Abstract
Background
Recent research indicates that CD133 are expressed in several kinds of stem cells, among which, its high expression in laryngeal carcinoma has caused wide concern. To further explore efficaciously targeting drugs to laryngeal carcinoma stem cells (CSCs), we transplanted a solid tumor from CSCs into abdominal subcutaneous tissue of nude mice, and then compared the biological characteristics of laryngeal solid tumors with or without cisplatin intervention.
Material/Methods
In this study, the expression of CD133 was detected in the Hep-2 cell line by flow cytometry. By applying magnetic cell sorting (MACS) technology, we reported the results of purifying CD133-positive cells from a Hep-2 cell line. Cell proliferation, colony formation, and tumor-forming ability were examined in vitro and in vivo to identify the marker of CSCs in Hep-2 cell line.
Results
Upon flow cytometry analysis, CD133 was expressed constantly on 40.12±1.32% in Hep-2 cell line. Cell proliferation and colony formation ability were higher in CD133-positive cells compared to CD133-negative cells, and the in vivo tumorigenesis experiment showed the same results as in vitro assay. The 2 subpopulations cells were both sensitive to DDP, among which, the effect of DPP on proliferation ability and tumor-forming ability of CD133-positive cells was obviously greater than that of CD133-negative cells.
Conclusions
Above all, our study revealed that CD133-positive cells have properties of higher proliferation, colony formation, and tumorigenesis in Hep-2 cell line, indicating that CD133 could be a marker to characterize laryngeal cancer stem cells.
MeSH Keywords: Carcinoma, Squamous Cell; Cell Line, Tumor; Cisplatin; Laryngeal Neoplasms; Mice, Nude
Background
Laryngeal carcinoma, a kind of malignancy with high prevalence, accounted for approximately 1–5% of all malignant tumors and 7–35% in eye, ear, nose, and throat (ENT) malignant tumors [1]. Although rapid progress has recently been made in treatment, the prognosis for laryngeal carcinoma patients remains unsatisfactory. Even though in clinical therapy radiochemotherapy can save the throat structure and function, it has limited efficacy when complications and resistance emerge after patients received lengthy treatment [2]. Therefore, understanding the mechanism underlying laryngeal carcinoma is essential for better management of laryngeal cancer patients. Furthermore, a tumor is considered a kind of stem cell disease, and the cancer stem cells (CSC) play a key role in tumor formation and growth [3–5].
CD133 is a 5-transmembrane glycoprotein with a molecular weight of 120 kDa. It is a kind of early antigen and it was identified as a marker of normal hematopoietic stem cells [6]. The expression of CD133 decreases dramatically as cells differentiate, and CD133 is selectively expressed in marrow, peripheral blood hematopoietic stem cells, and endothelial progenitor cells, but not in the mature endothelial cells [7]. Accumulating evidence shows that CD133 is a stem cell surface marker of many tumors. It can be an independent factor that plays a crucial role in separating and identifying stem cells and progenitor cells in some tumors [8–12].
In this study we focused on detecting the expression of CD133 in the Hep-2 cell line. The 3 subpopulations were separated by MACS technology according to the different surface antigen in Hep-2: CD133+ (CD133-positive cells), CD133− (CD133-negative cells), and unsorted cells, and their tumor formatting ability were compared. We detected the proliferation and differentiation ability of CD133 in vitro and the resistance for cisplatin (DDP) of laryngeal cancer stem cells to pinpoint the marker of laryngeal cancer stem cells and find a more effective laryngeal carcinoma targeted therapy.
Material and Methods
Reagent and instrument
Laryngeal cancer cell line Hep-2 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China); double-antibody RPMI1640 culture medium and fetal bovine serum (FBS) were obtained from Gibco™ (Invitrogen Co, Carlsbad, CA, USA); 0.25% trypsin was from TaKaRa (Dalian, China); immunomagnetic beads CD133, MTT, DMSO, and paraformaldehyde were purchased from Sigma (St. Louis, MO, USA); CD133 antibody was obtained from GeneTex (San Antonio, TX, USA); serum-free medium (SFM) is RPMI1640 culture medium with epidermal growth factor (EGF), basic fibroblast growth factor (bEGF), and insulin, which was also from Gibco™ (Invitrogen Co, Carlsbad, CA, USA).
Automatic CO2 constant temperature incubator, clean benches, inverted microscope, and fluorescence microscope purchased from Olympus (Japan), and ELIASA was purchased from Takara Shuzo (Otsu, Shiga, Japan).
