Abstract
The transplantation of glucose-responsive, insulin-producing cells offers the potential for restoring glycemic control in diabetic patients1. Pancreas transplantation and the infusion of cadaveric islets are currently implemented clinically2, but are limited by the adverse effects of lifetime immunosuppression and the limited supply of donor tissue3. The latter concern may be addressed by recently described glucose responsive mature β-cells derived from human embryonic stem cells; called SC-β, these cells may represent an unlimited human cell source for pancreas replacement therapy4. Strategies to address the immunosuppression concern include immunoisolation of insulin-producing cells with porous biomaterials that function as an immune barrier5,6. However, clinical implementation has been challenging due to host immune responses to implant materials7. Here, we report the first long term glycemic correction of a diabetic, immune-competent animal model with human SC-β cells. SC-β cells were encapsulated with alginate-derivatives capable of mitigating foreign body responses in vivo, and implanted into the intraperitoneal (IP) space of streptozotocin-treated (STZ) C57BL/6J mice. These implants induced glycemic correction until removal at 174 days without any immunosuppression. Human C-peptide concentrations and in vivo glucose responsiveness demonstrate therapeutically relevant glycemic control. Implants retrieved after 174 days contained viable insulin-producing cells.
Diabetes is a global epidemic afflicting over 300 million people8. While a rigorous regimen of blood glucose monitoring coupled with daily injections of exogenous insulin remains the leading treatment for patients with type 1 diabetes, they still suffer ill effects due to the challenges associated with daily compliance9,10. In addition, the process by which beta cells of the pancreatic islets of Langerhans release insulin in response to changes in blood glucose concentrations is highly dynamic and imperfectly simulated by periodic insulin injections10,11. The transplantation of donor tissue would achieve insulin independence for type 1 diabetics2,12,13. Recently, the in vitro differentiation of human pluripotent stem cells (hPSCs) into functional pancreatic β-cells was reported, providing for the first time a path to produce an unlimited supply of human insulin-producing tissue (Fig. 1a, Supplementary Fig. 1)4. Methods to relieve the need for life long immunosuppression are essential to enable broad clinical implementation of this new tissue source3,14,15.
Figure 1.
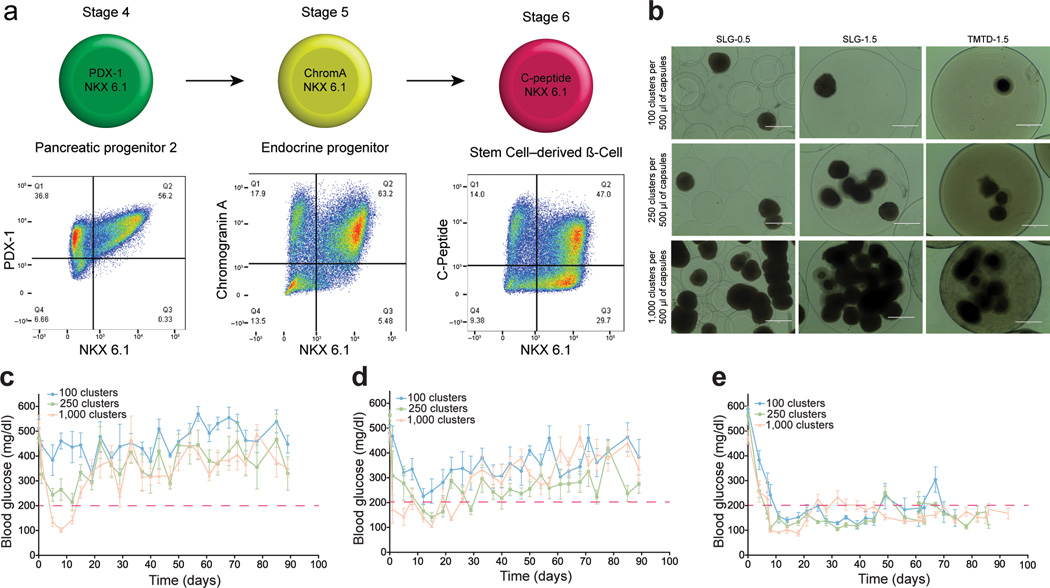
SC-β cells encapsulated with TMTD alginate sustain normoglycemia in STZ-treated immune competent C57BL/6J mice. (a) SC-β cells were generated using the differentiation protocol described4. FACS analysis shows surface markers on cells at indicated differentiation stages. Data is representative of 10 separate differentiations from the HUES8 stem cell line. (Editor: Stage 1–3 is previously described4 and not relevant to this manuscript) (b) Brightfield images of encapsulated SC-β cells.. Scale bar = 400 µm, N = 15. (c–e) SC-β cells encapsulated as shown in (b) were transplanted into the intraperitoneal space of STZ-treated C57BL/6 mice, and blood glucose concentrations were measured at indicated times. (c) 500 µm SLG20 alginate microcapsules; (d) 1.5 mm SLG20 alginate microspheres; (e) 1.5 mm TMTD alginate spheres. Three different doses of cell clusters (100, 250, and 1000 cluster per mouse) were implanted under each encapsulation condition. The red dashed line indicates the blood glucose cutoff for normoglycemia in mice. For reference 250 clusters equates to approximately 1 million cells. Error bars, mean ± s.e.m. Quantitative data shown is the average of N = 5 mice per treatment. All experiments were repeated three times for a total of N = 15 mice per treatment.
Cell encapsulation can overcome the need for immunosuppression by protecting therapeutic tissues from rejection by the host immune system7,16. The most commonly investigated method for islet encapsulation therapy is the formulation of isolated islets into alginate microspheres16–20. Clinical evaluation of this technology in diabetic patients with cadaveric human islets has only achieved glycemic correction for short periods16,21,22. Implants from these studies elicit strong innate immune-mediated foreign body responses (FBR) that result in fibrotic deposition, nutrient isolation, and donor tissue necrosis23,24. Similar results are observed with encapsulated xenogeneic islets and pancreatic progenitor cells in preclinical diabetic mouse or non-human primate models, where both the therapeutic efficacy of encapsulated cadaveric human islets and pig islets is hampered by immunological responses19,25,26.
A major contributor to the performance of encapsulated islet implants is the immune response to the biomaterials used for cell encapsulation5,7,17. We demonstrated that microsphere size can affect the immunological responses to implanted alginates27. More recently, we identified chemically-modified alginates such as triazole-thiomorpholine dioxide (TMTD, Supplementary Fig. 2) that resist implant fibrosis in both rodents and non-human primates28. Here we show that triazole-thiomorpholine dioxide (TMTD) alginate-encapsulated SC-β cells provide long-term glycemic correction and glucose-responsiveness without immune suppression in immune-competent C57BL/6J mice.
To ensure proper biocompatibility assessment in our studies we used immunocompetent C57BL/6J mice, because this strain is known to produce a strong fibrotic and foreign body response similar to observations made in human patients29. When implanted into the intraperitoneal space of non-human primates or rodents with robust immune systems such as C57BL/6J,30,31 conventional alginate microspheres elicit foreign body reactions and fibrosis30,31. However, 1.5 mm spheres of TMTD alginate mitigated fibrotic responses in non-human primates and C57BL/6J mice28. To determine whether encapsulation of SC-β cells can induce glycemic correction, we encapsulated cells with three different formulations: 500 µm alginate microcapsules conventionally used for islet encapsulation5,22, 1.5 mm alginate spheres27, and 1.5 mm TMTD alginate spheres (Supplementary Fig. 2). Each of these formulations containing three different doses of SC-β were transplanted into diabetic streptozotocin (STZ) treated C57BL/6J mice32,33, and we evaluated their ability to restore normoglycemia for 90 days for XX days (Fig. 1c–e). Naked, non-encapsulated SC-β cells were unable to provide glycemic correction in this diabetic model regardless of implantation site (Supplementary Fig. 3). Mice transplanted with SC-β cells encapsulated in 500 µm microcapsules showed the lowest levels of glycemic control, with only the highest dose of transplanted clusters able to restore normoglycemia for 15 days. SC-β cells encapsulated in 1.5 mm alginate spheres performed better than the 500 µm microcapsule formulation, with normoglycemia maintained for 20–30 days for the two higher doses, consistent with earlier results obtained using rat islets27. However, the 1.5 mm spheres were unable to sustain normoglycemia with SC-β cells as they could with rat islets, which can be rationalized by the increased xenogeneic immune responses against implants with human tissue compared to those with rat tissue. Sustained normoglycemia was achieved for over 70 days with 1.5 mm TMTD alginate spheres at all three doses tested (Fig. 1e). Human C-peptide concentrations detected in blood at 3, 6, and 9 weeks during the course of this study were consistent with the notion that transplanted SC-β cells remained functional; recipients of TMTD alginate spheres showed statistically significant higher levels of human C-peptide than the other treatment groups (Supplementary Fig. 4). The C-peptide levels measured in the TMTD alginate group are also consistent with previous studies using human cells to cure diabetic immunocompromised mouse models4,33–35.
