Abstract
Non-enzymatic glycosylation (glycation) plays an important role in the development of physiological and pathophysiological processes such as aging, diabetes, atherosclerosis, neurodegenerative diseases and chronic renal failure. Preventing glycation can minimize diabetic complications. Glycation can be prevented by the natural defence system in the body, synthetic inhibitors and natural inhibitors. Synthetic inhibitors may prevent glycation through several possible mechanisms. They might inhibit the glycation by interfering with the attachment of sugars with proteins, by inhibiting the late stage of glycation or by preventing Amadori product formation. Furthermore, their ability to scavenge free radicals and to break cross-links might be other mechanisms responsible for their potential to inhibit glycation. Naturally occurring phytochemicals/products have been found to be relatively non-toxic as compared to synthetic compounds, and are inexpensive and available in an ingestible form. A large number of plants and natural biomolecules have been shown to have antidiabetic effects. Several hypoglycaemic compounds have anti-oxidant properties. The present review describes the various ways in which glycation can be prevented.
Keywords: Glycation, diabetic complications, natural defence system, synthetic inhibitors, natural inhibitors
Introduction
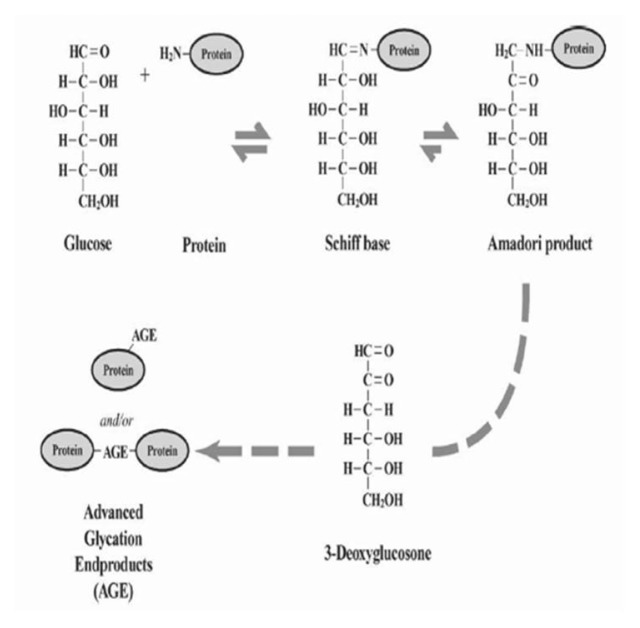
Glycation or Maillard reaction is a spontaneous, naturally occurring, non-enzymatic and complex network of reactions which is initiated by reaction of carbonyl group of a reducing sugar (e.g., glucose, galactose, fructose, mannose or ribose) with a free amino group, typically the ɛ-amino group of lysine residues and the α-amino group at the N-terminus of a protein to form an adduct commonly referred to as the Schiff base (1) (Fig. 1). (2)
Figure-1. Glycation of proteins.
