Abstract
Background
Sorafenib-induced dermatologic toxicity is common and consists primarily of dry skin, maculopapular rash, hand-foot skin reaction, and alopecia. Cutaneous squamous cell carcinoma (SCC) and inflammation of actinic keratosis (AK) were reported in 2 patients treated with sorafenib (Lacouture et al), but the scope of this observation has not been evaluated.
Patients and Methods
We reviewed medical records of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib at our institution from June 1, 2005, through April 4, 2007.
Results
We identified 7 cases of cutaneous SCC, 2 cases of SCC keratoacanthoma type, 2 cases of focal squamous atypia, and 3 cases of AKs. The time to development of SCC or AK from the start of sorafenib was 9.3 months (median, 6.5 months; range, 0.9-43 months). Ten of these 14 patients discontinued therapy with sorafenib: 7 patients as a result of disease progression, 2 patients as a result of nondermatologic toxicity, and 1 patient as a result of dermatologic toxicity. Four patients are continuing sorafenib therapy at reduced doses because of diarrhea and fatigue. One patient receiving sorafenib at a 25% dose reduction developed a second invasive SCC lesion on his forearm 6 months after the initial resection.
Conclusion
These data suggest that there could be an association between sorafenib therapy and the development of cutaneous SCC and inflammation of AK. This adverse event has important therapeutic implications. Full appraisal of this observation in prospective studies is warranted.
Keywords: Keratoacanthomas, Lymphocytic infiltration, Renal cell carcinoma
Introduction
Sorafenib has been approved for the treatment of metastatic renal cell carcinoma (mRCC),1 and based on its recent approval in advanced hepatocellular carcinoma, its use is expected to increase worldwide.2 Sorafenib was originally developed as a raf kinase inhibitor, but it also inhibits vascular endothelial growth factor (VEGFR)–2, VEGFR-3, platelet-derived growth factor receptor–β and phosphorylates Flt-3, c-Kit, and p38α.3 Preclinical evidence confirms that sorafenib has antiangiogenic properties. It is believed that angiogenesis inhibition is the main mechanism of action of this agent in human cancers. Sorafenib has been associated with dermatologic toxicity, such as a maculopapular or a desquamative rash and hand-foot skin reactions.1 The cutaneous reactions associated with tyrosine kinase inhibitors (TKIs) have been extensively reported4,5; however, the development of actinic keratoses (AK) or squamous cell carcinomas (SCC) secondary to therapy with a TKI, such as sorafenib, is a novel observation, initially reported by Lacouture et al in 2 patients who developed SCC and inflammation of AKs following treatment with sorafenib.6 Three patients developed keratoacanthomas after treatment with sorafenib.7
Patients and Methods
We reviewed the medical records of patients with mRCC who received single-agent sorafenib for ≥ 6 weeks from June 1, 2005, through April 4, 2007. Patients who received concurrent treatment with other antineoplastic agents were excluded from this analysis. The Institutional Review Board at M. D. Anderson Cancer Center approved this retrospective review. The goals of this study were to describe the spectrum of SCC and AKs observed in patients receiving sorafenib.
Results
Of 131 patients evaluated, 14 patients developed the spectrum of SCC/AK within 9.3 months of starting sorafenib (median, 6.5 months; range, 0.9-43 months). The median duration of follow-up for all 131 patients was 23 weeks (range, 6-183 weeks) from initiation of sorafenib. Patient characteristics and pathologic findings are summarized in Table 1. Sorafenib was given as first-line therapy in 9 patients and as salvage therapy in 5 patients. All patients started sorafenib at the standard dose of 400 mg twice daily, except for 1 patient with end-stage renal disease undergoing hemodialysis who received sorafenib 200 mg daily. Two patients reported a history of previous treatment for AK and SCC before the initiation of sorafenib, and 1 patient had been previously treated for basal cell carcinoma.
