |
| No MDA/H1N2 | None | H1N2-δ1(1:7)b | 10 | 5 |
| WIV-MDA/H1N2 | WIV | H1N2-δ1(1:7) | 10 | 5 |
| No MDA/H1N1 | None | H1N1pdm09(2:6)b | 10 | 5 |
| WIV-MDA/H1N1 | WIV | H1N1pdm09(2:6) | 10 | 5 |
| Study 2 | ||||
| No MDA/NC | None | None | 9 | - |
| No MDA/H1N1 | None | H1N1pdm09(2:6) | 8 | 5 |
| WIV-MDA/H1N1 | WIV | H1N1pdm09(2:6) | 8 | 5 |
| EXP-MDA/H1N1 | Experimental infectiona | H1N1pdm09(2:6) | 8 | 5 |
| No MDA/H1N2 | None | H1N2-δ1(1:7) | 8 | 5 |
| WIV-MDA/H1N2 | WIV | H1N2-δ1(1:7) | 8 | 5 |
| EXP-MDA/H1N2 | Experimental infectionc | H1N2-δ1(1:7) | 8 | 5 |
| NAT-MDA/H1N2 | Natural infectionc | H1N2-δ1(1:7) | 8 | - |
| Study 3 | ||||
| No MDA/WIV | None | WIV | 2 | - |
| WIV-MDA/WIV | WIV | WIV | 7 | - |
| No MDA/COM | None | Commercial | 2 | - |
| WIV-MDA/COM | WIV | Commercial | 7 | - |
Challenge strains for Studies 1&2, vaccine type for Study 3.
H1N2-δ1(1:7) = virus containing HA from A/swine/Minnesota/02011/08 H1N2 δ1 and the other seven genes from A/turkey/Ohio/313053/04 H3N2; H1N1pdm09(2:6)= virus containing the surface genes from A/California/04/2009 H1N1 and the other six genes from A/turkey/Ohio/313053/04 H3N2.
EXP-MDA pigs suckled sows which were previously naturally exposed and then experimentally boosted with live exposure to H1N1pdm09 and NAT-MDA pigs suckled sows which were previously naturally exposed to H1N1pdm09 only.
In vivo Study 2
In a follow-up study, to mimic a scenario likely to occur in the field, seropositive sows were boosted with a homologous virus strain and their piglets were challenged with homologous or heterologous virus. Six sows with IAV-specific antibodies elicited from previous natural exposure to H1N1pdm09 were subsequently boosted with WIV or additional exposure to wild-type virus to evaluate the differential effect of passive maternal antibody derived from WIV vaccination versus natural exposure on IAV infection in piglets. Sows were either vaccinated intramuscularly with 2 ml of H1N1pdm09 WIV (n=3) or infected intranasally with 2 ml of 1 × 106 TCID50/ml of the virus used in the WIV (n=2). Each sow was boosted using the same vaccine regimen as in Study 1. The remaining sow was not vaccinated or infected to derive piglets that would serve as controls. All sows delivered their litters without intervention, and piglets suckled their own dams.
Forty-five, two-week-old cross-bred, healthy pigs were obtained from the same source as in Study 1 and used as naïve controls and contacts without IAV-specific MDA. All piglets were evaluated, treated and cared for in the same manner as described for Study 1. At weaning, 40 pigs with MDA were divided into 5 groups and 16 pigs without MDA were divided into 2 groups (Table 1). Three pigs from each MDA status were combined into a non-challenged (NC) control group. Piglets with MDA from the non-boosted sow (NAT-MDA) were used to test if MDA elicited by prior natural exposure would have the same effect on IAV infection as MDA transferred after boosting the sows' IAV-specific immunity. Principal pigs were challenged with H1N2-δ1(1:7) or H1N1pdm09(2:6) as shown in Table 1. Indirect naïve contacts were placed with each inoculated group as described above, except in the NAT-MDA/H1N2 group. Challenge, sampling and necropsies were performed as described above for Study 1.
In vivo Study 3
Fourteen piglets born to the H1N1pdm09-WIV boosted sows from Study 2 were used to evaluate long-term MDA interference on WIV vaccination of weaned pigs (Table 1). When the piglets' HI antibody titers dropped below the positive cutoff reciprocal titer of 40, pigs were vaccinated with either the same adjuvanted H1N1pdm09 WIV vaccine as their dams (n=7) or with a commercial quadrivalent influenza vaccine (n=7) containing H1N1-γ, H1N2-δ1, H1N1-δ2 and H3N2-IV cluster viruses (Flusure® XP, Zoetis Animal Health, Florham Park, NJ) following the manufacturer's recommendations. Four naïve pigs obtained from the same source herd as the other studies were used as controls (n=2 per vaccine group). Vaccination was performed using 2 doses 2 weeks apart (at 16 and 18 weeks of age), and pigs were humanely euthanized at 28 days post-vaccination (dpv; at 20 weeks of age).
IAV antibody detection
To evaluate peripheral IAV-specific antibodies, serum samples were collected by venipuncture before weaning (3 days of age), before challenge (0 dpi), and at necropsy (5 dpi/15 dpc) in Studies 1 and 2. Serum was collected at 1, 9, 13, and 16 (pre-vaccination) weeks of age, and at 14 and 28 dpv in Study 3. To measure the transfer of antigen-specific local antibodies through colostrum, nasal washes (NW) were taken in Study 2 at 3 days of age and 0 dpi by flushing 3 ml of phosphate-buffered saline (PBS) into the nostrils and collecting the effluent.
To check for the transfer of MDA and to check for weaning of MDA in Study 3, an ELISA kit was used to detect anti-IAV nucleoprotein (NP) antibodies in serum (AI MultiS-Screen Ab Test, IDEXX, Westbrook, ME), according to the manufacturer's recommendation. Results were measured as presence or absence of antibodies based on the sample to negative (S/N) ratio, and means of each treatment group were used for comparison.