Laboratory animal
The laboratory animals are 4–6-year-old healthy male nude mice (BALB/c-nu/nu) with weight of 18–20 gram, which were purchased from Shanghai Slac Laboratory Animal Centre. They were raised in a SPF laminar flow room with constant 40–50% humidity and at constant 22–25°C temperature. Sterilized feed and water were provided.
Culture of the Hep-2 cell line
Hep-2 cell line was obtained from the ENT Department of Shanghai Tongji Hospital. The cells were cultured in RPMI-1640 immediately after removal from patients. At first, we cleaned the surrounding dead and fatty tissue and rinsed the specimen in D-Hanks solution. We soaked the fresh tissue in another petri dish using double-antibody RPMI-1640, and then cut the specimen into 1-mm3 pieces. Then we moved the specimens to 0.25% trypsin and oscillated them for 30 min at 37°C. The cell suspension was filtered through 0.15-mm nylon mesh. The solution was centrifuged at 1000 rpm for 10 min, and then the sediment was suspended in D-Hanks solution. Cells were then suspended again in medium containing 50% calf bovine serum after centrifugation twice, as before.
Magnetic cell sorting
After primary culture, laryngeal cancer cells were made into 100-μl suspension with concentration of 106 cells per ml. The suspension was kept at room temperature for 30 min after we added 10 μl of CD133-FITC antibody, and then washed the cells 3 times. Afterwards, percentage of CD133+ in laryngeal cancer cells was detected by using a flow cytometer to confirm the purity of the sorted cells.
Cell proliferation detection
CD133+ and unsorted cells were plated at a density of 2000 cells adjusted to 100-μl culture solution per well in 96-well plates to cultured at 37°C. The absorbance of different laryngeal cancer cell subpopulations was detected using MTT after continuous inoculation for 7 days. Three duplications were set to detect the absorbance at 490-nm wavelength using a microplate reader to draw a cell growth curve of mean absorbance value and culture time.
Cell clonogenicity assays
Soft agar colony-formation assay was performed as previously reported [13]. Briefly, sorted CD133+ and CD133− cells in logarithmic phase were dissociated into single cells by incubation in 0.05% trypsin and then cells were seeded into semisolid agarose medium (RPMI-1640 medium containing 10% heat-inactivated FCS and agarose-base layer, 0.6%; upper layer, 0.3%) at a density of 1×104 cells per well in 60-mm plates. Several drops of fresh medium were placed directly onto the surface of the upper layer each week. After 2–3 weeks of incubation at 37°C and 5% CO2 atmosphere, foci were viewed and counted. Photographs were taken under a microscope for analysis of representative colonies.
Cell cycle analysis
CD133+ and unsorted cells inoculated to 6-well plates separately, and then cell cycle was detected by using a flow cytometer when cell concentration was 80%. The cells were suspended to 2×105/ml in preheated RPMI-1640 culture solution. Afterward, 2 ml of solution was centrifuged and cells were suspended again in PBS after washing twice in PBS. Then the cells were added into precooled 70% ethyl alcohol for 30 min, and dyed with 1 ml of DNA dye in the dark for 30 min after being washed in PBS. Cell cycle analysis was done using flow cytometry after the cells were filtered through 48-μM nylon meshes. Experiments were performed in triplicate.
In vivo tumorigenicity study
Ninety nude mice were used in this study. CD133+ cells and CD133− cells were injected subcutaneously into the left upper abdomen of nude mice at densities of 106 cells, 105 cells, 104 cells, and 103 cells per mouse, and 10 mice were injected at each density. Unsorted Hep-2 cells were injected into 10 nude mice at densities of 106 cells per mouse as control. Tumor formation of these mice was observed each week for 4 weeks. Four weeks later, 30 mice inoculated at 106 cells per mouse were sacrificed, and the presence of each tumor nodule, tumor weight, and tumor volume were confirmed.
Statistical analysis
Comparison among the 3 groups was made using software SPSS 11.0 using the chi square test. Statistical significance was defined as P<0.05. Data are shown as mean ±SD.
Results
Isolating CD133+ Cells from the Hep-2 Cell Line by MACS
To evaluate the efficiency of magnetic separation, harvested cells were tested by flow cytometry analysis. Before isolation, the CD133+ fraction was 40.12±1.32% (Figure 1) and was raised to 94.16±2.31% (Figure 2) after isolation. The results verified that CD133+ cells and CD133− cells were effectively isolated by this method.