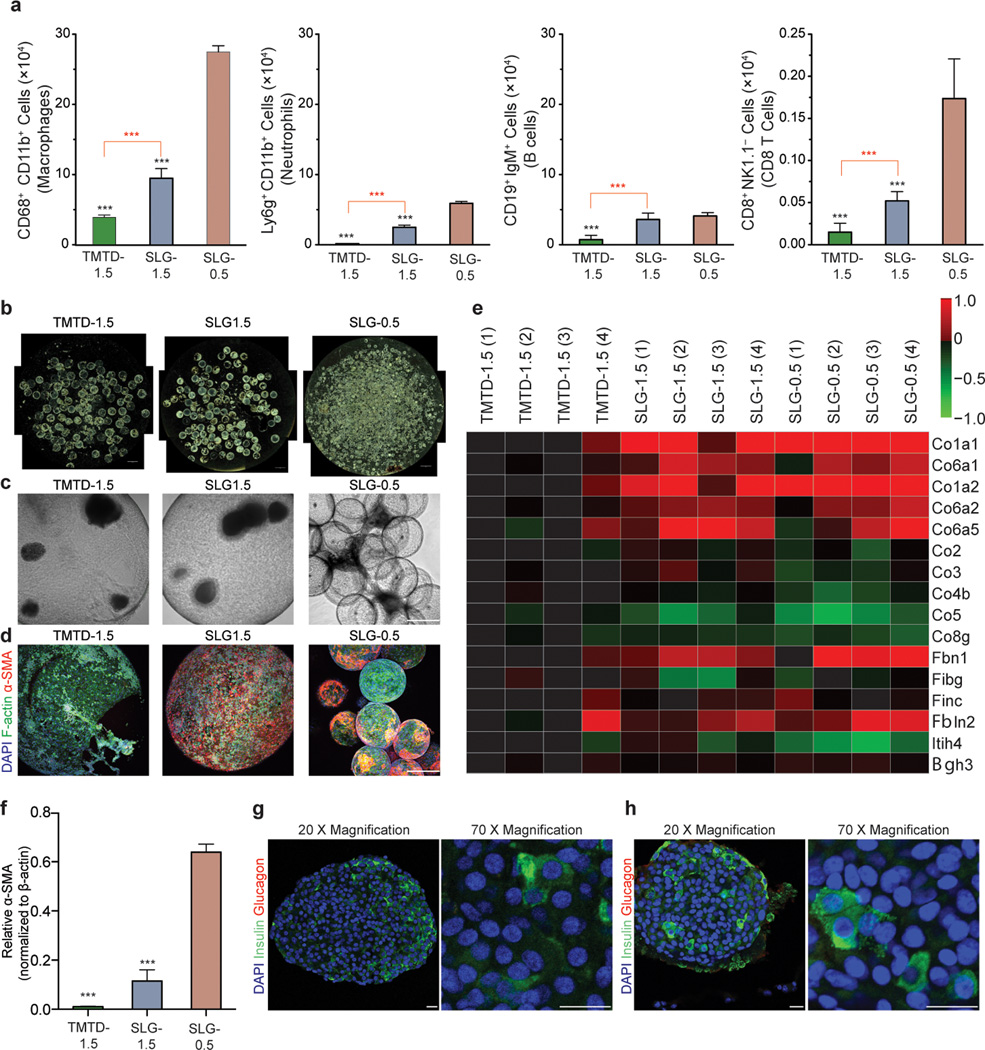
To evaluate the immune responses to these spheres, a separate cohort of encapsulated SC-β cells were implanted IP into C57BL/6 mice and retrieved after 14 days, a time point for monitoring foreign body responses to implanted materials. Cells associated with the outside of the spheres were isolated and analyzed by FACS (Fig. 2a). Statistically significant lower numbers of macrophages, neutrophils, B cells, and CD8+ T cells were associated with TMTD alginate encapsulated SC-β cells (formulation 3) compared to SLG20 (> 60% G, 75–220 kDa MW, NovaMatrix™) controls (formulation 1, 2). Implants retrieved after achieving glycemic correction for 80–90 days in STZ-C57BL/6J mice (implants from Fig. 1c–e) revealed that TMTD alginate spheres had much lower levels of fibrotic deposition (Fig. 2b–f). Immunofluorescence staining of these retrieved spheres for cellular deposition (DAPI, F-actin) and myofibroblasts (α-SMA) showed significantly lower levels of cellular deposition on TMTD alginate spheres (Fig. 2d, Supplementary Fig. 5 and 6). Proteomic analysis of these protein extracts detected 18 collagen isoforms, and 10 out of the 18 detected collagen proteins were significantly reduced in TMTD alginate transplants further showing that these materials are able to mitigate fibrotic responses (Fig. 2e). Western blot quantification of α-SMA protein extracted from the retrieved implants also support the conclusion that there is lower fibrosis on TMTD spheres (Fig. 2f, and Supplementary Fig. 5d). Finally, consistent with these results, histological processing and immunostaining of TMTD encapsulated SC-β clusters prior to transplantation and retrieved after over 90 days from STZ-treated C57BL/6J mice revealed cell clusters with positive staining for the mature SC-β cell marker human insulin; minimal glucagon staining was observed (Fig. 2g and h). Maintenance of insulin positive cells post-retrieval suggests that the SC-β cells retaining their differentiation state through the entire study.
Figure 2.
SC-β cells encapsulated with TMTD alginate elicit weaker immunological and fibrotic responses in immune competent C57BL/6J mice. (a) FACS analysis of encapsulated SC-β cell implants retrieved 14 days after intraperitoneal transplantation in C57BL/6 mice, N = 10. Red asterisks specify statistical significance between indicated groups while black asterisks specify significance against the SLG 0.5 group. (b–d) Dark-field (N=15) (b), brightfield (N=15) (c) and Z-sacked confocal immunofluorescence imaging (DAPI shown blue, F-actin shown in green, α-SMA shown in red) (d) of implants retrieved after 90 days from the STZ-treated C57BL/6J mice presented in Figure 1c–e, (N = 15) (e) The implants containing 250 clusters were retrieved from the STZ-treated C57BL/6J mice shown in Figure 1c–e 90 days after implantation at XX days after implantation. Implant lysates were subjected to proteomic analysis XXX analysis. (N = 4 per mice treatment; analysis was performed on two separate cohorts for a total of 8 mice per treatment). Each column in the heatmap represents an individual mouse from the respective treatment group. (f) α-SMA protein isolated from implants retrieved from the STZ-treated C57BL/6J mice shown in Figure 1c–e was analyzed by immunoblot. Graph shows lower levels of α-SMA for TMTD 1.5 mm spheres. (N = 5) (g-h) Pre-transplantation (g) and post-retrieval (h) histological sectioning and immunostaining of encapsulated SC-β cells from implants retrieved at 90 XXXX days after implantation from the STZ-treated C57BL/6J mice shown in Figure 1c–e (N = 15). Scale bar = 20 µm. All experiments were performed at least three times with the exception of FACS and proteomics quantification, which were each repeated twice. One-way ANOVA with Bonferroni multiple comparison correction, = p < 0.0001. Scale bar in a = 2 mm. Scale bar in b and c = 300 µm.