For use in the HI assay, sera were heat-inactivated at 56 °C for 30 min, treated with a 20% suspension of kaolin (Sigma–Aldrich, St. Louis, MO) followed by adsorption with 0.5% turkey red blood cells (RBCs). HI assays were performed with H1N2-δ1 and H1N1pdm09 antigens for Studies 1 and 2, and with H1N1pdm09, an H1N1-γ (A/Swine/Ohio/511445/2007), an H1N1-δ2 (A/Swine/Illinois/00685/2005), and an H3N2-IV (A/Swine/Minnesota/01146/2006) for Study 3 according to standard techniques (Kitikoon et al., 2014). Antibody titers were reported as geometric means. Enzyme-linked immunosorbent assays (ELISA) were performed as previously described (Vincent et al., 2012) to evaluate the levels of total isotype specific antibodies against H1N2-δ1(1:7) and H1N1pdm09(2:6) in serum, NW, and BALF. Briefly, Immulux-HB™ 96-well plates (Dynex, Bustehrad, Czech Republic) were coated with concentrated H1N2-δ1(1:7) or H1N1pdm09(2:6) diluted to 100 HA units/50μl/well. Anti-swine IgG (Kirkegaard and Perry, Gaithersburg, MD) and anti-swine IgA (Bethyl Laboratories, Montgomery, TX) were used for the assays. Serum samples were diluted to 1:2,000. BALF and NW samples were treated with 10mM Dithiothreitol (DTT; Sigma–Aldrich, St. Louis, MO) and diluted to 1:4. The optical density (O.D.) was measured at 405 nm wavelength with an automated ELIS A reader. Antibody levels were reported as the mean O.D. of duplicate wells for each sample, and means of each treatment group were compared for each antibody isotype (IgA or IgG).
For the neutralization assay, NW were treated with 10mM DTT at an initial dilution of 1:2. Samples were then two-fold serially diluted in serum-free MEM supplemented with tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin and antibiotics in 96-well plates. Neutralization assays were performed with both challenge viruses as previously described (Gauger and Vincent, 2014). Reciprocal titers for NW were log2-transformed, and reported as the geometric mean.
Multi-parameter flow cytometry (MP-FCM)
Whole blood samples were collected from all piglets and sows in vacutainer cell preparation tubes with sodium citrate (CPT™, BD Diagnostics, Franklin Lakes, NJ) before challenge (0 dpi). Peripheral blood mononuclear cells (PBMC) were isolated and tested for activation markers by MP-FCM after in vitro stimulation with both challenge viruses, using a previously described method (Platt et al., 2011). Briefly, PBMC of each pig were incubated with either RPMI 1640 media (Mediatech, Inc., Herndon, VA) as non-antigen stimulation control, ConA at 5 μg/ml as mitogen control, undiluted conditioned media from uninfected MDCK culture as mock-stimulation control, or one of the challenge viruses (H1N2-δ1(1:7) or H1N1pdm09(2:6)) as recall antigens in duplicate. Monoclonal primary antibodies for surface antigens CD4 (IgG2b, clone 74-12-4,VMRD, Inc., Pullman, WA), CD8α (IgG2a, clone 76-2-11, VMRD, Inc., Pullman, WA), γδ TCR (Rat IgG2a, cat# 551543, BD Pharmingen, San Diego, CA), CD25 (IgG1, clone PGBL25A,VMRD, Inc., Pullman, WA), and for intracellular antigens IFN-γ (polyclonal antibody, cat# ASC4032, Invitrogen, Carlsbud, CA) and IL-10 (IgG1, cat# ASC9109, Invitrogen, Carlsbud, CA) were used with corresponding fluorochrome-conjugated secondary antibodies. Four T cell subsets (CD4 single positive, CD4; CD8 single positive, CD8; CD4:CD8 double positive, CD4+CD8; and γδ TCR positive, γδ) were analyzed for the IAV-specific response of activation markers CD25, IFN-γ and IL-10 expression. The net percentage increase of each marker was calculated by subtracting the increase of marker-positive cell percentage of mock-stimulated samples from the increase of virus-stimulated samples of the same subset for the same pig. Net negative results were adjusted to 0 before statistical analysis.
Viral Replication and Shedding
Nasal swabs (NS; Nylon Minitip Flocked Dry Swabs, Copan Diagnostics, Murrieta, CA) were taken from principal pigs daily from 0 to 5 dpi and from indirect contact pigs on 0 to 5, 7, 9, and 11 dpc to evaluate viral shedding as previously described (Gauger et al., 2011). NS specimens were filtered (0.45 mm) and plated onto confluent phosphate-buffered saline (PBS)-washed MDCK cells in 24-well plates for virus isolation, as previously described (Vincent et al., 2012). For viral titration, virus isolation-positive NS and BALF samples were 10-fold serially diluted in serum-free MEM supplemented with 1μg/ml TPCK-trypsin and antibiotics, and plated onto confluent MDCK cells in 96-well plates in triplicate as previously described (Vincent et al., 2012). Cells were fixed and plates were stained for IAV nucleoprotein after 48 h incubation as previously described (Gauger and Vincent, 2014). The TCID50/ml virus titers were calculated for each sample by the Reed and Muench method (Reed and Muench, 1938).
Pathological examination of the trachea and lungs
At necropsy, lungs were evaluated for the percentage of the lung affected with lesions typical of IAV infection and a percentage of the lung affected surface was calculated based on weighted proportions of each lobe to the total lung volume (Halbur et al., 1995). For histopathologic examination, trachea and lung tissue samples fixed in 10% buffered formalin were routinely processed and stained with hematoxylin and eosin. Microscopic lesions were evaluated and scored by a veterinary pathologist blinded to treatment groups following previously described parameters (Gauger et al., 2012). Individual scores were summed to give composite scores for lung and trachea microscopic lesions. Influenza A virus-specific NP antigen was detected in lung and trachea using an immunohistochemical (IHC) method and slides were scored as previously described (Gauger et al., 2012).
Cytokine assays
Cytokine levels in cell-free lung lavage were determined using a multiplex ELISA according to manufacturer's recommendations (SearchLight; Aushon Biosystems, Billerica, MA). Samples were analyzed in duplicate and results were averaged. Data is reported as the mean levels ± SEM for pigs in each treatment group.
Statistical analysis
Mean macroscopic lesions, composite microscopic lung and trachea scores, O.D. for ELISAs, log10-transformed virus titers, log2 transformations of HI and neutralization reciprocal titers were analyzed using analysis of variance (ANOVA), with P≤0.05 considered significant (Prism software; GraphPad, La Jolla, CA). Variables with significant effects were subjected to pairwise mean comparisons using the Tukey-Kramer test.
Results
Serology
Pigs in the MDA-negative groups remained seronegative throughout the study. MDA were efficiently transferred from dams to their piglets, as measured by serum HI titers and IAV-specific IgG levels at 3 days of age (data not shown), and before and after challenge (Fig. 1A-F). No HI cross-reactivity against heterologous challenge viruses was detected in any of the MDA-positive groups in Studies 1 or 2 (data not shown). However, serum IgG cross-reactive against the heterologous virus was detected in the WIV-derived MDA groups before and after challenge (Figs. 1E-F), but not in any other group. All naïve pigs placed into indirect contact with heterologous challenged pigs seroconverted by 15 dpc, with geometric mean HI titers ranging from 30 to 278. None of the pigs in indirect contact with WIV-MDA pigs that were challenged with homologous H1N1 in Study 2 seroconverted by HI assay.