Table 1. Patient Characteristics and Pathologic Findings*.
| Patient Characteristic | Number of Patients (N = 14) |
|---|---|
| Race | |
| White | 14/14 (100%) |
| Median Age, Years (Range) | 65 (53-73) |
| Sex | |
| Male | 11/14 (78%) |
| RCC Type | |
| Conventional | 14/14 (100%) |
| Patients with Previous Systemic Therapy | 5/14 (36%) |
| Concurrent Medical Diagnoses | |
| Melanocytic nevi | 1/14 (7%) |
| Previous treatment of SCC or AK | 2/14 (14%) |
| Hypertension | 8/14 (57%) |
| Type II diabetes mellitus or impaired glucose tolerance | 5/14 (36%) |
| Pathology | |
| Invasive SCC | 7/14 (64%) |
| Focal squamous atypia | 3/14 (21%) |
| Keratoacanthomas, SCC type | 2/14 (14%) |
| Actinic keratoses | 2/14 (14%) |
| Location of Lesions | |
| Head, neck, and upper extremities | 21/32 (65%) |
| Back and lower extremities | 11/32 (34%) |
Number of skin lesions = 32.
Abbreviations: AK = actinic keratoses; RCC = renal cell carcinoma; SCC = squamous cell carcinoma
Ten of the 14 patients reported here discontinued therapy with sorafenib: 7 patients because of disease progression, 2 patients because of nondermatologic toxicity, and 1 patient because of dermatologic toxicity. Four patients are continuing sorafenib at reduced doses because of diarrhea and fatigue after the eruption of AK/SCC. One patient taking sorafenib at 25% dose reduction developed a second invasive SCC lesion on his forearm 6 months after the initial resection; another patient receiving sorafenib at 50% dose reduction developed 4 additional SCC lesions. There were no additional incidences of AK or SCC. After disease progression with sorafenib, 8 patients received sunitinib, 1 patient received interferon-α, and 1 patient received no further systemic therapy, but to date, none of these patients has developed additional AK or SCC.
Several representative slides were also analyzed for the level of keratinocyte proliferation. The lesions shown in this study do show evidence of proliferation, namely epidermal thickening and mitotic figures (Figure 1). We also noted a degree of lymphocyte proliferation at the interface between the tumor and dermis (Figure 2).
Figure 1. High-Powered Image of Squamous Cell Carcinoma.
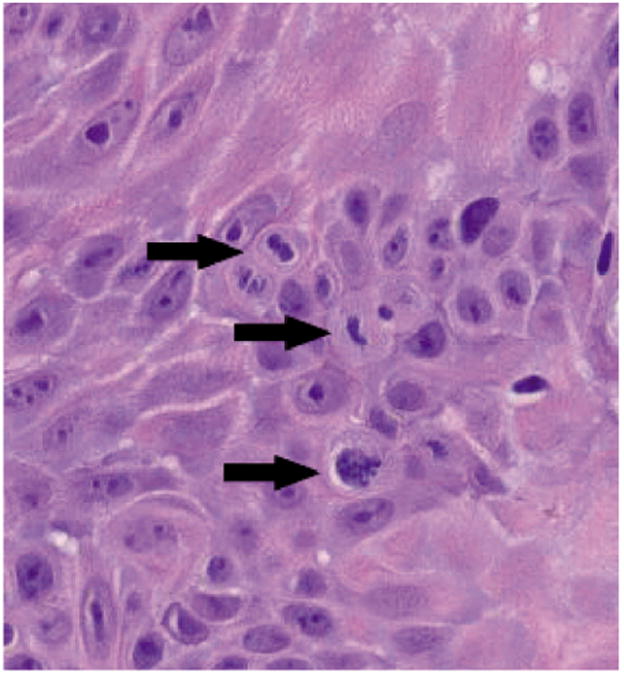
Hematoxylin and eosin stain, magnification × 20. Arrows show 3 mitotic figures in this field.
Figure 2. Low-Powered Image Showing a Dense Lymphocytic Infiltrate in the Interface Between the Tumor and Dermis.