We next characterized the ability of TMTD alginate spheres to shield the encapsulated SC-β cells from the host immune system. Freeze-fracture cryogenic scanning electron microscopy (cryo-SEM) of the spheres display a heterogeneous pore structure with pore sizes ranging from sub-micron to 1–3 µm in size, a range capable of preventing permeation by cells and large proteins (Supplementary Fig. 2b). Intravital imaging of transplanted spheres after 14 days in B6.129S6-Ccr6tm1(EGFP)Irw/J mice, in which T, B, and dendritic cells express EGFP, showed localization of CCR6+ cells to regions of spheres containing SC-β cells, but an inability for these cells to make contact and initiate cytotoxic events (Supplementary Fig. 7).
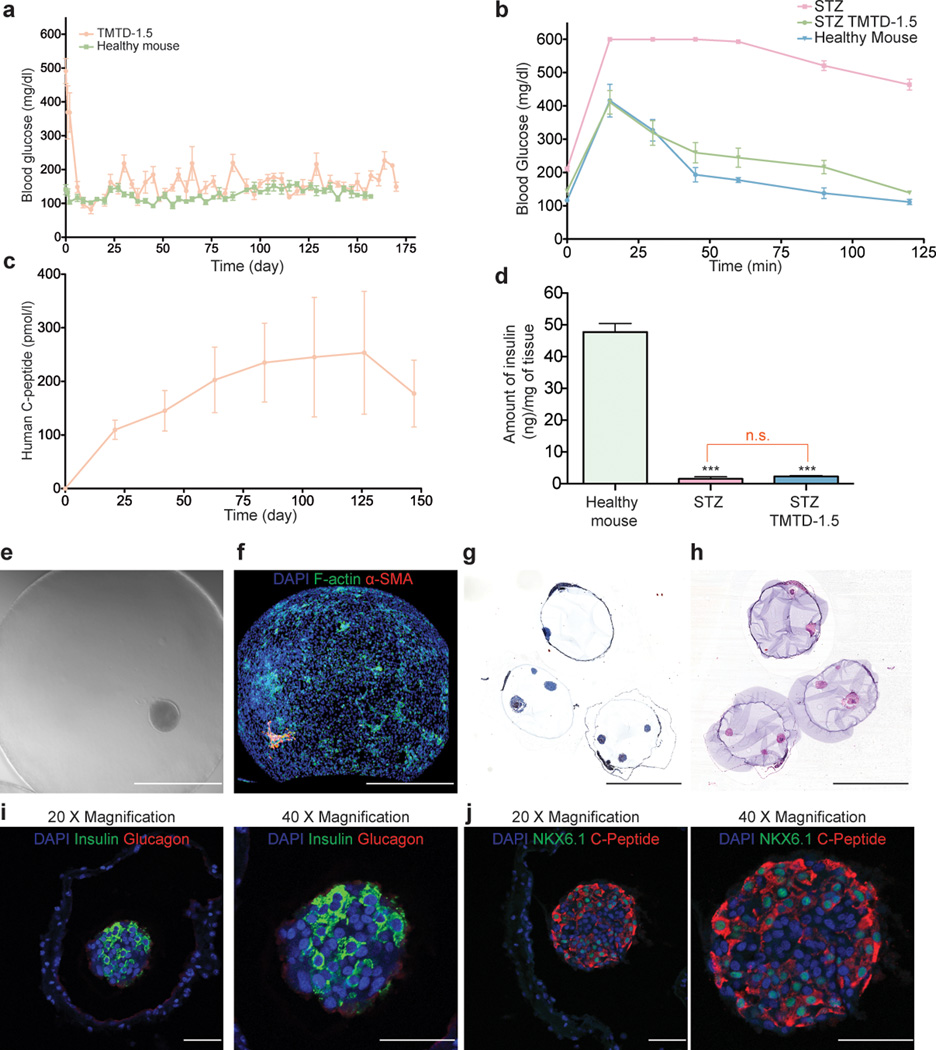
The achievement of long-term glycemic correction in diabetic animal models has previously been defined as sustained normoglycemia for more than 100 days32,36. To evaluate the capacity of TMTD alginate encapsulated SC-β cell transplants to sustain normoglycemia, we tracked a separate cohort of transplanted STZ-treated C57BL/6J diabetic mice for 174 days, at which point implants were retrieved prior to the onset of complications of STZ treatment on the mice (Fig. 3a). Transplanted mice maintained glycemic correction over the entire period, even at the end of the experiment, and closely matched the blood glucose levels of wild type C57BL/6J mice tracked over a similar period. In addition, fasted human C-peptide levels over 100 pmol/L were recorded at multiple points throughout the study (Fig. 3c) and are comparable to C-peptide levels of fasted wild–type mice. We also performed a glucose challenge on these mice 150 days post-transplantation, and encapsulated SC-β cells restored normoglycemia at a rate comparable to wild type mice (Fig. 3b). Host pancreas insulin concentrations confirmed that STZ treatment induced hyperglycemia XXXX, and that there was a lack of endogenous pancreas regeneration in STZ-treated mice (Fig. 3d). SC-β cell implants retrieved after 6 months displayed no signs of fibrotic overgrowth, with minimal collagenous and cellular deposition evident on the capsule surface (Fig. 3e–h). Since spheres retrieved after 3 months showed minimal levels of fibrosis, this suggests that TMTD alginate mitigates immunological responses by inhibiting the activation of innate immune cell populations. Finally, immunostaining of implants retrieved 174 days post-transplantation again showed SC-β clusters staining positive for human insulin; these cells showed minimal co-localization with glucagon staining (Fig. 3i). Insulin-positive cells also stained positive for the nuclear factor Nkx6.1, a β cell marker, adding further evidence that SC-β cells maintained their differentiation state throughout the experiment (Fig. 3j).
Figure 3.
SC-β cells encapsulated with TMTD alginate sustain long-term normoglycemia in STZ-treated C57BL/6J mice. (a) Blood glucose levels were measured in STZ-treated C57BL/6 mice implanted with SC-β cells encapsulated with TMTD alginate at a dose of 250 clusters/mouse. Blood glucose levels were also measured of a Healthy C57BL/6J mouse cohort not treated with STZ. Shown are cohorts of N = 5 mice; experiment was repeated 3 times for a total of 15 mice per condition. (b) The mice shown in (a), together with a cohort of STZ-treated non-implanted mice, were subjected to IVGTT 174 days after implantation. (c) Human C-peptide levels were measured in the blood of the STZ-treated C57BL/6 mice implanted with SC-β cells shown in (a). (d) Quantification of mouse insulin extracted from the pancreas of mice (N = 5) in each treatment group. TMTD alginate treated mice had their pancreas removed after 174 days post-implantation, while the pancreas of healthy and STZ-treated non-implanted C57BL/6 mice were taken at 8–10 weeks. at XX time point. (e–f) Brightfield (e) and Z-sacked confocal immunofluorescence imaging (e) (DAPI shown blue, F- actin shown in green, α-SMA shown in red) of implants retrieved at XX time point from the mice presented in (a–c). Scale bar = 400 µm (g-h). Masson’s thrichrome (g), and H&E (h) histological analysis of implants retrieved after 174 days from the mice presented in (a–c); Scale bar = 2 mm. (i-j) immunofluorescence analysis of implants retrieved after 174 days from the mice presented in (a–c). (i) DAPI shown blue, Insulin shown in green, and Glucagon shown in red. Scale bar = 50 µm (j) DAPI shown blue, NKX6.1 shown in green, and C-peptide shown in red. Scale bar = 50 µm. Error bars, mean ± s.e.m. N = 5 mice per treatment and all experiments were performed at least three times for a total of 15 mice per treatment. Insulin extraction data: one-way ANOVA with Bonferroni multiple comparison correction, = p < 0.0001.