Fig. 1.
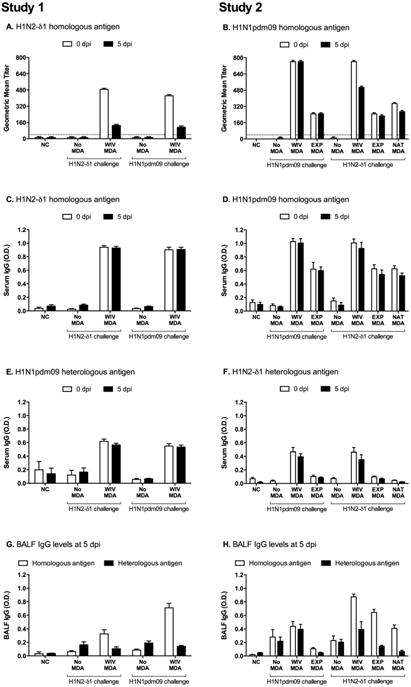
Serum and lung IAV-specific antibody levels due to maternally-derived antibodies (MDA) induced by vaccinating naïve dams with H1N2-δ1(1:7) WIV (Study 1) or boosting seropositive dams with H1N1pdm09 WIV or experimental infection (EXP) or from a non-boosted naturally exposed sow (NAT-MDA; Study 2). Reciprocal geometric mean HI titers against the homologous vaccine antigens are shown for 0 and 5 days post infection (dpi) for Study 1 (A) and Study 2 (B). Mean optical density (O.D.) in whole-virus ELISAs for serum IgG against H1N2-δ1 antigen (C, F) and H1N1pdm09 antigen (D, E). Treatments in Study 1 (A, C, E, G) were: pigs with no MDA or H1N2-δ1(1:7) WIV-MDA, challenged with homologous H1N2-δ1(1:7) or heterologous H1N1pdm09(2:6) virus. Treatments in Study 2 (B, D, F, H) were: pigs with no MDA, H1N1pdm09 WIV-MDA, H1N1pdm09 EXP-MDA, or H1N1pdm09 NAT-MDA challenged with homologous H1N1pdm09(2:6) or heterologous H1N2-δ1(1:7).
Titers of HI MDA were low in pigs from Study 3 at 13 weeks of age and below the detection limit by 16 weeks of age, however most of the pigs (11/14) still had detectable levels of IAV-specific serum IgG until the day of vaccination (Fig. 2A). MDA-positive pigs vaccinated with the same WIV vaccine as their mothers showed no increase in neutralizing antibody levels against the vaccine strain or against a H1N1-γ strain (Fig. 2B and D). MDA-positive pigs vaccinated with the commercial vaccine only had a detectable antibody response against the swine H3N2 and H1N1-δ2 antigens (Fig. 2C and E), components of the vaccine that do not cross-react by HI assay with the H1N1pdm09-specific MDA, but titers were lower than titers in the MDA-negative controls that received the commercial vaccine.
Fig. 2.
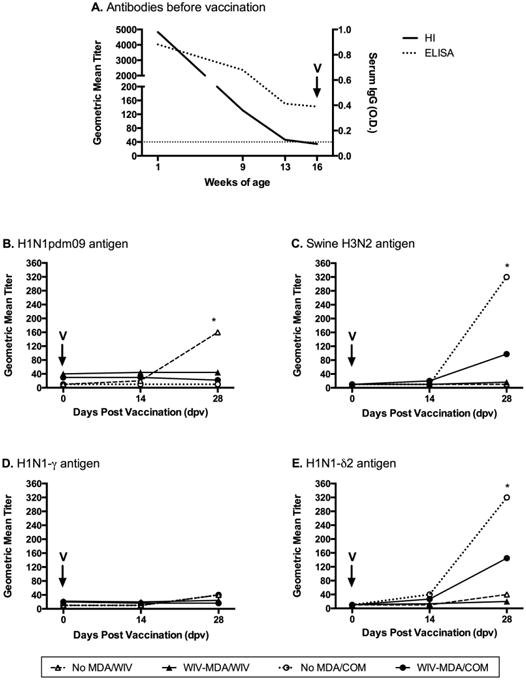
Serum IAV-specific antibody levels in pigs vaccinated with H1N1pdm09 whole inactivated virus vaccine (WIV) or commercial quadrivalent vaccine (COM) in the presence of IAV-specific maternally-derived antibodies (MDA). Pigs were vaccinated at 16 weeks of age, when MDA HI titers dropped below the limit of detection (dotted line). (A) Reciprocal geometric mean HI titers against H1N1pdm09 antigen and mean optical density (O.D.) in influenza A virus nucleoprotein (NP)-specific ELISA at 1, 9, 13 and 16 weeks of age; reciprocal geometric mean HI titers at 0, 14 and 28 days post vaccination against H1N1pdm09 (B), H3N2-IV (C), γ-H1N1 (D), and δ2-H1N1 (E) antigens. Asterisks indicate significant difference between MDA-statuses within the same vaccine (P ≤ 0.05).
Mucosal antibody response
Animals that suckled colostrum with MDA elicited from boosting prior natural exposure immunity (WIV and EXP) had significant levels of IAV-specific IgA in NW at 3 days of age, but this was not observed for animals that suckled sows with MDA elicited only from prior natural exposure (NAT-MDA; Fig. S1). Low titers of homologous neutralizing antibodies were also detected in the nasal washes collected from 3 day-old piglets with MDA (Fig. S1).
At 5 dpi, there were significant levels of homologous IgG in the lungs of all MDA-positive pigs (Fig. 1G-H). No IgG cross-reactivity against the heterologous virus was detected in pigs with WIV-MDA in Study 1 (Fig. 1G), but low cross-reactivity was observed in Study 2, although not statistically significant (Fig. 1H). IAV-specific IgA was not detected in any of the groups (data not shown).
Cell mediated immunity (CMI) transfer via colostrum
The transfer of IAV-specific CMI to homologous and heterologous viruses through colostrum was assessed in each T-cell subset by MP-FCM prior to challenge. Although some minor differences were observed between different MDA status groups, overall antigen-driven increases in the expression of CD25, IFN-γ and IL-10 were nearly undetectable in all T-cell populations of weaned piglets (Fig. S2). The piglets had almost no CD4CD8 population, and therefore these results were not included (data not shown). IAV-specific CMI results in sows were added to graphs as positive values for illustration purpose.