Figure 1.
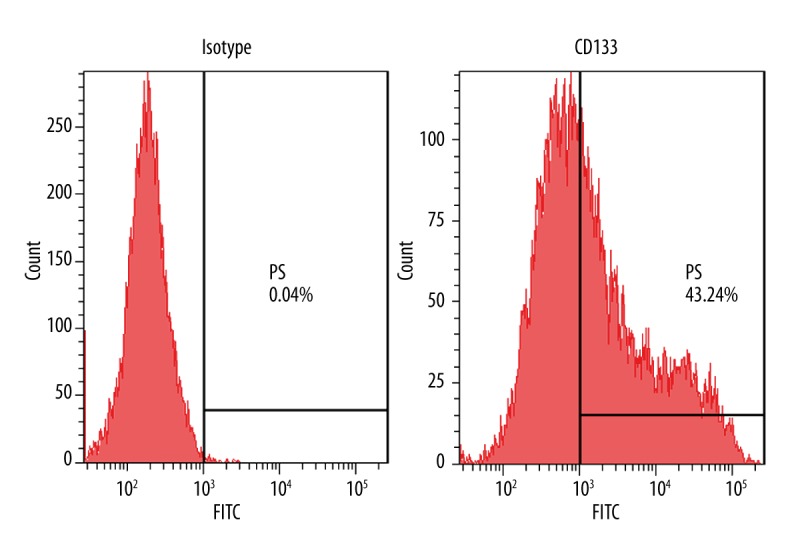
Expression of CD133 in Hep-2 cell lines. Flow cytometry analysis of CD133 in Hep-2cell lines. Data are representative of 3 independent experiments.
Figure 2.
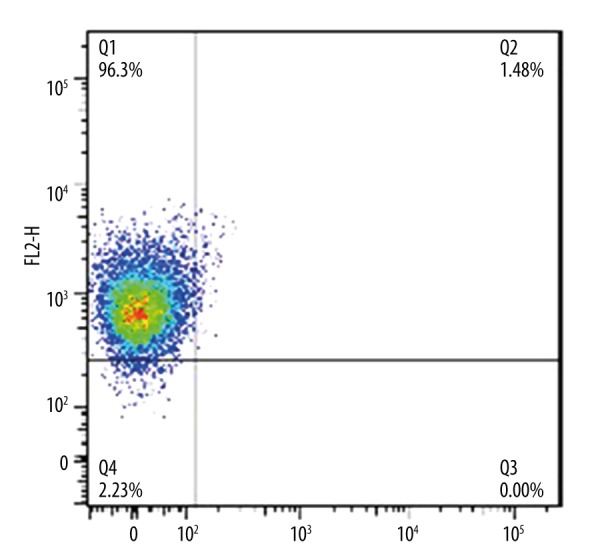
Hep-2 cells isolation situation. Purity of CD133 positive cells were confirmed after magnetic cell sorting assessed by flow cytometry. Data are representative of triplicate experiments.
In vitro proliferation of CD133+ cells and CD133− cells
The absorbance at 490 nm in 2 subpopulations of cells 1 day, 2 days, and 3 days after injection was detected by MTT assay. By comparing the proliferation of CD133+ cells and CD133− cells, we found that absorbance of CD133+ cells and CD133− cells showed a significant difference from the first day (P<0.05) (Figure 3). The results indicated that the cell proliferation ability in 2 populations showed significant difference at the first day after injection.
Figure 3.
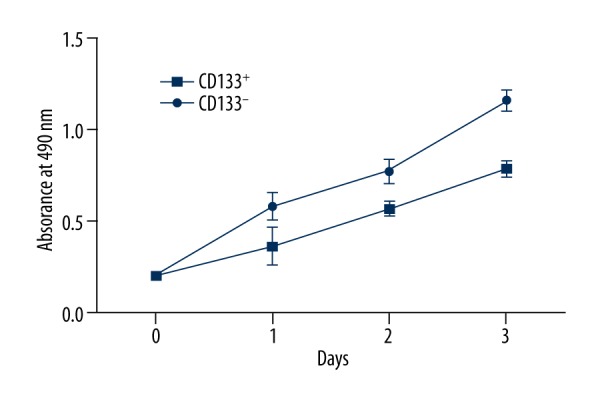
Growth curve of CD133+ cells and CD133− cells. Cell proliferation assay was performed by MTT analysis. Data are shown as mean ±SD and P<0.05 was considered as statistical significance.