Here we showed that encapsulated SC-β cells can achieve glucose-responsive, long-term glycemic correction (174 days with the mice still euglycemic at the end of the experiment) in an immune-competent diabetic animal with no immunosuppression utilizing a modified alginate capable of mitigating the foreign body response. To our knowledge, this is the first demonstration of long-term glycemic correction with SC-β cells and the longest duration of sustained normoglycemia achieved with any encapsulated insulin-producing human cell in a robust FBR rodent model. Previously, we showed that alginate spheres with large diameters (> 1.5 mm) could mitigate fibrosis and support euglycemia for 6 months with encapsulated rat islets in STZ-treated C57BL/6J mice27. In this report, we show that these same large spheres, when encapsulating human cells, can only support euglycemia for less than one month and become fibrosed in this animal model (Fig. 1d and Fig. 2d–f). These results highlight the added barrier erected by the increased immunological responses against xenogeneic human cell implants in this model, comparable to the aggressive immune responses of an autoimmune diabetic patient, and the need to develop materials that can mitigate these responses. Here, the combination of both increased sphere size and alginate derivatization was critical to mitigate these immunological responses. The TMTD alginate used here is part of a group of imidazole-modified alginates that mitigate foreign body responses in non-human primates for up to 6 months28. Taken together, these results lay the groundwork for studies in autoimmune animal models and future human studies with these formulations with the goal of achieving long-term replacement therapy for type 1 diabetes. We believe that encapsulated human SC-β cells have the potential to provide insulin independence for patients suffering from this disease.
MATERIALS AND METHODS
Materials/Reagents
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) and cell culture reagents from Life Technologies (Grand Island, NY), unless otherwise noted. Antibodies: Alexa Fluor 488-conjugated anti-mouse CD68 (Cat. #137012, Clone FA-11) and Alexa Fluor 647-conjugated anti-mouse Ly-6G/Ly-6C (Gr-1) (Cat. #137012, Clone RB6-8C5) were purchased from BioLegend Inc. (San Diego, CA). Cy3-conjugated anti-mouse alpha smooth muscle actin antibody was purchased from Sigma Aldrich (St. Louis MO). Filamentous actin (F-actin)-specific Alexa Fluor 488-conjugated Phalloidin was purchased from Life Technologies (Grand Island, NY). Anti-Glucagon cat# ab82270, Anti-insulin cat# ab7842, Goat Anti-Guinea pig IgG H&L conjugated Alexa Fluor® 488 ca#ab150185, and Goat Anti-Mouse IgG H&L conjugated Alexa Fluor® 594 ca#ab150116 were purchased from abcam (Cambridge, MA). Anti human C-peptide cat #GN-1D4, and NKX 6.1 cat # F55A12 was purchased from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, Iowa). A sampling of spheres used in study were submitted for endotoxin testing by a commercial vendor (Charles River, Wilmington, MA) and the results showed that spheres contained < 0.05 EU/ml of endotoxin levels.
Reproducibility
The batches of SC-β cells used for experiments presented in this manuscript ranged between 40–65% NKX6.1/C-peptide double positive cells and a representative FACS plot for a single batch of cells is presented in Figure 1a. In brief, all materials were implanted intraperitoneally and retrieved at specified times from immunocompetent streptozotocin induced diabetic C57BL/6J or B6.129S6-Ccr6tm1(EGFP)Irw/J male mice in accordance with approved protocols and federal guidelines. No blinding or randomization was used. Sample processing, staining, FACS, and imaging were performed as detailed below. Shown are representative images in all cases at least from N = 15 mice per treatment group. FACS analysis was performed on two separate cohorts of N = 5 per treatment group (total of 10 animals per treatment). Blood glucose correction, serum C-peptide analysis, and in vivo Glucose tolerance test studies were performed on three separate cohorts of N = 5 per treatment group (total of 15 animals per treatment). Western Blot analysis was performed on retrievals from three separate cohorts of N = 5 per treatment group (total of 15 animals per treatment). Long-term blood glucose monitoring study was terminated at approximately 6 months (174 days) to avoid potential complications associated with tumors forming from the STZ treatment, which increase in incidence longer than 6 months post STZ administration. Quantified data shown are group mean values ± SEM.
Statistical analysis
Data are expressed as mean ± SEM, and N = 5 mice per time point and per treatment group and all experiments were at least repeated twice and in most cased three times (total sample size ranges between 10–15 animals per treatment group). We have specified in each figure caption the number of N’s for each experiment and treatment group. These sample sizes where chosen based on previous literature. All animals were included in analyses and cohorts where randomly selected. Investigators where not blind to performed experiments. Due to technical limitations (instrument throughput) for proteomics analysis 12 samples were analyzed per experiment by randomly selecting a collection of four samples per treatment group. FACS, Elisa, and western blot data was analyzed for statistical significance either by unpaired, two-tailed t-test, or one-way ANOVA with Bonferroni multiple comparison correction, unless indicated otherwise, as implemented in GraphPad Prism 5; *: p < 0.05, **: p < 0.001, and ***: p < 0.0001.
hESC maintenance and directed differentiation in stirred suspension culture
The HUES8 hESC line was maintained undifferentiated as previously reported4 in mTESR1 (StemCell Technologies Inc.; 05850) in 500ml stir flasks (Corning, VWR; 89089-814) placed on a 9-Position stir plate set at rotation rate of 70rpm in a 37°C incubator, 5% CO2, and 100% humidity. Cells were seeded at 0.5×106 cells/ml in mTESR with 10µM Y27632 (Abcam; ab120129), with mTESR1 media changed (w/o Y27632) on the second day and cultures were split every third day to keep cluster diameter size <300µm. Cells were differentiated with a modified previously published protocol4 by seeding at 0.5×106/ml and differentiation starting 72 hours later.
Media changes were as follows - Day1: S1 + 100ng/ml ActivinA (R&D Systems; 338-AC) + 3µM Chir99021 (Stemgent; 04-0004-10)). Day2: S1 + 100ng/ml ActivinA. Days 4,6: S2 + 50ng/ml KGF (Peprotech; AF-100-19)). Days 7,8: S3 + 50ng/ml KGF + 0.25µM Sant1 (Sigma; S4572) + 2µM Retinoic acid (RA) (Sigma; R2625) + 200nM LDN193189 (Day7 only) (Sigma; SML0559) + 500nM PdBU (EMD Millipore; 524390) + 10µM Y27632. Days 9, 11, 13: S3 + 50ng/ml KGF + 0.25µM Sant1 + 100nM RA + 10µM Y27632 + 5ng/ml ActivinA. Days 15, 16: BE5 + 0.25µM Sant1 + 50nM RA + 1µM XXI (EMD Millipore; 565790) + 10µM Alk5i II (Axxora; ALX-270-445) + 1µM GC1 (R&D systems; 211110-63-3) + 20ng/ml Betacellulin (Thermo Fisher Scientific; 50932345) + 10µM ZnSO4 + 100nM LDN + 3nM Staurosporine (EMD Millipore; 569396) + 10µM Y27632. Days 18, 20: BE5 + 1µM XXI + 10µM Alk5i II + 1µM GC1 + 20ng/ml Betacellulin + 10µM ZnSO4 + 100nM LDN + 3nM Staurosporine + 10µM Y27632. Days 22–36 (media change every second day): CMRLM + 10µM Alk5i II + 1µM GC1 + 10µM Trolox (Calbiochem; CAS 53188-07-1).
Basal media formulation used for directed differentiation were as follows:
S1 media: MCDB131 (Cellgro; 15-100-CV) + 8mM D-(+)-Glucose (Sigma; G7528) + 2.46g/L NaHCO3 (Sigma; S3817) + 2% FAF-BSA (Proliant; 68700) + ITS-X (Invitrogen; 51500056) 1:50.000 + 2mM Glutamax (Invitrogen; 35050079) + 0.25mM Vitamin C (Sigma Aldrich; A4544) + 1% Pen/Strep (Cellgro; 30-002-CI).