Clinical disease
Challenge with either virus did not result in apparent clinical signs in MDA-negative pigs or homologous MDA-positive pigs. Pigs challenged in the presence of heterologous WIV-derived MDA showed moderate to severe coughing, increased respiratory rate and respiratory distress. Two pigs in the WIV-MDA/H1N2 group (heterologous) of Study 2 showed severe coughing, lethargy, and dyspnea, and died from respiratory disease at 2 dpi. All infected animals showed a peak in rectal temperatures at 1 dpi when compared to non-challenged pigs, except for pigs with homologous MDA in both studies and pigs with MDA elicited from only natural infection (Fig. S3).
Lung and trachea pathology
Naïve pigs challenged with either IAV strain had moderate lung pathology typical of IAV infection. In Studies 1 and 2, pigs challenged with H1N1pdm09(2:6) or H1N2-δ1(1:7) in the presence of homologous WIV-MDA had macroscopic scores similar to non-challenged pigs (Tables 2 and 3). In contrast, the heterologous challenged pigs developed enhanced macroscopic pneumonia, compared to their MDA-negative counterparts (Tables 2 and 3). Notably, there was no enhancement in macroscopic lesions in pigs with natural or booster experimental infection-MDA that were challenged with heterologous virus. Additionally, EXP-MDA resulted in reduced macroscopic lung pathology following homologous challenge (Table 3).
Table 2.
Mean percentage macroscopic pneumonia, composite microscopic pneumonia, trachea microscopic scores ± standard error of the mean (SEM) for groups with maternally-derived antibodies (MDA) from sows vaccinated with H1N2-δ1 whole inactivated virus vaccine (WIV-MDA) or without MDA (No MDA) at 5 days post infection (dpi) with homologous H1N2-δ1(1:7) or heterologous H1N1pdm09(2:6) in Study 1.
| Challenge Groups | Macroscopic percentage (%) | Microscopic scores (0-22) | Trachea histopathology (0-8) |
|---|---|---|---|
| Homologous | |||
| No MDA/NC | 0.0 ± 0.0a | 0.6 ± 0.2a | 0.0 ± 0.0a |
| No MDA/H1N2 | 13.4 ± 2.6b | 7.2 ± 0.7b | 1.5 ± 0.4b |
| WIV-MDA/H1N2 | 2.8 ± 1.1a | 1.5 ± 0.5a | 1.1 ± 0.4a,b |
| Heterologous | |||
| No MDA/NC | 0.0 ± 0.0a | 0.6 ± 0.2a | 0.0 ± 0.0a |
| No MDA/H1N1 | 16.8 ± 2.3b | 10.6 ± 0.9b | 1.0 ± 0.2a,b |
| WIV-MDA/H1N1 | 24.7 ± 2.1c | 9.8 ± 0.6b | 2.0 ± 0.4b |
Statistically significant differences identified with different lowercase letters (P ≤ 0.05).
Table 3.
Mean percentage macroscopic pneumonia, composite microscopic pneumonia, and trachea microscopic scores ± standard error of the mean (SEM) from pigs with maternally-derived antibodies (MDA) from exposed sows vaccinated with H1N1pdm09 whole inactivated virus (WIV-MDA) vaccine or experimentally infected with H1N1pdm09 (EXP-MDA), or from a non-boosted naturally exposed sow (NAT-MDA), or without MDA (No MDA) at 5 days post infection (dpi) with homologous H1N1pdm09(2:6) or heterologous H1N2-δ1(1:7) in Study 2.
| Challenge Groups | Macroscopic percentage (%) | Microscopic scores (0-22) | Trachea histopathology (0-8) |
|---|---|---|---|
| Homologous | |||
| No MDA/NC | 0.4 ± 0.1a | 1.3 ± 0.1a | 0.1 ± 0.1a |
| No MDA/H1N1 | 24.6 ± 2.3b | 15.2 ± 0.6b | 17 ± 03b,c |
| WIV-MDA/H1N1 | 0.7 ± 0.4a,c | 5.3 ± 0.1c | 0.8 ± 0.6a,b |
| EXP-MDA/H1N1 | 8.2 ± 1.8c | 10.4 ± 0.9d | 2.4 ± 0.2c |
| Heterologous | |||
| No MDA/NC | 0.4 ± 0.1a | 1.3 ± 0.1a | 0.1 ± 0.1a |
| No MDA/H1N2 | 15.2 ± 2.0b | 13.4 ± 0.7b | 3.1 ± 0.5b |
| WIV-MDA/H1N2 | 28.9 ± 8.1c | 13.4 ± 1.0b | 3.0 ± 0.6b |
| EXP-MDA/H1N2 | 17.6 ± 2.0b,c | 12.0 ± 0.8b | 2.3 ± 0.4b |
| NAT-MDA/H1N2 | 16.2 ± 2.9b | 17.7 ± 1.1c | 3.5 ± 0.6b |
Statistically significant differences in the same column identified with different lowercase letters (P ≤ 0.05).
Pigs with WIV-MDA had lower microscopic lesion scores after homologous challenge when compared to the MDA-negative challenged groups in both studies, indicating at least partial protection. The presence of WIV-derived MDA at the time of heterologous challenge did not result in enhanced microscopic pneumonia and scores were not statistically different from the control groups, which is in contrast to macroscopic lesions for this group (Table 2 and 3). WIV-MDA pigs with macroscopic evidence of VAERD demonstrated less peribronchiolar lymphocytic cuffing and interstitial pneumonia compared to our traditional WIV VAERD model, although necrotizing bronchiolitis typical of influenza infection was apparent (Gauger et al., 2012)). Pigs that suckled from an H1N1pdm09 naturally exposed sow that was not boosted showed higher microscopic pneumonia scores than MDA-negative infected counterparts (Table 3).