In vitro cell colony forming capacity of CD133+ cells
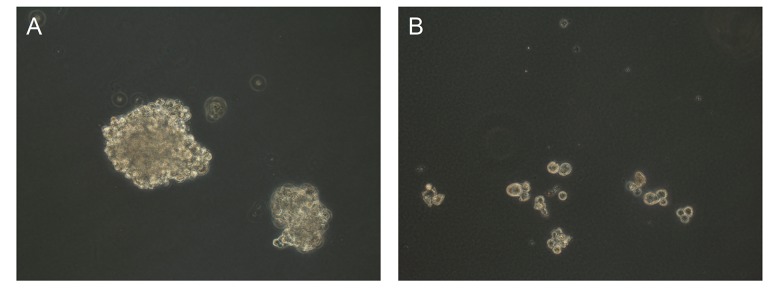
Colony-forming assay confirmed the high self-renewal ability of CD133+ cells; in comparison to CD133− cells, CD133+ cells were able to form more tumor colonies (Figure 4).
Figure 4.
Colony formation assay of sorted CD133+ cells and CD133− cells. Photographs are representative of triplicate experiments. (A) CD133+ cells; (B) CD133− cells.
Tumor-forming ability of CD133+ cells, CD133− cells and unsorted cells in vivo
Only 30% of CD133+ cells injected mice (3 of 10) formed tumors at a density of 103 cells per mouse. Five of 10 mice from the CD133+ cells group and 3 of 10 mice from the CD133− cells group formed tumors when 104 cells were injected into each mouse. All of the CD133+ cells and CD133− cells formed nodules at a density of ≥105 cells per mouse. Four weeks later, the presence of each tumor nodule, tumor weight, and tumor volume in mice injected with CD133+ cells were confirmed. As shown in Table 1, the tumor weight and tumor volume of the CD133+ group were greater than in the 2 other groups (P<0.05), but there was no significant difference within the CD133+ and the unsorted cells group (P>0.05).
Table 1.
Tumor weight, volume and forming time among three groups (mean ±SD).
| Groups | No. of nude mice | Tumor weight (g) | Tumor volume (mm3) | Tumor forming time (d) |
|---|---|---|---|---|
| CD133+ cells | 10 | 3.523±0.36 | 62.35±2.13 | 2–4 |
| CD133− cells | 10 | 2.961±0.29 | 53.47±2.51 | 4–6 |
| Unsorted cells | 10 | 3.024±0.31 | 55.26±2.85 | 4–6 |
Proliferation ability of CD133+ cells and CD133− cells under DDP treatment
MTT analysis was detected after 48 h of culturing in the presence of 5, 10, 15, and 20 μM DDP and the absorbance at 490 nm in 2 subpopulations of cells is shown in Figure 5. As a result, at the presence of DDP, CD133+ cells showed stronger proliferation ability than CD133− cells, indicating the cells in these 2 subpopulations are significantly different (P<0.05).
Figure 5.
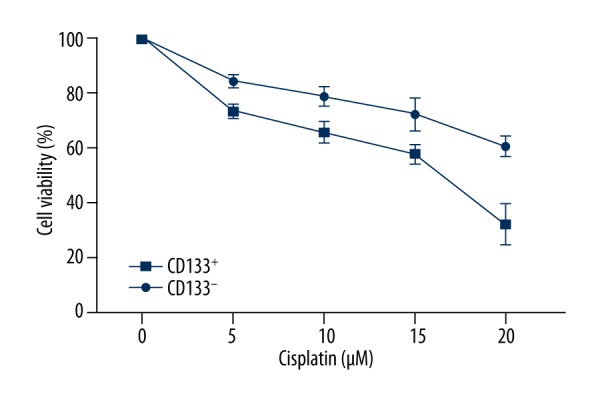
Sensitivity of CD133+ cells and CD133− cells to chemotherapy agents. MTT analysis is detected after 48 h of culture in the presence of 5, 10, 15, and 20 μM DDP. Data are expressed as mean ±SD and P<0.05 was considered as statistical significance.
Tumor forming ability of different subpopulations cells under DDP treatment
All of the CD133+ cells, CD133− cells, and unsorted cells formed nodules at a density of ≥105 cells per mouse and there was no change after DDP was injected. Four weeks later, all mice were sacrificed, and the presence of each tumor nodule, as well as the tumor weight and tumor volume, were confirmed (Table 2). The results show that the tumor weight and tumor volume of the CD133+ group were greater than in the 2 other groups (P<0.05).