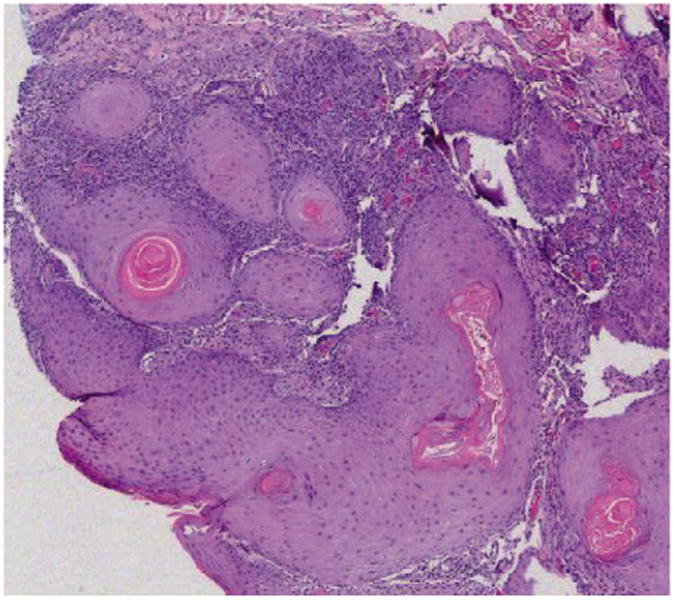
Hematoxylin and eosin stain, magnification × 4.
Discussion
In this report, we present the largest case series of patients developing AK and/or cutaneous SCC during therapy with sorafenib. The mechanism through which sorafenib may be implicated in the development of AK and cutaneous SCC has not been elucidated. The spontaneous resolution of AK after discontinuation of sorafenib and the photodistribution of most of the lesions on sun-damaged skin suggests that these cases could represent inflammation of pre-existing lesions. Inflammation of AK during therapy with certain cytotoxic agents is a well-known process termed “AK recall.” Significant inflammation secondary to therapy can lead to resolution of AK, as has been exploited with the use of topical 5-fluorouracil to treat AK.
In our study, ≥ 3 patients developed SCC in locations that suggest a de novo process. Robert et al hypothesized that chemotherapeutic agents causing acral erythema induce actinically damaged keratinocytes to proliferate.4 They noted the increased mitoses in the basal layer of the skin. Therefore, it is plausible that sorafenib, with known association to acral erythema, could also induce proliferation elsewhere, leading to AK and cutaneous SCC. Although we use the terms “acral erythema” and “hand-foot skin reactions” interchangeably, it should be noted that the particular pattern of skin reactions observed in patients on sorafenib is unique and is well-characterized by Yang et al.8
Inhibition of the A-raf isoform results in increased expression of p53, p27, matrix metalloproteinases, and Bcl-2; however, inhibition of another raf isoform, B-raf, leads instead to decreased apoptosis.8 Transfection of moderate raf activators, such as A-raf and raf-1 into NIH-3T3 cells has led to cell proliferation associated with induction of cyclin expression and Cdk activity, whereas ectopic expression of the much more potent B-raf has led to apoptosis.9 Cell culture models with overexpression of different raf isoforms might explain the clinical dichotomy we observe with sorafenib: keratinocytes proliferate to form SCC, whereas RCC tumor cells undergo presumed apoptosis. Another plausible explanation for the development of SCC in our patients is immunosuppression secondary to sorafenib. Although we observe cellular proliferation in the SCC tumors, the range of cellular proliferation in SCC varies considerably. To prove that sorafenib induces SCC by increasing cellular proliferation would require examining multiple SCC lesions before and after treatment. SCC is common in patients who undergo allografts; however, in this setting, the usual time to the development of SCC is much longer,10 with features of human papilloma virus infection present in some patients.