Virus levels in lung and nasal secretions
Virus was not detected in NC pigs at any time throughout the studies, or in the nasal swabs of any pigs on the day of challenge. Pigs with H1N2-δ1 WIV-MDA challenged with homologous virus in Study 1 showed lower virus titers in nasal secretions when compared with the MDA-negative infected controls (Table 4), although virus transmitted to naïve, indirect contact pigs (Fig. 3A). Similarly, H1N1pdm09-MDA elicited from WIV or secondary wild-type exposure provided effective protection against the nasal shedding of homologous virus in Study 2, as limited virus was detected in NS collected from these treatment groups on any dpi (Table 5). Notably, only one WIV-MDA/H1N1 pig shed virus after challenge. None of the pigs in indirect contact with this group shed virus (Fig. 3B). However, pigs in the EXP-MDA/H1N1 indirect contact group became infected (Fig. 3B). In contrast, neither H1N1pdm09 nor H1N2-δ1 MDA protected against shedding of heterologous challenge virus, as there was no difference in titers from MDA-negative controls on dpi 1-4 (Tables 4 and 5), and all pigs in indirect contact became infected (Fig. 3A-B). By 5 dpi, MDA elicited from H1N1pdm09-WIV vaccination resulted in significantly lower nasal viral shedding from pigs challenged with heterologous H1N2-δ1 in Study 1 (Table 5).
Table 4.
Virus titers in nasal swabs at 1 to 5 days post infection (dpi) and bronchoalveolar lavage fluid (BALF) at 5 dpi from pigs with maternally-derived antibodies (MDA) from sows vaccinated with H1N2-δ1(1:7) WIV or without MDA and challenged with homologous H1N2-δ1(1:7) or heterologous H1N1pdm09(2:6) in Study 1. Values shown as mean ± standard error of the mean (SEM).
| Challenge Groups | Nasal swabsx | BALFx | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 dpi | 2 dpi | 3 dpi | 4 dpi | 5 dpi | 5 dpi | |
| No MDA/NC | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| Homologous | ||||||
| No MDA/H1N2 | 1.5 ± 0.4b | 1.9 ± 0.4b | 2.3 ± 0.4b | 2.8 ± 0.3b | 2.8 ± 0.2b | 3.9 ± 0.5b |
| WIV-MDA/H1N2 | 0.0 ± 0.0a | 0.1 ± 0.1a | 0.8 ± 0.4a | 0.8 ± 0.4a | 0.1 ± 0.1a | 2.7 ± 0.7b |
| Heterologous | ||||||
| No MDA/H1N1 | 1.2 ± 0.2b | 1.0 ± 0.1b | 1.0 ± 01a,b | 1.5 ± 0.2b | 1.0 ± 0.2b | 0.7 ± 0.4a |
| WIV-MDA/H1N1 | 1.3 ± 0.3b | 0.5 ± 02a,b | 1.4 ± 0.2b | 1.2 ± 0.2b | 1.0 ± 0.2b | 1.3 ± 0.4a |
Virus titers represented as log10 TCID50/ml.
Statistically significant differences in the same column identified with different lowercase letters (P ≤ 0.05).
Fig. 3.
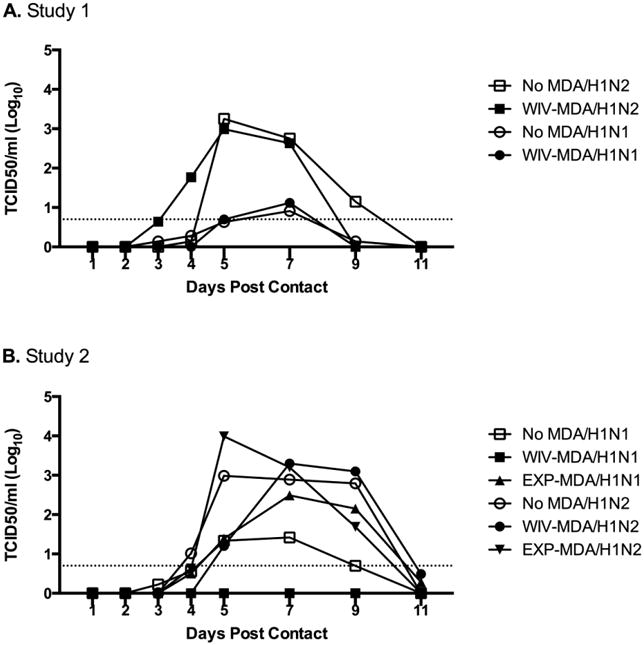
Mean virus titers in nasal swabs of naïve sentinel pigs at 1 to 5, 7, 9, and 11 days post indirect contact (dpc) with pigs with maternally-derived antibodies (MDA) challenged with homologous or heterologous virus. Treatments in Study 1 (A) were: pigs with no MDA and with MDA from vaccination of naïve sows with H1N2-δ1(1:7) WIV, challenged with homologous H1N2-δ1(1:7) or heterologous H1N1pdm09(2:6). Treatments in Study 2 (B) were: pigs with no MDA, and with MDA from H1N1pdm09 WIV vaccination of seropositive sows, from experimental infection of seropositive sows with H1N1pdm09 (EXP-MDA), or from a non-boosted H1N1pdm09 naturally exposed sow (NAT-MDA) challenged with homologous H1N1pdm09(2:6) or heterologous H1N2-δ1(1:7). The dotted line indicates the assay's limit of detection.
Table 5.
Virus titers in nasal swabs from pigs with maternally-derived antibodies (MDA) from exposed sows vaccinated with H1N1pdm09 whole inactivated virus (WIV-MDA) vaccine or experimentally infected with H1N1pdm09 (EXP-MDA), or from a non-boosted H1N1pdm09 naturally exposed sow (NAT-MDA), and without MDA (No MDA) at 1 to 5 days post infection (dpi) with homologous H1N1pdm09(2:6) or heterologous H1N2-δ1(1:7) in Study 2. Values shown as mean ± standard error of the mean (SEM).
| Challenge Groups | Nasal swabsx | BALFx | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 dpi | 2 dpi | 3 dpi | 4 dpi | 5 dpi | 5 dpi | |
| No MDA/NC | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| Homologous | ||||||
| No MDA/H1N1 | 1.2 ± 0.3b | 1.1 ± 0.2b | 1.7 ± 0.2b | 2.3 ± 0.2b | 1.0 ± 0.2b | 4.2 ± 0.3b |
| WIV-MDA/H1N1 | 0.0 ± 0.0a | 0.1 ± 0.1a | 0.0 ± 0.0a | 0.1 ± 0.1a | 0.1 ± 0.1a | 0.0 ± 0.0a |
| EXP-MDA/H1N1 | 0.0 ± 0.0a | 0.6 ± 0.3a,b | 0.6 ± 0.4a | 0.7 ± 0.3a | 0.4 ± 0.1a,b | 3.7 ± 0.6b |
| Heterologous | ||||||
| No MDA/H1N2 | 2.8 ± 0.3b | 2.6 ± 0.2b | 3.3 ± 0.3b | 3.1 ± 0.2b | 3.0 ± 0.2b | 5.1 ± 0.3b |
| WIV-MDA/H1N2 | 2.5 ± 0.6b | 2.1 ± 0.4b | 3.0 ± 0.3b | 2.9 ± 0.2b | 1.6 ± 0.4c | 6.0 ± 0.2b,c |
| EXP-MDA/H1N2 | 2.8 ± 0.4b | 2.6 ± 0.5b | 3.0 ± 0.2b | 2.7 ± 0.3b | 2.8 ± 0.4b | 6.8 ± 0.2c |
| NAT-MDA /H1N2 | 1.1 ± 0.5c | 3.1 ± 0.4b | 2.3 ± 0.4b | 2.8 ± 0.1b | 2.5 ± 0.4b,c | 6.1 ± 0.2b,c |
Virus titers represented as log10 TCID50/ml.