Table 2.
Tumor weight, volume and forming time among three groups under DDP treatment (mean ±SD).
| Groups | No. of nude mice | Tumor weight (g) | Tumor volume (mm3) | Tumor forming time (d) |
|---|---|---|---|---|
| CD133+ cells | 10 | 2.963±0.31 | 54.61±0.28 | 2–4 |
| CD133− cells | 10 | 2.547±0.28 | 46.31±0.35 | 4–6 |
| Unsorted cells | 10 | 2.612±0.24 | 48.34±0.42 | 4–6 |
Discussion
The cancer stem cells (CSCs) hypothesis holds that tumors originate from a few cancer stem cells. In correlation studies started from hematological cancer, Florian et al. reported that acute myelogenous leukemia (AML) stem cells have the same phenotype as CD34+/CD38− [14]. Recent research indicates that the cells composing a tumor are hierarchically organized with respect to their potential to initiate and sustain tumor growth. Only rare subsets of tumor cells, defined as CSCs, have the capacity to form tumors [15]. Cells phenotypes as CD44+CD24−/low were confirmed as breast cancer stem cells [16]; cells that express the human neural stem cells marker CD133 were reported as brain tumor stem cels l [17,18]; and cells CD133+CD44+α2β1hi were proved as prostatic tumor stem cells [19]. Recent advances in stem cell research have facilitated the demonstration of the existence of CSCs in kidney cancer, liver cancer, lung cancer, pancreatic cancer, and various solid tumor cell lines [3]. The origin of CSCs is unclear, so researchers prefer to define CSCs by functions and characteristics. Generally speaking, the characteristic of CSCs are: 1) their capacity for self-renewal and multipotent differentiation; 2) not belonging to a terminally differentiated cell, in the state of undifferentiation or poorly differentiated, or keeping constant number and strong migration ability; 3) at the silent stage; 4) could prevent apoptosis; and 5) inherent resistance to drugs. Recent studies were focused on screening and identifying specificity marker of CSCs [20].
Studies on laryngeal cancer stem cells are at the initial phase relative to other cancer stem cells. Whether laryngeal cancer stem cells have heterogeneity and whether any cancer stem cells exist among the heterogeneous populations remain to be determined. We studied the heterogeneous mechanism of laryngeal cancer stem cells by combining the primary culture and single-cell culture in vitro. The primary culture can reflect the biological characteristics in vivo because the cells, tissue, and organs were cultured directly and tumor cells were cultured in vitro before the first subculture. The tumor forming ability is the most important factor in CSC identification, so we chose the Hep-2 cell lines with stem cells characteristics and strong tumor forming ability as the study object. In combined recent studies on solid cancer stem cells, we investigated the expression of CD133 in Hep-2 cell line, the proliferation ability and tumor formation ability of CD133+ cells, and explored the possibility that CD133 acts as the marker of laryngeal cancer stem cells by observing the biological characteristics of tumor cells under DDP treatment. Our results indicated that CD133+ cells showed stronger tumor forming ability, shorter tumor forming time, and faster tumor growth than CD133− cells and unsorted cells.
Drug resistance is one of the characteristics of CSCs, and it has been reported several times that CSC is the key reason for limited effect of chemotherapy [2]. Drug-resistance molecules such as Brcp1/ABCG2, MRP1/2 are normally expressed to different degrees in lung, digestive tract, liver, and kidney, and these molecular play key roles in maintaining organism physiologic barriers such as the blood-brain barrier, blood-testis barrier, maternal fetal barrier, and blood cerebrospinal fluid barrier [21]. ABC overexpression transporters on tumor stem cell membranes can effectively attenuate the inhibition effect and lethal effect of antitumor drugs due to its ability to discharge metabolite, toxic substance, nucleotide, sterols analogues, and drugs. Our study revealed that CD133+ cells possess stronger proliferation ability than CD133− cells under DDP treatment, which indicates that the lethal effect of antitumor drugs on laryngeal cancer stem cells were weakened significantly.
Conclusions
In conclusion, we found CD133 could be a marker useful in characterizing laryngeal cancer stem cells because of the CSC characteristic of CD133+ cells. Moreover, CD133− cells also possess proliferation ability, which indicates that there are other undiscovered stem cell markers in the Hep-2 cell line, and more research is needed to find them for use in further combinations of those various markers in laryngeal cancer cells.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest concerning this article.