The initial reaction between glucose and protein amino groups forms a reversible Schiff base that rearranges to a ketoamine or Amadori product. With time, these Amadori products form AGEs via dicarbonyl intermediates. (2)
The Schiff bases undergo Amadori rearrangement and through a series of further rearrangement, cyclizations etc. form a variety of diverse compounds collectively described as advanced glycation end products (AGEs). (3, 4) AGE formation is accompanied by the formation, among others of a number of reactive oxygen species (ROS), α-oxoaldehydes, that further react and damage the proteins and other important biological molecules
The formation of AGEs is increased under diabetic conditions. (5) AGE formation is highly increased in patients with diabetes mellitus type 2 as compared to type 1 diabetic patients. (6) Glycation is one of the major pathways for the formation of AGEs. The highly reactive carbonyl species, including glyoxal, methylglyoxal and 3-deoxyglucosone are formed in all the steps of the glycation process, and also during the process of lipid peroxidation, glucose autoxidation and the polyol pathway in diabetes mellitus (type 2). (7, 8) Glyoxal than further leads to production many AGEs that include Nɛ-(carboxymethyl) lysine (CML), (9) glyoxal-derived lysyl dimer (GOLD), (10) Nω-(carboxymethyl) arginine (CMA) (11) or S-carboxymethylcysteine. (12) And methylglyoxal leads to the formation of Nɛ-(carboxyethyl) lysine (CEL), (13) methylglyoxal-derived lysyl dimer (MOLD), (14) argpyrimidine, (15) methylglyoxal-derived hydroimidazolone MG-H1, (16) etc, whereas 3-deoxyglucosone leads to the formation of pyrraline, (17) pentosidine, (18) imidazolone or also CML. (19) Glucose-derived dicarbonyl precursors are the chief sources of formation and hence, intracellular accumulation of AGEs. (20, 21)
AGEs accumulation has been found to be implicated in several chronic diseases including typical diabetic complications, atheroscelerosis, Alzhimer’s disease, rheumatoid arthritis and chronic heart failure. (22, 23) Interaction of AGEs with their receptors (RAGEs) causes the oxidative stress and initiation of inflammation cascade. The inflammation cascade involves the activation of MAPK pathway, NF-Kb, IL-6, TNF-α, expression of ICAM-1 and VCM-2. All of these are collectively responsible for diabetic complications. (24) Increased glycation and build up of tissues AGEs can alter enzymatic activity, decrease ligand binding, modify protein half-life and alter immunogenicity, so they have been considered to be involved in pathogenesis of diabetic conditions. Glycation has been shown to play an important role in the development of physiological and pathophysiological processes such as aging, diabetes, atherosclerosis, neurodegenerative diseases and chronic renal failure. (25)
Diabetes is one of the most common serious metabolic diseases worldwide. All kinds of diabetes are characterized by hyperglycaemia, lipidaemia, oxidative stress and long-term complications affecting the eyes, nerves, blood vessels, skin, and kidneys. Hyperglycaemia is considered to be the driving factor of glycation and gradual build-up of AGEs in body tissues. Although, intracellular AGEs may be involved in the activation of intracellular signalling pathways and modification of the functions of intracellular proteins, there are several evidences that accumulation of AGEs is an important causative factor for the pathogenesis of diabetes, cataracts, atherosclerosis, diabetic nephropathy, and neurodegenerative diseases. (2, 26–30) Furthermore, enhanced production of free radicals leads to oxidative stress during diabetic conditions.
Inhibition of glycation and prevention of diabetes
In experimental diabetic animal models, blocking of the Maillard reaction has shown to be beneficial against diabetes. Conversely, several antidiabetic agents have been found to reduce the formation of AGEs. Glycation and accumulation of tissue AGEs have a great role in diabetes and its pathology. Thus, inhibiting any step of glycation and preventing the formation of intermediate and end products, scavenging of free radicals, detoxification of liver enzymes, etc., can help in the prevention of diabetic complications. The antidiabetic potential of a possible drug can be considered to be related with inhibition of glycation.
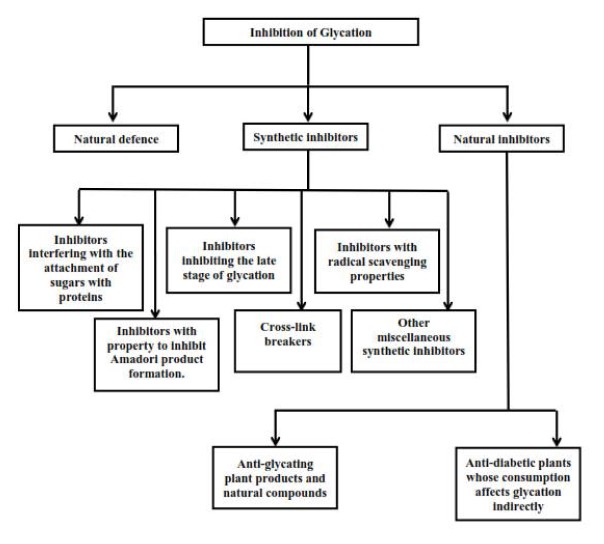
Controlling the blood sugar level is a very effective and natural method to inhibit glycation in diabetes, and inhibition of protein glycation is a complex process that could occur at any step in the formation of AGEs. Hence, several mechanisms can be considered to deal with and prevent AGE formation in the body. And, this protection is supported by detoxifying liver enzymes, as well as plasma amines and antioxidants. Basically, glycation can be prevented by natural defence system present inside the body or by inhibitors that may be synthetic or natural (Fig. 2).