Statistically significant differences identified with different lowercase letters (P ≤ 0.05).
Consistent with the reduced viral shedding, MDA against H1N1pdm09 provided protection against homologous virus replication in the lungs, as no virus was detected in the lungs of WIV-MDA/H1N1 pigs at 5 dpi in Study 2 (Table 5), but the same was not observed for WIV-MDA pigs after homologous challenge with H1N2-δ1 in Study 1 (Table 4). H1N1pdm09 virus titers in the lungs of control pigs without MDA were different between studies, even though inoculum back titrations were similar (data not shown), with titers in Study 1 lower than in Study 2 (Table 4 and 5).
Cytokine concentrations in BALF were evaluated at 5 dpi in Studies 1 and 2, and different patterns were observed between the two studies. Overall, pigs in Study 2 showed higher levels of all cytokines when compared to Study 1 (Fig. S4); however, the pattern of distinct cytokine dysregulation seen in our previous VAERD studies (Gauger et al., 2012; Gauger et al., 2011) was not observed.
Discussion
Currently, protection of pigs against IAV relies primarily on the use of inactivated vaccines, most commonly in breeding females (Vincent et al., 2008b). Sows vaccinated prior to farrowing often have high HI antibody titers against vaccine virus(es) that may lead to long-lasting maternally-derived anti-IAV antibodies in piglets (Markowska-Daniel et al., 2011). MDA has been shown to protect piglets against clinical disease with homologous infection (Choi et al., 2004; Kitikoon et al., 2006; Loeffen et al., 2003), but the level of protection may be greatly influenced by the virus strain, the levels of MDA and the age at the time of infection (Loeffen et al., 2003). Additionally, the expanded genetic diversity of IAV in swine in the U.S. over the past two decades has led to the co-circulation of numerous genetic and antigenic virus strains (Lorusso et al., 2013; Olsen, 2002; Vincent et al., 2008b), making it difficult to rely on vaccine-derived MDA to cross-protect nursery pigs against heterologous viruses. Here, we demonstrated different outcomes following IAV challenge of piglets depending on the method used to elicit IAV-specific MDA, and the challenge virus used (homologous versus heterologous to MDA). While WIV-induced MDA provided better protection than natural exposure and boosted-exposure MDA against homologous challenge, WIV-induced MDA were also associated with enhanced clinical signs when pigs were exposed to a heterologous H1 virus, irrespective to the previous serological status of the sows.
The epitheliochorial placenta of sows does not allow for the transfer of immunoglobulin from sow to piglets in utero; therefore, pigs are born agammaglobulinemic (Sterzl et al., 1966). Hence, newborn swine depend on the ingestion of colostrum to passively acquire antibodies (referred to as maternally-derived antibody, MDA) as a first line of defense against pathogens in the first weeks of life. In this study, IAV-specific antibodies were successfully transferred to the litters of vaccinated or naturally/experimentally infected sows, as all pigs that suckled from these sows had detectable levels of serum HI and IgG antibodies against the immunizing strains prior to challenge. Previous studies have shown that maternally-derived lymphoid cells are also absorbed from the digestive tract of piglets after colostrum ingestion, and transported to peripheral blood and various tissues, such as liver, lungs, lymph nodes, spleen and gastrointestinal tract (Tuboly and Bernath, 2002; Williams, 1993). However, we did not detect an IAV-specific IFN-γ and IL-10 recall response in peripheral T cells from 3 weeks-old piglets that suckled colostrum from vaccinated sows (Fig. S2). Both antigen-specific IgG and monomeric IgA have been shown to transudate into the lungs (presumably from the periphery) of piglets after colostrum ingestion, with levels peaking at 3 days after birth (Nechvatalova et al., 2011). We were able to detect significant levels of anti-H1N1pdm09 IgA in the nasal cavity of MDA-positive piglets at 3 days of age, but levels dropped bellow the detection limit by 3 weeks of age (data not shown). The presence of maternally-derived IgA in the nasal wash of newborn piglets must be interpreted with caution, as this could be a result of aspiration or spill during colostrum feeding.
Homologous IAV-specific MDA typically protects piglets against disease but often does not prevent infection or reduce viral shedding (Choi et al., 2004; Kitikoon et al., 2006; Loeffen et al., 2003). A previous study showed that pigs challenged in the presence of homologous MDA shed virus for a longer period of time, and these pigs had an inhibition or delay in induction of an active antibody and cellular immune response (Loeffen et al., 2003). Here, challenge with either H1N1pdm09 or H1N2-δ1 resulted in no apparent clinical signs in pigs with homologous MDA, and reduced lung and trachea lesions (Tables 2 and 3). Notably, matched MDA elicited from WIV administration in sows previously exposed to IAV also protected piglets from homologous infection and prevented transmission, as only one principal pig in this group shed low titers of virus after challenge, and there was no transmission to indirect naïve contact pigs. Secondary experimental infection of seropositive sows also resulted in transfer of immunity that provided protection of their litters from development of clinical disease following homologous challenge, but there was no protection against infection or transmission. This was likely due to the lower levels of IAV-specific peripheral antibodies in the sows that resulted in lower levels transferred to their piglets.