Source of support: This work was funded by Shanghai Science and Technology Commission, China. Grant Number: 12ZR1428500
Reference
- 1.Shao Y, Chen Y, Li Q. [The research progress of laryngeal cancer recurrence transfer factors]. World Health Digest. 2013;31:14–15. [in Chinese] [Google Scholar]
- 2.Gorenc M, Kozjek NR, Strojan P. Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Rep Pract Oncol Radiother. 2015;20(4):249–58. doi: 10.1016/j.rpor.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao L, Fan X, Jing W, et al. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-kappaB-HIF-1alpha pathway. Oncotarget. 2015;6:6627–40. doi: 10.18632/oncotarget.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–38. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung Y, Kim WY. Cancer stem cell targeting: are we there yet? Arch Pharm Res. 2015;38:414–22. doi: 10.1007/s12272-015-0570-2. [DOI] [PubMed] [Google Scholar]
- 6.Arndt K, Grinenko T, Mende N. CD133 is a modifier of hematopoietic progenitor frequencies but is dispensable for the maintenance of mouse hematopoietic stem cells. Proc Natl Acad Sci USA. 2013;110:5582–87. doi: 10.1073/pnas.1215438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Zhang F, Feng B, et al. Hematopoietic stem cell capture and directional differentiation into vascular endothelial cells for metal stent-coated chitosan/hyaluronic acid loading CD133 antibody. Tissue Eng Part A. 2015;21:1173–83. doi: 10.1089/ten.tea.2014.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jing F, Kim HJ, Kim CH, et al. Colon cancer stem cell markers CD44 and CD133 in patients with colorectal cancer and synchronous hepatic metastases. Int J Oncol. 2015;46:1582–88. doi: 10.3892/ijo.2015.2844. [DOI] [PubMed] [Google Scholar]
- 9.Sahlberg SH, Spiegelberg D, Glimelius B, et al. Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PLoS One. 2014;9:e94621. doi: 10.1371/journal.pone.0094621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Wu L, Shen XM, et al. Expression patterns of cancer stem cell markers ALDH1 and CD133 correlate with a high risk of malignant transformation of oral leukoplakia. Int J Cancer. 2013;132:868–74. doi: 10.1002/ijc.27720. [DOI] [PubMed] [Google Scholar]
- 11.Liu TJ, Sun BC, Zhao XL, et al. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013;32:544–53. doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- 12.Al-Zoubi A, Jafar E, Jamous M, et al. Transplantation of purified autologous leukapheresis-derived CD34+ and CD133+ stem cells for patients with chronic spinal cord injuries: long-term evaluation of safety and efficacy. Cell Transplant. 2014;23:S25–34. doi: 10.3727/096368914X684899. [DOI] [PubMed] [Google Scholar]
- 13.Li YJ, Zhang XP, Chen ZC, et al. LCRG1 suppresses tumor growth in vivo by liposome-mediated gene transfer. Chinese Journal of Cancer Research. 2002;14:113–17. [Google Scholar]
- 14.Florian S, Sonneck K, Hauswirth AW, et al. Detection of molecular targets on the surface of CD34+/CD38– stem cells in various myeloid malignancies. Leuk Lymphoma. 2006;47:207–22. doi: 10.1080/10428190500272507. [DOI] [PubMed] [Google Scholar]
- 15.Pathania R, Ramachandran S, Elangovan S, et al. DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis. Nat Commun. 2015;6:6910. doi: 10.1038/ncomms7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright MH, Calcagno AM, Salcido CD, et al. Brca1 breast tumors contain distinct CD44+/CD24– and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfenninger CV, Roschupkina T, Hertwig F, et al. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67:5727–36. doi: 10.1158/0008-5472.CAN-07-0183. [DOI] [PubMed] [Google Scholar]
- 18.Haus DL, Nguyen HX, Gold EM, et al. CD133-enriched Xeno-Free human embryonic-derived neural stem cells expand rapidly in culture and do not form teratomas in immunodeficient mice. Stem Cell Res. 2014;13:214–26. doi: 10.1016/j.scr.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miki J, Furusato B, Li H, et al. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153–61. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- 20.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–78. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez JP, Wang-Rodriguez J, Chang C, et al. Gefitinib inhibition of drug resistance to doxorubicin by inactivating ABCG2 in thyroid cancer cell lines. Arch Otolaryngol Head Neck Surg. 2007;133:1022–27. doi: 10.1001/archotol.133.10.1022. [DOI] [PubMed] [Google Scholar]