Figure-2.
Inhibition of glycation: Possible strategies.
Natural defence
Several researchers have proposed the concept of an enzymatic defence against glycation that offers protection against cell damage mediated by glycation. This concept is based on the enzymatic activities that involves the prevention/suppression of glycation adducts formation and catalysis of glycated protein repairing. Glyoxalase system (both I and II), aldehyde reductase, aldose reductase and the liver enzyme, α-ketogluteraldehyde dehydrogenase are considered to be included in these natural enzymatic defence mechanisms against glycation and AGEs accumulation. (31, 32)
Amadoriosis, an exclusive enzyme found in Aspergillus, catalyzes the deglycation of Amadori products. (33) Human fructosamine-3-kinase is able to reverse the non-enzymatic glycation at an early stage. (34) Reactive dicarbonyl compounds are detoxified by oxoaldehyde dehydrogenase and aldehyde reductase that are NADPH dependent. (35, 36) During the reduction of peroxide or superoxide, gluthaothione (GSH) system work both as antioxidant and coenzyme. Furthermore, GSH has an important role to facilitate the detoxification of methyglyoxal (MG) in glyoxylase pathway. (32) Numerous plasma amines are present in body that may react with sugar and Amadori carbonyl groups to decrease AGEs.
Antiglycating compounds
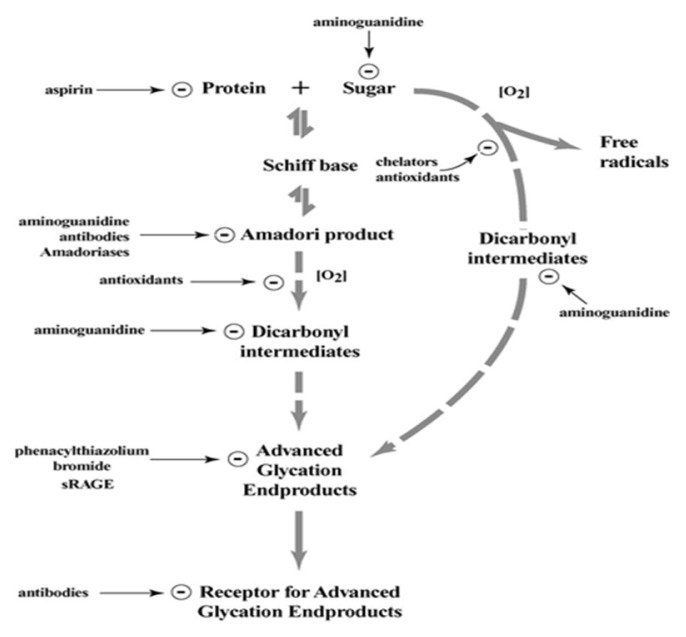
Several types of antiglycating agents have been described. (37) These can interfere with different potential sites to inhibit glycation and AGE formation (Fig. 3). (38) Some may have the ability to compete for the amino groups on the protein. (38, 39) Others can directly bind to the protein or to the glycation intermediates to stop the progression up to the AGE formation stage. (38) Otherwise, they may have the property to eliminate the open chain form of glycating sugars. (38) Furthermore, several probable AGE inhibitors have been proposed. (40, 41) Various inhibitors have been developed and some of them are in advanced clinical studies/trials. (42, 43) The concept of inhibitory mechanism is primarily concentrated on blocking of the sugar attachment to proteins, attenuating glycooxidation and oxidative stress through trapping or scavenging some glycation intermediates including ROS, reactive nitrogen species (RNS) and dicarbonyls, as well as breakage of AGEs crosslinks. (5) Another means of inhibition or protection from AGEs can include creating antibodies for Amadori products, chelation of transition metals, deglycation enzymes, dissolution of crosslinking or blocking 17 of RAGEs. Primarily, inhibitors can be divided into two groups: synthetic and natural inhibitors.