The presence of WIV-derived MDA led to a very different outcome when pigs were challenged with a virus heterologous to the vaccine strain, regardless of the sows being previously exposed or not. The WIV-MDA not only failed to protect the piglets against infection and disease, it resulted in enhanced clinical signs and pathology associated with VAERD (Gauger et al., 2012), albeit at an intermediate level of enhancement only apparent for macroscopic pathology, as VAERD-affected piglets demonstrated similar mean microscopic lung lesion scores compared to the no-MDA challenged control groups. Notably, when previously exposed sows were boosted with WIV vaccine, the resulting disease enhancement in their piglets seemed more pronounced, and two piglets succumbed at 2 dpi with severe respiratory disease. In both studies, WIV-induced MDA was shown to cross-react with the heterologous virus by whole virus ELISA (Fig. 1), a parameter frequently observed in VAERD (Gauger et al., 2012; Gauger et al., 2011; Rajao et al., 2014). Antibody-dependent enhancement (ADE) or antibody mediated complement fixation may play an important role in the enhanced pathology associated with VAERD, in which the presence of non-protective antibodies and antigen-antibody complexes could lead to increased virus uptake, enhanced replication, and dysregulated inflammation (Huisman et al., 2009). Remarkably, even though MDA induced through natural IAV exposure without boosting did not protect piglets against heterologous challenge with H1N2-δ1, it did not result in VAERD. The same was observed in piglets with MDA elicited after boosting sows with secondary IAV exposure, which did not seem to increase the levels of IAV-specific IgG transferred via colostrum (Fig. 1), consistent with the transfer of lower antibodies levels mentioned above. The implication that antibodies are the immune component driving VAERD is consistent with the report that cross-reacting, non-neutralizing serum IgG directed towards the HA conserved stalk domain increased acute infection by enhancing virus fusion in vitro (Khurana et al., 2013). Importantly, the lack of maternally-derived IAV-specific CMI at the time of challenge suggests that antibodies alone may trigger the inciting events leading to VAERD. However, IAV-specific CMI responses likely are involved in the severity of the enhancement, as evidenced here by the microscopic lesions with less leucocyte recruitment and reduced cytokine responses compared to the traditional VAERD model (Gauger et al., 2011).
In addition to the limited protection and risk of enhanced respiratory disease, previous studies have shown MDA interference on the immune response to vaccination in piglets (Kitikoon et al., 2006; Markowska-Daniel et al., 2011; Sandbulte et al., 2014; Vincent et al., 2012). In herds that routinely vaccinate breeding females during gestation, high titers of MDA can be detected by HI in offspring until 14 weeks of age (Markowska-Daniel et al., 2011), thus, vaccination of weaned pigs may be negatively impacted. Here, MDA elicited from WIV-boosting of naturally exposed sows capable of neutralizing homologous virus were detected until 13 weeks of age, but NP-specific antibodies were still detected at 16 weeks of age. Notably, although homologous HI antibody titers were low or undetectable at the time of vaccination of these pigs, the interference with vaccine-induced immunity was still evident. Commercial multivalent vaccines induced no HI response against strains similar to those in the vaccine, and there were reduced responses to other distant strains in the vaccine (Fig. 2). Similar results were observed previously when pigs were vaccinated at various ages in the presence of MDA: a higher antibody response was observed if pigs were older at the time of first vaccination, and the response was dependent on weaning of MDA levels (Markowska-Daniel et al., 2011). Our data show that high titers of homologous vaccine-elicited MDA can reduce infection, transmission, and/or disease following homologous exposure. Importantly, MDA alone made piglets susceptible to VAERD upon heterologous infection. Swine IAV diversity has increased rapidly, at a pace that current control strategies, such as WIV vaccination, are not able to follow. Beside the potential for failure of protection from WIV or WIV-elicited MDA against antigenically distant viruses, there is an practical risk for VAERD to occur in the field. Thus, the selection of WIV vaccine, the timing of vaccination in sows, and immune status are crucial to assure adequate MDA coverage. In addition, the use of diagnostic and surveillance data to monitor genetic and antigenic evolution of viruses circulating in swine populations, along with improved methodologies for evaluation of vaccine responses, are needed to develop more effective intervention strategies against this important pathogen.
Supplementary Material
Highlights.
Maternally derived antibodies from WIV protected pigs against homologous infection
MDA from natural exposure did not protect pigs against heterologous challenge
MDA from WIV led to enhanced respiratory disease upon heterologous challenge
Acknowledgments
We thank Michelle Harland, Gwen Nordholm, and Zahra Olson for technical assistance; Jason Huegel, Jason Crabtree, and Tyler Standley for assistance with animal studies; and Dr. Susan Brockmeier for assisting with bacterial screening. This study was supported by USDA-ARS, USDA-APHIS, and by National Institute of Allergy and Infectious Diseases grant R21AI098079. Dr. Rajao was a CNPq-Brazil fellowship recipient.
USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Choi YK, Goyal SM, Joo HS. Evaluation of transmission of swine influenza type A subtype H1N2 virus in seropositive pigs. Am J Vet Res. 2004;65:303–306. doi: 10.2460/ajvr.2004.65.303. [DOI] [PubMed] [Google Scholar]
- Gauger PC, Vincent AL. Serum virus neutralization assay for detection and quantitation of serum-neutralizing antibodies to influenza A virus in swine. In: Spackman E, editor. Animal Influenza Virus. Springer; New York, NY: 2014. pp. 313–324. [DOI] [PubMed] [Google Scholar]
- Gauger PC, Vincent AL, Loving CL, Henningson JN, Lager KM, Janke BH, Kehrli ME, Jr, Roth JA. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Vet Pathol. 2012;49:900–912. doi: 10.1177/0300985812439724. [DOI] [PubMed] [Google Scholar]
- Gauger PC, Vincent AL, Loving CL, Lager KM, Janke BH, Kehrli ME, Jr, Roth JA. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine. 2011;29:2712–2719. doi: 10.1016/j.vaccine.2011.01.082. [DOI] [PubMed] [Google Scholar]
- Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- Huisman W, Martina BE, Rimmelzwaan GF, Gruters RA, Osterhaus AD. Vaccine-induced enhancement of viral infections. Vaccine. 2009;27:505–512. doi: 10.1016/j.vaccine.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, Vincent AL, Golding H. Vaccine-Induced Anti-HA2 Antibodies Promote Virus Fusion and Enhance Influenza Virus Respiratory Disease. Sci Transl Med. 2013;5:200ra114. doi: 10.1126/scitranslmed.3006366. [DOI] [PubMed] [Google Scholar]
- Kitikoon P, Gauger PC, Vincent AL. Hemagglutinin inhibition assay with swine sera. In: Spackman E, editor. Animal Influenza Virus. Springer; New York, NY: 2014. pp. 295–301. [DOI] [PubMed] [Google Scholar]
- Kitikoon P, Nilubol D, Erickson BJ, Janke BH, Hoover TC, Sornsen SA, Thacker EL. The immune response and maternal antibody interference to a heterologous H1N1 swine influenza virus infection following vaccination. Vet Immunol Immunopathol. 2006;112:117–128. doi: 10.1016/j.vetimm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Lewis NS, Anderson TK, Kitikoon P, Skepner E, Burke DF, Vincent AL. Substitutions near the hemagglutinin receptor-binding site determine the antigenic evolution of influenza A H3N2 viruses in U.S. swine. J Virol. 2014;88:4752–4763. doi: 10.1128/JVI.03805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffen WL, Heinen PP, Bianchi AT, Hunneman WA, Verheijden JH. Effect of maternally derived antibodies on the clinical signs and immune response in pigs after primary and secondary infection with an influenza H1N1 virus. Vet Immunol Immunopathol. 2003;92:23–35. doi: 10.1016/s0165-2427(03)00019-9. [DOI] [PubMed] [Google Scholar]
- Lorusso A, Vincent AL, Gramer ME, Lager KM, Ciacci-Zanella JR. Contemporary epidemiology of North American lineage triple reassortant influenza A viruses in pigs. In: Richt JA, Webby RJ, editors. Swine Influenza. Springer Berlin Heidelberg; Berlin, DE: 2013. pp. 113–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso A, Vincent AL, Harland ML, Alt D, Bayles DO, Swenson SL, Gramer MR, Russell CA, Smith DJ, Lager KM, Lewis NS. Genetic and antigenic characterization of H1 influenza viruses from United States swine from 2008. J Gen Virol. 2011;92:919–930. doi: 10.1099/vir.0.027557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loving CL, Brockmeier SL, Vincent AL, Gauger PC, Zanella EL, Lager KM, Kehrli ME., Jr Cross-fostering to prevent maternal cell transfer did not prevent vaccine-associated enhanced respiratory disease that occurred following heterologous influenza challenge of pigs vaccinated in the presence of maternal immunity. Viral Immunol. 2014;27:334–342. doi: 10.1089/vim.2014.0034. [DOI] [PubMed] [Google Scholar]
- Loving CL, Lager KM, Vincent AL, Brockmeier SL, Gauger PC, Anderson TK, Kitikoon P, Perez DR, Kehrli ME., Jr Efficacy of inactivated and live-attenuated influenza virus vaccines in pigs against infection and transmission of emerging H3N2 similar to the 2011-2012 H3N2v. J Virol. 2013 doi: 10.1128/JVI.01038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loving CL, Vincent AL, Pena L, Perez DR. Heightened adaptive immune responses following vaccination with a temperature-sensitive, live-attenuated influenza virus compared to adjuvanted, whole-inactivated virus in pigs. Vaccine. 2012;30:5830–5838. doi: 10.1016/j.vaccine.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska-Daniel I, Pomorska-Mol M, Pejsak Z. The influence of age and maternal antibodies on the postvaccinal response against swine influenza viruses in pigs. Vet Immunol Immunopathol. 2011;142:81–86. doi: 10.1016/j.vetimm.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis. 2007;44:1084–1088. doi: 10.1086/512813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechvatalova K, Kudlackova H, Leva L, Babickova K, Faldyna M. Transfer of humoral and cell-mediated immunity via colostrum in pigs. Vet Immunol Immunopathol. 2011;142:95–100. doi: 10.1016/j.vetimm.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Olsen CW. The emergence of novel swine influenza viruses in North America. Virus Res. 2002;85:199–210. doi: 10.1016/s0168-1702(02)00027-8. [DOI] [PubMed] [Google Scholar]
- Platt R, Vincent AL, Gauger PC, Loving CL, Zanella EL, Lager KM, Kehrli ME, Jr, Kimura K, Roth JA. Comparison of humoral and cellular immune responses to inactivated swine influenza virus vaccine in weaned pigs. Vet Immunol Immunopathol. 2011;142:252–257. doi: 10.1016/j.vetimm.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Pyo HM, Hlasny M, Zhou Y. Influence of maternally-derived antibodies on live attenuated influenza vaccine efficacy in pigs. Vaccine. 2015;33:3667–3672. doi: 10.1016/j.vaccine.2015.06.044. [DOI] [PubMed] [Google Scholar]
- Rajao DS, Loving CL, Gauger PC, Kitikoon P, Vincent AL. Influenza A virus hemagglutinin protein subunit vaccine elicits vaccine-associated enhanced respiratory disease. 2014 doi: 10.1016/j.vaccine.2014.07.059. [DOI] [PubMed] [Google Scholar]
- Reed IJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- Sandbulte MR, Platt R, Roth JA, Henningson JN, Gibson KA, Rajao DS, Loving CL, Vincent AL. Divergent immune responses and disease outcomes in piglets immunized with inactivated and attenuated H3N2 swine influenza vaccines in the presence of maternally-derived antibodies. Virology. 2014:464–465. 45–54. doi: 10.1016/j.virol.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Sterzl J, Rejnek J, Travnicek J. Impermeability of pig placenta for antibodies. Folia Microbiol (Praha) 1966;11:7–10. doi: 10.1007/BF02877148. [DOI] [PubMed] [Google Scholar]
- Tuboly S, Bernath S. Intestinal absorption of colostral lymphoid cells in newborn animals. Adv Exp Med Biol. 2002;503:107–114. doi: 10.1007/978-1-4615-0559-4_12. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Lager KM, Janke BH, Gramer MR, Richt JA. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet Microbiol. 2008a;126:310–323. doi: 10.1016/j.vetmic.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. Swine influenza viruses: a North American perspective. In: Maramorosch K, Shatkin AJ, Murphy FA, editors. Adv Virus Res. Academic Press; Burlington, MA: 2008b. pp. 127–154. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Ma W, Lager KM, Janke BH, Webby RJ, Garcia-Sastre A, Richt JA. Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine. Vaccine. 2007;25:7999–8009. doi: 10.1016/j.vaccine.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AL, Ma W, Lager KM, Richt JA, Janke BH, Sandbulte MR, Gauger PC, Loving CL, Webby RJ, Garcia-Sastre A. Live attenuated influenza vaccine provides superior protection from heterologous infection in pigs with maternal antibodies without inducing vaccine-associated enhanced respiratory disease. J Virol. 2012;86:10597–10605. doi: 10.1128/JVI.01439-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PP. Immunomodulating effects of intestinal absorbed maternal colostral leukocytes by neonatal pigs. Can J Vet Res. 1993;57:1–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