S2 media: MCDB131 + 8mM D-Glucose + 1.23g/L NaHCO3 + 2% FAF-BSA + ITS-X 1:50.000 + 2mMl Glutamax + 0.25mM Vitamin C + 1% Pen/Strep
S3 media: MCDB131 + 8mM D-Glucose + 1.23g/L NaHCO3 + 2% FAF-BSA + ITS-X 1:200 + 2mMl Glutamax + 0.25mM Vitamin C + 1% Pen/Strep
BE5 media: MCDB131 + 20mM D-Glucose + 1.754g/L NaHCO3 + 2% FAF-BSA + ITS-X 1:200 + 2mM Glutamax + 1% Pen/Strep + Heparin 10µg/ml (Sigma; H3149).
CMRLM (CMRL 1066 Modified media): CMRL 1066 w/ Phenol Red and NaHCO3, w/o L-Glutamine and HEPES (Invitrogen;11530-037) + 2% FAF-BSA + 2mM Glutamax + 1% Pen/Strep + 20nM Human insulin (Sigma-Aldrich; I9278) + 70nM Human Apo-Transferrin (Sigma-Aldrich; T2036) + Heparin 10µg/ml (Sigma; H3149) + 10µM ZnSO4 (Sigma Aldrich; Z0251) + 5mM Sodium Pyruvate (Invitrogen; 11360-070) + 1:2000 Chemically Defined Lipid Concentrate (Invitrogen; 11905-031) + 1:1000 Medium Trace Elements A (Cellgro; 25-021-CI) + 1:1000 Medium Trace Elements B (Cellgro; 99-175-CI) + 15µM Ethanolamine (Sigma- Aldrich; E0135)
In Vitro glucose stimulated insulin secretion
Glucose stimulated insulin secretion to assess in vitro function was performed as previously described4. Approximately 5×105 SC-β cells after 7–14 days in stage 6 were washed with Krebs buffer (krb; 128 mM NaCl, 5 mM KCl, 2.7 mM CaCl2, 1.2 mM MgCl2, 1 mM Na2HPO4, 1.2 mM KH2PO4, 5 mM NaHCO3, 10 mM HEPES (Life Technologies; 15630080), and 0.1% BSA (Proliant; 68700) in deionized water) then incubated for 2 hr in 2 mM glucose (Sigma; G7528) in krb. After incubation, cells were sequentially incubated 30 min each with 2 mM, 20 mM, 2 mM, 20 mM, 2 mM, and 20 mM glucose in krb then with 30 mM KCl in krb. The supernatants after each 30 min incubation collected and insulin quantified using the Human Ultrasensitive Insulin ELISA (ALPCO Diagnostics; 80-INSHUU-E01.1) with measurement by a FLUOstar optima spectrophotometer (BMG Labtech) at 450 nm. Data was normalized with viable cell counts from a Vi Cell XR (Beckman Coulter).
TMTD Alginate Synthesis
A complete description of methods and characterization are provided for TMTD alginate in reference 27, where the material is referred to as Z1-Y15. Briefly, 3.5 g of 4-Propagylthiomorpholine 1,1-Dioxide (1 equiv. 20 mmol) was added to a solution of 2.5g TBTA (0.2 equiv, 4 mmol), 750µL Triethylamine (0.5 equiv, 10 mmol), 250mg Copper(I)-iodide (0.06 equiv, 1.3 mmol) in 50ml methanol. The mixture was cooled to 0 °C and 5.25 ml of 11-Azido-3,6,9-trioxaundecan-1-amine (1 equiv, 20 mmol) was added. The reaction was agitated overnight at 55 °C, and the solvent was removed under reduced pressure the next day. The crude reaction was purified by reverse phase (water/acetonitrile) flash chromatography on a C18 column, yielding purified TMTD amine. This product was then reacted with ultrapure alginate follows: 1.5 g of UP-VLVG (1 equiv, > 60% G, ~25 kDa MW, NovaMatrix™) was dissolved in 45ml of water and 675 mg of 2-Chloro-4,6-dimethoxy-1,3,5-triazine (CDMT, 0.5 equiv) and 840 µL of N-Methylmorpholine (NMM, 1 equiv) was added. Then 7.65 mmol of the TMTD amine was dissolved in 22.5 ml acetonitrile and added to the mixture. The reaction was stirred overnight at 55 °C. The solvent was removed under reduced pressure and the solid was dissolved in water. The solution was filtered through a pad of cyano functionalized silica (Silicycle) and the water was removed under reduced pressure to concentrate the solution. It was then dialyzed against a 10,000 MWCO membrane in DI water overnight. The water was removed under reduced pressure to give the functionalized alginate.
Fabrication of alginate hydrogel spheres and cell encapsulation
Prior to sphere fabrication, buffers were sterilized by autoclave and alginate solutions were sterilized by filtration through a 0.2 um filter. Aseptic processing was implemented for fabrication by performing capsule formation in a type II class A2 biosafety cabinet to maintain sterility of manufactured microcapsules/spheres for subsequent implantation. An electrostatic droplet generator was set up in the biosafety cabinet as follows: an ES series 0–100 kV, 20 Watt high voltage power generator (Gamma ES series, Gamma High Voltage Research, FL, USA) is connected to the top and bottom of a blunt tipped needle (SAI Infusion Technologies, IL, USA).
This needle is attached to a 5 mL luer lock syringe (BD, NJ, USA) which is clipped to a syringe pump (Pump 11 Pico Plus, Harvard Apparatus, MA, USA) that is oriented vertically. The syringe pump pumps alginate out into a glass dish containing a 20 mM barium 5% mannitol solution (Sigma Aldrich, MO, USA). The settings of the PicoPlus syringe pump are 12.06 mm diameter and 0.2 mL/min flow rate. After the capsules are formed, they are then collected and then washed with HEPES buffer (NaCl 15.428g, KCl 0.70g, MgCl2*6H2O 0.488g, 50 mL of HEPES (1 M) buffer solution (Gibco, Life Technologies, California, USA) in 2L of DiH2O) 4 times. The alginate capsules are left overnight at 4 °C. The capsules are then washed 2 times in 0.8% saline and kept at 4 °C until use.
To solubilize alginates, SLG20 (NovaMatrix, Sandvika, Norway) was dissolved at 1.4% weight to volume in 0.8% saline. TMTD alginate was initially dissolved at 5% weight to volume in 0.8% saline, and then blended with 3% weight to volume SLG100 (also dissolved in 0.8% saline) at a volume ratio of 80% TMTD alginate to 20% SLG100.
For formation of 0.5 mm spheres were generated with a 25G blunt needle, a voltage of 5kV and a 200 µl/min flow rate. For formation of 1.5 mm spheres, an 18 gauge blunt tipped needle (SAI Infusion Technologies) was used with a voltage of 5–7 kV.