The patients reported in this study, the majority being of elderly age, could naturally develop AK and SCC as a result of the aging process. Previous treatments with multiple agents could induce a state of immunosuppression, thereby increasing the risk for the development of SCC; however, most of the patients affected had not previously received any immunosuppressive agent. In vivo work by Hipp et al and Zhao et al suggests that sorafenib might inhibit an antigen-specific cytotoxic T-cell response.11-12 This might decrease the ability of the immune response to reject the SCC. It is noteworthy to mention that sorafenib, but not sunitinib, reduced cytokine production by dendritic cells and their ability to stimulate a T-cell response via inhibition of PI3K, mitogen-activated protein kinases, and nuclear factor–κB signaling.11 Immunohistochemical evaluation of our own SCC samples reveals a dense lymphocytic infiltrate in the interface between the tumor and dermis.
Conclusion
The data reported here have therapeutic implications. Oncologists who treat patients with sorafenib should be aware of this potential complication, as early recognition of AK/SCC is crucial for prompt intervention, to lessen the additional morbidity in these patients. We recommend that patients who receive sorafenib should be queried about the development of any new skin lesions and should have periodic dermatologic evaluation. Furthermore, these patients should be recommended to avoid excessive sun exposure. Prospective studies are needed to elucidate the mechanisms underlying the pathogenesis of AK/SCC as an adverse event associated with this agent.
Figure 3.
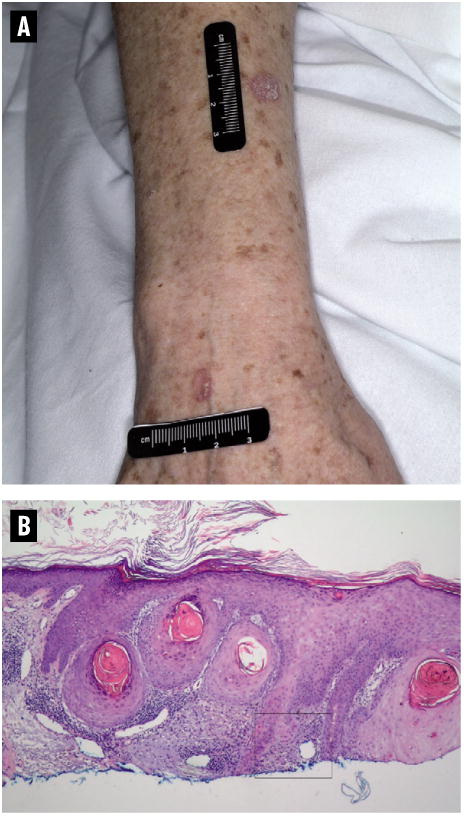
A & B Patient with Squamous Cell Carcinoma After Treatment with Sorafenib
A. Erythematous nodules in a 66-year-old white woman who developed 3 cutaneous squamous cell carcinomas (1 on the back, 1 on the right forearm, and 1 on the right dorsal hand) and multiple actinic keratoses 15 weeks after initiation of therapy with sorafenib. All lesions have been excised with clear margins. After discontinuation of sorafenib because of disease progression, the patient had no further dermatologic toxicity. B. Routine histopathology of case described above demonstrating well-differentiated invasive squamous cell carcinoma (hematoxylin and eosin stain, magnification × 4).
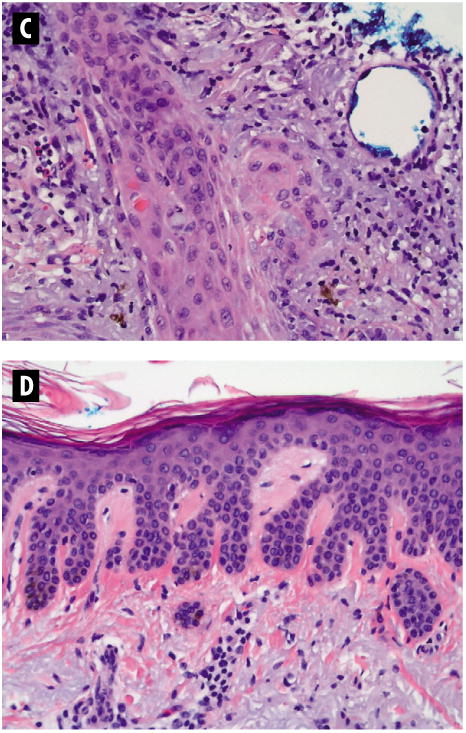
C & D Histopathology of Squamous Cell Carcinoma and Actinic Keratosis Lesions
C. Same as Figure 3B, with greater magnification (hematoxylin and eosin [H & E] stain, magnification × 20). D. Routine histopathology of a lesion demonstrating hyperplastic actinic keratosis (H & E stain, magnification × 20).