Figure-3.
Potential sites where pharmacological compounds can act to inhibit protein glycation and AGE-mediated damage. (2)
Synthetic inhibitors
(1) Inhibitors interfering with sugar attachment with proteins
It has been found that only a few of the synthetic inhibitors have the property to interfere with the initial attachment of reducing sugars to the amino groups of proteins. For example, aspirin inhibits the glycation process by acetylating free amino groups of proteins, therefore it can block the attachment of reducing sugars with amino groups. (44, 45) An anti-inflammatory drug, diclofenac can make a covalent interaction with proteins and thus, can block the attachment of sugars with proteins. It has been shown to block at least one of the major glycation sites of human serum albumin. (46) Inositol is a synthetic and potent antiglycating agent because glucose can be scavenged out by it, and it was found that the glycation process in the human eye lens protein decreased by 57–67% in the presence of inositol. (47) Arginine and arginine-lysine can prevent the alterations of rat tail tendon that are induced by glycation because of competitive attachment of these amino acids to glucose. (48) Metformin is a well-known blood sugar lowering agent and has been reported to have moderate inhibitory effects on early stage of glycation. (40) Pioglitazone and pentoxifylline are other synthetic drugs that exert the same inhibitory effect on the early stage of glycation. (41)
(2) Inhibitors inhibiting the late stage of glycation
Some synthetic inhibitors have the ability to scavenge both reactive carbonyls and reactive free radicals formed during glycation or can block the formation of Amadori products. Aminoguanidine (AG) and pyridoxamine are potent carbonyl and free radical scavengers, and have been widely studied to investigate their AGE inhibiting property. AG was the first AGE inhibitor to be studied both in vitro and in vivo. (49) AG has a significant potential to react with dicarbonyl intermediates formed during glycation. (49, 50) Thus, AG helps in preventing diabetic complications. (51) AG is a potent AGE inhibitor and also prevents diabetic complications that include nephropathy, neuropathy and vasculopathy. (51) Pyridoxamine and thiamine pyrophosphate are potential dicarbonyl scavengers and have a strong inhibitory effect on AGE formation. Pyridoxamine offers more protection against AGE formation (52–54) as compared to AG (55) by trapping dicarbonyl compounds. (53, 56) Thiamine pyrophosphate has also comparable inhibitory effect on AGEs formation. (57) Buformin (58) and carnosine (59, 60) can prevent in vitro protein glycation and cross-linking.
(3) Inhibitors with radical scavenging properties
Some compounds have been reported to retard or suppress AGE formation because of their possible radical scavenging properties. Calcium antagonists (61), amlodipine (62), kinetin (63) and quinine (64) are good examples.