Immediately prior to encapsulation, the cultured SC-β clusters were centrifuged at 1,400 rpm for 1 minute and washed with Ca-free Krebs-Henseleit (KH) Buffer (4.7mM KCl, 25mM HEPES, 1.2mM KH2PO4, 1.2mM MgSO4×7H2O, 135mM NaCl, pH≈7.4, ≈290 mOsm). After washing, SC-β cells were centrifuged again and all supernatant was aspirated. The SC-β pellet was then re-suspended in the SLG20 or TMTD alginate solutions (described above) at cluster densities of 1,000, 250, and 100 clusters per 0.5 ml alginate solution. Spheres were crosslinked using a BaCl2 gelling solution and their sizes were controlled as described above. Immediately after crosslinking, the encapsulated SC-β clusters were washed 4 times with 50 mL of CMRLM media and cultured overnight in a spinner flask at 37°C prior to transplantation. Due to an inevitable loss of SC-β clusters during the encapsulation process, the total number of encapsulated clusters were recounted post-encapsulation
Transplantation surgeries
All animal protocols were approved by the MIT Committee on Animal Care, and all surgical procedures and post-operative care was supervised by MIT Division of Comparative Medicine veterinary staff. Immune-competent male (6–14 weeks of age) STZ-induced diabetic C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) or male (6–10 weeks of age) B6.129S6-Ccr6tm1(EGFP)Irw/J mice (Jackson Laboratory, Bar Harbor, ME) were anesthetized with 3% isoflurane in oxygen and had their abdomens shaved and sterilized using betadine and isopropanol. Preoperatively, all mice also received a 0.05 mg/kg dose of buprenorphine subcutaneously as a pre-surgical analgesic, along with 0.3 mL of 0.9% saline subcutaneously to prevent dehydration. A 0.5 mm incision was made along the midline of the abdomen and the peritoneal lining was exposed using blunt dissection. The peritoneal wall was then grasped with forceps and a 0.5–1 mm incision was made along the linea alba. A desired volume of spheres (all materials without islets, as well as SLG20 spheres encapsulating rat islets) were then loaded into a sterile pipette and implanted into the peritoneal cavity through the incision. The incision was then closed using 5-0 taper-tipped polydioxanone (PDS II) absorbable sutures. The skin was then closed over the incision using a wound clip and tissue glue.
Blood glucose monitoring
To create insulin-dependent diabetic mice, healthy C57BL/6J mice were treated with Streptozotocin (STZ) by the vendor (Jackson Laboratory, Bar Harbor, ME) prior to shipment to MIT. The blood glucose levels of all the mice were retested prior to transplantation. Only mice whose 1 hour-fasted blood glucose levels were above 400 mg/dL for two consecutive days were considered diabetic and underwent transplantation.
Blood glucose levels were monitored two to three times a week following transplantation of islet-containing alginate capsules. To measure the blood glucose of animals we used clinically approved and commercially available handheld glucose meters (Clarity One, Clarity Diagnostic Test Group, Boca Raton, FL) with a measurement range of 0–600 mg/dL. These readers only require very low volumes of blood, (< 2 µl) making it feasible for us to perform multiple blood glucose measurements per week from mice. A small drop of blood was collected from the tail vein using a lancet and tested using a commercial glucometer (Clarity One, Clarity Diagnostic Test Group, Boca Raton, FL). Mice with unfasted blood glucose levels below 200 mg/dL were considered normoglycemic. Monitoring continued until experimental time points had been reached, at which point they were euthanized and the spheres were retrieved.
Human c-peptide monitoring
Human c-peptide levels were monitored every three weeks following transplantation of SC-β containing alginate capsules. Mice were fasted for 1 hour before blood collection, at which point approximately 100–150 µL of blood was collected retro-orbitally into a serum collection tube. Collected blood was centrifuged for 10 minutes at 13000 rpm, serum was removed, and stored at 20 °C until assayed. Serum was assayed for human c-peptide using the Alpco human c-peptide kit (Catalog #: 80-CPTHU-E10) according to the manufacturer’s instructions.
Retrieval of cells, tissues, and materials
At desired time points post-implantation or transplantation (with encapsulated islets), as specified in figures, mice were euthanized by CO2 administration, followed by cervical dislocation. In certain instances, 5 ml of ice cold PBS was first injected in order perform an intraperitoneal lavage to rinse out and collect free-floating intraperitoneal immune cells. An incision was then made using the forceps and scissors along the abdomen skin and peritoneal wall, and intraperitoneal lavage volumes were pipetted out into fresh 15 ml falcon tubes (each prepared with 5 ml of RPMI cell culture media). Next, a wash bottle tip was inserted into the abdominal cavity. KREBS buffer was then used to wash out all material spheres from the abdomen and into petri dishes for collection. After ensuring all the spheres were washed out or manually retrieved (if fibrosed directly to intraperitoneal tissues), they were transferred into 50 mL conical tubes for downstream processing and imaging. After intraperitoneal lavage and sphere retrieval, remaining fibrosed tissues were also excised for downstream FACS and expression analyses.
Imaging of the retrieved material spheres
For phase contrast imaging retrieved materials were gently washed using Krebs buffer and transferred into 35 mm petri dishes for phase contrast microscopy using an Evos Xl microscope (Advanced Microscopy Group).
For bright-field imaging of retrieved materials, samples were gently washed using Krebs buffer and transferred into 35 mm petri dishes for bright-field imaging using a Leica Stereoscopic microscope.
Confocal Immunofluorescence
Immunofluorescence imaging was used to determine immune populations attached to spheres. Materials were retrieved from mice and fixed overnight using 4% paraformaldehyde at 4°C. Samples where then washed twice with KREBS buffer, permeabilized for 30 min using a 0.1% Triton X100 solution, and subsequently blocked for 1 hour using a 1% bovine serum albumin (BSA) solution. Next, the spheres were incubated for 1 hour in an immunostaining cocktail solution consisting of DAPI (500 nM), specific marker probes (1:200 dilution) in BSA. After staining, spheres were washed three times with a 0.1% Tween 20 solution and maintained in a 50% glycerol solution. Spheres were then transferred to glass bottom dishes and imaged using an LSM 700 point scanning confocal microscope (Carl Zeiss Microscopy, Jena Germany) equipped with 5 and 10× objectives. Obtained images where adjusted linearly for presentation using Photoshop (Adobe Inc. Seattle, WA).
Proteomic Analysis
For this assay four samples were randomly selected from each treatment group for protein analysis.
Reduction, Alkylation and Tryptic Digestion
Retrieved samples were suspended in urea cell lysis buffer (8 M urea, Tris pH 8.0) and incubated at 4 °C overnight. Equivalent amounts of protein were reduced (10 mM dithiothreitol, 56 °C for 45 min) and alkylated (50 mM iodoacetamide, room temperature in the dark for 1 h). Proteins were subsequently digested with trypsin (sequencing grade, Promega, Madison, WI), at an enzyme/substrate ratio of 1:50, at room temperature overnight in 100 mM ammonium acetate pH 8.9. Trypsin activity was quenched by adding formic acid to a final concentration of 5%. Peptides were desalted using C18 SpinTips (Protea, Morgantown, WV) then lyophilized and stored at −80 °C.
TMT Labeling
Peptides were labeled with TMT 6plex (Thermo) per manufacturer’s instructions. Lyophilized samples were dissolved in 70 µL ethanol and 30 µl of 500 mM triethylammonium bicarbonate, pH 8.5, and the TMT reagent was dissolved in 30 µl of anhydrous acetonitrile. The solution containing peptides and TMT reagent was vortexed, incubated at room temperature for 1 h. Samples labeled with the six different isotopic TMT reagents were combined and concentrated to completion in a vacuum centrifuge. For the first analysis samples were labeled using the TMT 6plex channels as follows: 126 – TMTD-1.5 biological replicate 1; 127- TMTD-1.5 biological replicate 2; 128 – SLG-1.5 biological replicate 1; 129 – SLG-1.5 biological replicate 2; 130 – SLG-0.5 250 biological replicate 1; and 131 – SLG-0.5 biological replicate 2. For the second analysis samples were labeled using the TMT 6plex channels as follows: 126 – TMTD-1.5 biological replicate 3; 127- TMTD-1.5 biological replicate 4; 128 – SLG-1.5 biological replicate 3; 129 – SLG-1.5 biological replicate 4; 130 – SLG-0.5 biological replicate 3; and 131 – SLG- 0.5 biological replicate 4.