Figure 4. Case Study of a Patient Treated with Sorafenib.
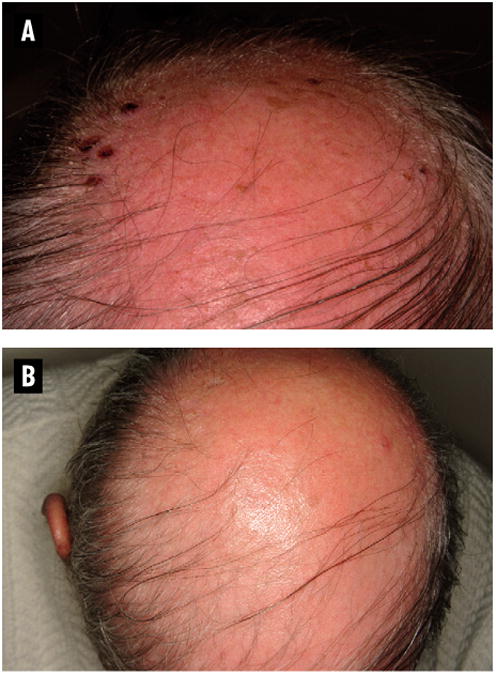
A 59-year-old white man developed multiple hemorrhagic crusts symmetrically over his scalp, helices of both ears, and both nasal ala within 2-3 weeks of starting sorafenib (Figure 4A). Five biopsies were performed from the scalp and face. Two showed squamous cell carcinoma (SCC). The remainder showed actinic keratosis, but because of the superficial depth of the biopsy, histopathology could not rule out squamous cell carcinoma in situ. Two days after discontinuation of sorafenib, the patient reported marked improvement of all lesions (Figure 4B). After a 2-week interruption of therapy, physical examination revealed only verrucous, fissured plaques over both nasal ala, with no remaining lesions on the scalp or ears. Sorafenib was restarted at 50% dose reduction. The patient developed 2 new SCC lesions on the left hand and forearm 4 months after the initial diagnosis of SCC.
References
- 1.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal cell carcinoma. N Eng J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzeferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Eng J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the Raf/ MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 5.Clark SH, Duvic M, Prieto VG. Mycosis fungoides-like reaction in a patient treated with Gleevec. J Cutan Pathol. 2003;30:279–81. doi: 10.1046/j.0303-6987.2003.053.x. [DOI] [PubMed] [Google Scholar]
- 6.Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783–5. doi: 10.1111/j.1365-2230.2006.02223.x. [DOI] [PubMed] [Google Scholar]
- 7.Kong HH, Cowen EW, Azad NS, et al. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol. 2006;56:171–2. doi: 10.1016/j.jaad.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CH, Lin WC, Chuang YC, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2007;158:592–6. doi: 10.1111/j.1365-2133.2007.08357.x. [DOI] [PubMed] [Google Scholar]
- 9.Mc Cubrey J, Steelman LS, Chappell WH, et al. Roles of Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochimica Biophysica Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassarino DS, Miller WJ, Auerbach A, et al. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification – part two. J Cutan Pathol. 2006;33:261–79. doi: 10.1111/j.0303-6987.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 11.Hipp MM, Hilf N, Walter S, et al. Sorafenib but not sunitinib affects the function of dendritic cells and the induction of primary immune responses. Blood. 2008;111:5610–20. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 12.Zhao W, Gu YH, Song R, et al. Sorafenib inhibits activation of human peripheral blood T cells by targeting LCK phosphorylation. Leukemia. 2008;22:1226–33. doi: 10.1038/leu.2008.58. [DOI] [PubMed] [Google Scholar]