(4) Inhibitors with property to inhibit Amadori product formation
Some antiglycating compounds have the ability to inhibit the formation of Amadori products. Tenilsetam is able to attach with sugar-derived moieties of glycated proteins. (65) Thus, reactive sites are blocked and this stops the further polymerization reactions. Some researchers have also reported the inhibition of formation of Amadori products and reduction in the level of AGEs by pencillamine. (66) Ethanol can be metabolized into acetaldehyde in vivo which forms a stable complex with Amadori products, thus ethanol may exert inhibitory effect on AGE formation. (67)
(5) Cross-link breakers
Cross-linking between AGEs and proteins is the reason behind the stiffening of arteries and cardiovascular damages. (68) Hence, breakage of AGE cross-links is a very good method to prevent diabetic complications caused by cross-linking. The concept of AGE breakers suggests that they can release albumin from preformed AGE-albumin-collagen complexes and can be able to dissociate the immunoglobulin adducts from red cells of diabetic rats. (69) Another view implicates the involvement of AGE breakers in the prevention of cross-linking and/or reversing of the cross-links once they are formed. (70) One more way by which AGE breakers can work is metal chelation. (71) N-phenacylthiazolium bromide was the first cross-link breaker reported. (72) Alagebrium (ALT-711) is a small synthetic compound and is able to decrease cardiovascular stiffening (73) and (74) in diabetic rats. Furthermore, TRC4186 is a pyridinium analog that is able to break AGE cross-links. (75)
(6) Other miscellaneous synthetic inhibitors
Some anti-inflammatory drugs including acetylsalicylic acid, ibuprofen, indomethacin and diclofenac act as antiglycating agents because they inhibit oxidative stress. (76) Specific iron chelators like desferoxamine was also helpful in treating diabetes. (77) Aldose reductase inhibitors (ARIs) can block excessive glucose metabolism. Eplarestat is an ARI and it brings about the lowering of the level of fructose-3-phosphate in diabetic patients (78) and also lowers down imidazolone and carboxymethyl lysine (AGEs) due to decreased peroxidation of lipids. (79) Angiotensin II Receptor Blocker and Angiotensin converting Enzyme Inhibitors (ACEI) also have inhibitory effects on AGEs. The possible mechanisms of action may include chelating of metal ions, scavenging of free radicals, trapping of carbonyl compounds and/or inhibition of carbonyl compound production. (80)
Natural inhibitors
Although synthetic compounds are strong antiglycating agents or strong inhibitors of AGE formation, they might exert severe adverse effects. For example, the clinical trials of AG were terminated because of safety concerns because AG was found to have many adverse effects like gastrointestinal disturbances, rare vasculitis, anaemia and flu-like symptoms. (81) Its interference with vitamin B6 metabolism is another major limitation. (80) Metformin, another synthetic antiglycating compound exerts several adverse effects including nausea and diarrhoea. (82) Therefore, recently much interest has been developed in the search of natural phytochemicals from plants that effectively inhibit glycation and have fewer side effects. (83) Naturally occurring phytochemicals/products have been found to be relatively safe for human consumption as compared to synthetic compounds and are relatively non-toxic, inexpensive and are available in an ingestible form. A large number of plants and natural biomolecules have been discussed in literature for their antidiabetic effects. (83–85) Some plant extracts have been tested for antiglycating activities. (86) However, the mechanism is often not completely understood.
It is well established that glycation and AGEs formation are accompanied and accelerated by oxidative stress, therefore antioxidant compounds may be promising agents for the prevention of glycation and AGE formation. Polyphenolic compounds especially flavonoids have received most attention with regard to their antidiabetic properties. (85) Anthocyanins are flavonoids with high antioxidant capacity.