LC-MS/MS
Peptides were then loaded on a precolumn and separated by reverse phase HPLC (Thermo Easy nLC1000) over a 140 minute gradient before nanoelectrospray using a QExactive mass spectrometer (Thermo). The mass spectrometer was operated in a data-dependent mode. The parameters for the full scan MS were: resolution of 70,000 across 350–2000 m/z, AGC 3e6, and maximum IT 50 ms. The full MS scan was followed by MS/MS for the top 10 precursor ions in each cycle with a NCE of 32 and dynamic exclusion of 30 s. Raw mass spectral data files (.raw) were searched using Proteome Discoverer (Thermo) and Mascot version 2.4.1 (Matrix Science). Mascot search parameters were: 10 ppm mass tolerance for precursor ions; 0.8 Dathe fragment ion mass tolerance; 2 missed cleavages of trypsin; fixed modification was carbamidomethylation of cysteine; variable modification was methionine oxidation. TMT quantification was obtained using Proteome Discoverer and isotopically corrected per manufacturer’s instructions.
Histological processing for H&E and Masson’s Trichrome staining
Retrieved materials where fixed overnight using 4% paraformaldehyde at 4°C. After fixation, alginate sphere or retrieved tissue samples were washed using 70% alcohol. The materials where then mixed with 4 degrees calcium-cooled Histogel (VWR, CA # 60872-486). After the molds hardened, the blocks were processed for paraffin embedding, sectioning and staining according to standard histological methods.
Histological immunostaining
Parafin embedded sectioned samples were stained for the following: human insulin (Anti-insulin cat# ab7842, abcam), human c-peptide (C-peptide cat #GN-1D4, Developmental Studies Hybridoma Bank, University of Iowa), NKX 6.1 (cat # F55A12, Developmental Studies Hybridoma Bank, University of Iowa), human glucagon (Anti-Glucagon cat# ab82270, abcam). Cellular nuclei were stained with DAPI (Life Technologies).
Parafin slides were deparaffinized through subsequent incubations in the following solvents (Xylene 5min 2× 100% ETOH 2min ×2 95% 2 min ×2 70% 2min ×2 d-water). Antigen retrieval was done by incubating sections for 30min in ice cooled PBS, and then blocking with 3% horse serum to block for 30 min. Antibody mixtures were then applied as follows: Primary A - Mix together KKX 6.1, 1 to 500 3% & horse serum and c-peptide, 1 to 500. Primary B - Mix together Human insulin 1 to 500 and glucagon 1 to 200, incubate for 2 hours and then Wash in PBS 10min×4. Secondary A - Add anti-mouse AF594 1 to 500 and anti-rat AF488 1 to 500. Secondary B - Add anti-guinea pig AF488 1 to 500 with anti-mouse AF594 1 to 500 incubate for 30min then wash 10min 4×. Slides were then stained with DAPI and coverslips mounted using prolong gold antifade (Life Technologies, Carlsbad, CA)
Western Blotting
Protein was extracted directly from materials for western blot analysis. For protein analyses, retrieved materials were prepared by immersing materials in Pierce RIPA buffer (Cat. #89901, Thermo Scientific) with protease inhibitors (Halt Protease inhibitor single-use cocktail, Cat. #78430, Thermo Scientific) on ice, and then lysed by sonication (for 30 seconds on, 30 seconds off, twice at 70% amplitude). Samples were then subjected to constant agitation for 2 hours at 4°C. Lysates were then centrifuged for 20 min at 12,000 rpm at 4°C, and protein-containing supernatants were collected in fresh tubes kept on ice. In samples from fat tissue, an excess of fat (a top layer on the supernatant) was first removed before supernatant transfer. 20 µg protein (quantified by BCA assay, Pierce BCA protein assay kit, Cat. #23225, Thermo Scientific) for each lane was boiled at 95 °C for 5 min and electrophoresed on SDS-polyacrylamide gels (Any kD 15-well comb mini-gel, Biorad, Cat. #456-9036) and then blotted onto nitrocellulose membranes (Biorad, Cat. #162-0213). Blots were probed with anti-αSmooth Muscle actin antibody (1:400 dilution, Rabbit polyclonal to alpha smooth muscle actin; Cat. #ab5694, AbCam), and anti-β-actin antibody (1:4000 dilution, monoclonal anti-β-actin antibody produced in mouse; Cat. #A1978, Sigma Aldrich) as a loading control followed by donkey anti-rabbit (1:15,000 dilution, Cat. #926-32213, Li-Cor) and goat anti-mouse (1:15,000 dilution, Cat. #926-68070, Li-Cor) fluorophore-conjugated secondary antibodies. Antibody-antigen complexes were visualized using Odyssey detection (Li-Cor, Serial No. ODY-2329) at 700 and 800 nm wavelengths.
FACS analysis
Single-cell suspensions of cells from the surfaces of freshly retrieved capsules were prepared using a gentleMACS Dissociator (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s protocol. Single-cell suspensions were prepared in a passive PEB dissociation buffer (1X PBS, pH 7.2, 0.5% BSA, and 2 mM EDTA) and suspensions were passed through 70 µm filters (Cat. #22363548, Fisher Scientific, Pittsburgh, PA). This process removed the majority of cells adhered to the surface (>90%). The cells were then centrifuged at 300–400g at 4°C and resuspended in a minimal volume (~50 µl) of eBioscience Staining Buffer (Cat. #00-4222) for antibody incubation. All samples were then co-stained in the dark for 25 min at 4°C with two of the fluorescently tagged monoclonal antibodies specific for the cell markers CD68 (1 µl (0.5 µg) per sample; CD68-Alexa647, Clone FA-11, Cat. #11-5931, BioLegend), Ly-6G (Gr-1) (1 µl (0.5 µg) per sample; Ly-6G-Alexa-647, Clone RB6-8C5, Cat. #108418, BioLegend), CD11b (1 µl (0.5 µg) per sample; or CD11b-Alexa-488, Clone M1/70, Cat. #101217, BioLegend), CD19 (1 µl (0.5 µg) per sample; CD19-Alexa-647, Clone 6D5, Cat. #115522, BioLegend), IgM (1 µl (0.5 µg) per sample; IgM-FITC, Clone RMM-1, Cat. #406506, BioLegend), CD8 (1 µl (0.5 µg) per sample; CD8-Alexa647, Clone 53-6.7, Cat. #100724, BioLegend), or NK1.1 (1 µl (0.5 µg) per sample; NK1.1-Alexa488, Clone PK136, Cat. #108718, BioLegend). Two ml of eBioscience Flow Cytometry Staining Buffer (Cat. #00-4222, eBioscience) was then added, and the samples were centrifuged at 400–500g for 5 min at 4°C. Supernatants were removed by aspiration, and this wash step was repeated two more times with staining buffer. Following the third wash, each sample was resuspended in 500 µl of Flow Cytometry Staining Buffer and run through a 40 µm filter (Cat. #22363547, Fisher Scientific) for eventual FACS analysis using a BD FACSCalibur (Cat. #342975), BD Biosciences, San Jose, CA, USA). For proper background and laser intensity settings, unstained, single antibody, and IgG (labeled with either Alexa-488 or Alexa- 647, BioLegend) controls were also run.
Intravital Imaging
For intravital imaging, SC-β containing hydrogels of 0.5 mm and 1.5 mm sizes were fabricated with Qdot 605 (Life technologies, Grand Island, NY) and surgically implanted into B6.129S6- Ccr6tm1(EGFP)Irw/J mice as described above. After 14 days post implantation, the mice were placed under isoflurane anesthesia and a small incision was made at the site of the original surgery to expose beads. The mice were placed on an inverted microscope and imaged using a 25×, N.A. 1.05 objective on an Olympus FVB-1000 MP multiphoton microscope at an excitation wavelength of 860 nm. Z-stacks of 200 µm (10 µm steps) were acquired at 2-minute intervals for time series of 20 – 45 minutes depending on the image. The mice were kept under constant isoflurane anesthesia and monitored throughout the imaging session. Obtained images were analyzed using Velocity 3D Image Analysis Software (Perkin Elmer, Waltham, MA).
In vivo glucose challenges (GSIS)
Mice were fasted overnight (12 hours) prior to glucose challenge. On the day of the challenge, fasting blood glucose levels were measured and then mice were injected via tail-vein with a 30 g/L solution of glucose at a dose of 200 mg/kg. Blood glucose was then monitored every 15 minutes for 2 hours.
Pancreas removal and insulin quantification
After 174 days, mice treated with SC-β cells encapsulated in TMTD-alginate were euthanized and the pancreas of each mouse removed. Each pancreas was weighed and then placed into vial with a stainless steel ball while keeping samples frozen in liquid nitrogen. A volume of 3 ml of acid ethanol was added to each vial and samples were homogenized on a GenoGrinder at 1000 rpm at 1 min increments until tissue was pulverized. Sample vials are held by aluminum blocks that can be placed in liquid nitrogen between each cycle to keep it cold. Vials were then centrifuged at 2400 rpm at 4 °C for 30 min. The supernatant (now containing insulin) was removed and stored, while the vial is filled with more acid ethanol and vortexed. The vials were left overnight shaking at 4°C. Again, vials were centrifuged at 2400 rpm at 4°C for 30 min and the supernatant was collected and added to the previously stored supernatant. Acid ethanol was again added to the vials, vortexed, incubated overnight, centrifuged, and supernatant collected and combined. Supernatant solution was evaporated using a Genevac EZ-2 plus. Samples were stored at −80°C until used. Prior to insulin quantification, samples were resuspended in PBS and quantified using a mouse insulin ELISA kit (Alpco catalog #: 80-INSMS-E10) according to manufacturer’s instructions. This same procedure was repeated for healthy, wild type C57BL/6J mice and a STZ treated C57BL/6J mice.
Supplementary Material
Acknowledgments
This work was supported jointly by the JDRF and Leona M. and the Harry B. Helmsley Charitable Trust (Grant 3-SRA-2014-285-M-R), National Institutes of Health (Grants EB000244, EB000351, DE013023, CA151884, and UC4DK104218), and through a generous gift from the Tayebati Family Foundation. OV was supported by JDRF and DOD/CDMRP postdoctoral fellowships (Grants 3-2013-178 and W81XWH-13-1-0215, respectively). JRM was supported by a fellowship from the Harvard Stem Cell Institute. The authors would like to acknowledge Roman Bogorad for useful discussions and assistance and the use of resources at the Koch Institute Swanson Biotechnology Center for technical support, specifically, the Hope Babette Tang Histology, Microscopy, Flow Cytometry, and Animal Imaging and pre-clinical testing core facilities. We acknowledge the use of imaging resources at the Harvard University Center for Nanoscale Systems, W.M. Keck Biological Imaging Facility (Whitehead Institute), and assistance from Dr. Wendy Salmon.
Footnotes
AUTHOR CONTRIBUTIONS
A.V., O.V., and D.A. designed experiments, analyzed data, and wrote the manuscript. M.G., J.M. F.P., and D.M. provided SC-β cells. A.V., O.V., M.G., J.M., F.P., A.B., J.D., J.L., M.C., K.O., S.J., E.L., S.A-D., S.G., J.M., M.B, and J.H-L. performed experiments. H.T. performed statistical analyses of data sets and aided in the preparation of displays communicating data sets. J.O., D.G., G.W., D.M., R.L. provided conceptual advice and technical support. R.L. and D.A. supervised the study. All authors discussed the results and assisted in the preparation of the manuscript.
COMPETING FINANCIAL INTERESTS
FP, JM, MG and DM declare a financial interest via a patent filed by Harvard and HHMI on the production of stem cell derived beta cells. A.V., O.V., J.D., R.L., and D.A. declare financial interest via patents filed by MIT on the material and hydrogel capsule technology.
References
- 1.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Hirshberg B. Lessons learned from the international trial of the edmonton protocol for islet transplantation. Current diabetes reports. 2007;7:301–303. doi: 10.1007/s11892-007-0048-9. [DOI] [PubMed] [Google Scholar]
- 4.Pagliuca FW, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 6.Soon-Shiong P, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:950–951. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 7.Dolgin E. Encapsulate this. Nat Med. 2014;20:9–11. doi: 10.1038/nm0114-9. [DOI] [PubMed] [Google Scholar]
- 8.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Pickup JC. Insulin-pump therapy for type 1 diabetes mellitus. N Engl J Med. 2012;366:1616–1624. doi: 10.1056/NEJMct1113948. [DOI] [PubMed] [Google Scholar]
- 10.Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14:45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson RP. Islet Transplantation as a Treatment for Diabetes - A Work in Progress. New England Journal of Medicine. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 13.Qi M, et al. Five-year follow-up of patients with type 1 diabetes transplanted with allogeneic islets: the UIC experience. Acta diabetologica. 2014;51:833–843. doi: 10.1007/s00592-014-0627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro AM. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. The review of diabetic studies : RDS. 2012;9:385–406. doi: 10.1900/RDS.2012.9.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel G. Biomedicine. Stem cell recipe offers diabetes hope. Science. 2014;346:148. doi: 10.1126/science.346.6206.148. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs-Tulleneers-Thevissen D, et al. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia. 2013;56:1605–1614. doi: 10.1007/s00125-013-2906-0. [DOI] [PubMed] [Google Scholar]
- 17.Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Advanced drug delivery reviews. 2014;67–68:35–73. doi: 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Lum ZP, et al. Prolonged reversal of diabetic state in NOD mice by xenografts of microencapsulated rat islets. Diabetes. 1991;40:1511–1516. doi: 10.2337/diab.40.11.1511. [DOI] [PubMed] [Google Scholar]
- 19.Schneider S, et al. Long-term graft function of adult rat and human islets encapsulated in novel alginate-based microcapsules after transplantation in immunocompetent diabetic mice. Diabetes. 2005;54:687–693. doi: 10.2337/diabetes.54.3.687. [DOI] [PubMed] [Google Scholar]
- 20.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Advanced Materials. 2006;18:1345–1360. [Google Scholar]
- 21.Basta G, et al. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: four cases. Diabetes Care. 2011;34:2406–2409. doi: 10.2337/dc11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calafiore R, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29:137–138. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 23.de Groot M, Schuurs TA, van Schilfgaarde R. Causes of limited survival of microencapsulated pancreatic islet grafts. The Journal of surgical research. 2004;121:141–150. doi: 10.1016/j.jss.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Tuch BE, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32:1887–1889. doi: 10.2337/dc09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott RB, et al. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplantation proceedings. 2005;37:3505–3508. doi: 10.1016/j.transproceed.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 26.Omer A, et al. Survival and maturation of microencapsulated porcine neonatal pancreatic cell clusters transplanted into immunocompetent diabetic mice. Diabetes. 2003;52:69–75. doi: 10.2337/diabetes.52.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Veiseh O, et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam H, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang J, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nature Biotechnology. 2015 doi: 10.1038/nbt.3462. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolb M, et al. Differences in the fibrogenic response after transfer of active transforming growth factor-beta1 gene to lungs of "fibrosis-prone" and "fibrosis-resistant" mouse strains. Am J Respir Cell Mol Biol. 2002;27:141–150. doi: 10.1165/ajrcmb.27.2.4674. [DOI] [PubMed] [Google Scholar]
- 30.Dang TT, et al. Spatiotemporal effects of a controlled-release anti-inflammatory drug on the cellular dynamics of host response. Biomaterials. 2011;32:4464–4470. doi: 10.1016/j.biomaterials.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King A, Sandler S, Andersson A. The effect of host factors and capsule composition on the cellular overgrowth on implanted alginate capsules. Journal of biomedical materials research. 2001;57:374–383. doi: 10.1002/1097-4636(20011205)57:3<374::aid-jbm1180>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Pepper AR, et al. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol. 2015;33:518–523. doi: 10.1038/nbt.3211. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, et al. Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation. 2009;87:983–991. doi: 10.1097/TP.0b013e31819c86ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezania A, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rezania A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 36.Wang T, et al. An encapsulation system for the immunoisolation of pancreatic islets. Nat Biotechnol. 1997;15:358–362. doi: 10.1038/nbt0497-358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.