Anti-diabetic plants and their role in the prevention of diabetes
Plants are considered to be an excellent source of drugs for the treatment of various diseases and pathological conditions and several currently available drugs are obtained directly or indirectly from them. Many plants have been used by the local Indian tribes for antidiabetic therapeutics since a long time, but only a few of them have been scientifically studied e.g. Flemingia macrophylla, Potentilla fulgens L., Albizzia lebbek, Curcuma amada, Gymnoptela cochinchinesis and Ixeris gracilis DC. (87)
Aqueous/ethanolic extracts of Allium cepa (skin), Illicium religiosum (bark and wood), Fagopyrum esculentum (hull) and Origanum officinalis (leaf) have been shown to have effective antiglycating and antioxidant properties. The results also showed that their antiglycating activities significantly correspond to their antioxidative capacities. (88) Ethyl ether extract of dried Allium cepa powder has been shown to have anti-hyperglycaemic effect in alloxanized diabetic rabbits. (89) Oral administration of ethyl-ether extract of garlic, Allium sativum causes significant reduction in blood sugar level in alloxan treated diabetic rabbits. (90) One major component of garlic extract, allicin is a sulfur-containing compound and has been shown to have significant hypoglycemic activity. (91) The age-related increase in collagen cross-linking and fluorescent products in C57BL/6 mice can be decreased by green tea extract. (92) Aloe vera also has antidiabetic and lipid-lowering properties, since oral administration of its extract significantly reduced fasting blood glucose level and improved lipid profile status in streptozotocin-induced diabetic rats. (93) The methanol extract of Salacia chinensis stems provides strong inhibition against the formation of Amadori compounds and AGEs in addition to its anti-hyperglycemic action. (94) The seeds of Acacia arabica induced hypoglycemic effect in mice by initiating the release of insulin from pancreatic beta cells. (94) The aqueous extract of Aegle marmelos leaves reduces blood sugar, urea and serum cholesterol levels in diabetic rats as compared to the control. (95)
Azadirachta indica is widely distributed throughout India. The leaf extracts of this plant have been shown to have anti-hyperglycemic effect in diabetic rats. (96) Beta vulgarosides isolated from roots of Beta vulgaris, have been proved to enhance glucose tolerance in OGTT conducted in rats. (97) Ocimum santalum is considered to be a sacred plant in Indian culture. Its aqueous extract significantly reduces the blood sugar level in diabetic rats. (98) The aqueous extract of Mangifera indica also proved to have hypoglycemic activity which may be due to the reduction of the intestinal absorption of glucose. (99) Momordica charanta is considered to be very popular antidiabetic and antihyperglycemic vegetable in India as well as in other Asian countries. The hypoglycemic effect of extracts of its fruit pulp, seed, leaves and whole plant have been proved in various animal models. (100) Phyllanthus amarus is a herb and is used by the local people of south India in treating diabetes. Potent antioxidant activity has been found in the methanolic extract of P. amarus. In diabetic rats, its extract reduced the blood sugar level. (101) In addition, several studies have reported the AGE formation and crosslinking inhibiting potential of curcumin in diabetic rats. (40)
Brassica juncea is one of the most commonly used spices in India. Oral feeding of its diet, has been reported to induce hypoglycaemia, stimulation of glycogen synthetase, suppression of glycogen phosphatase and other gluconeogenic enzymes, (102) and it has been found to have antioxidant and hypolipidemic activity. (103–105) Fruit powder of Capparis decidua was orally given to alloxanized diabetic rats and caused significant hypoglycaemia. (106) In addition, its antioxidant and hypolipidemic activities also have been reported. (106–108) Citrullus colocynthis is an annual herb and is widely cultivated throughout India. Aqueous extract of its fruit has been shown to increase insulin secretion in a dose dependent manner. (109) Dried leaf powder of Gymnema sylvestris induces anti-hyperglycaemic effects, decrease in the activity of gluconeogenic enzymes and reversal of pathological effects during hyperglycaemic phase in alloxan treated diabetic mice. (110) Oral administration of aqueous extract of its leaves has been found to induce the regeneration of β cells in streptozotocin treated diabetic rats. (111)
Plants and natural products with antiglycating potential
Numerous medicinal herbs and dietary plants have been reported to possess antiglycating potential of similar (112) or even of higher order (113, 114) than that of AG. Several evidences have demonstrated that their antiglycating potential is correlated significantly with the total phenolics present in their extracts. (115–119) Methanol extracts of whole plants of Calendula officinalis and fruits of Juglans regia have shown antiglycating activity with respect to bovine serum albumin (BSA) in vitro, and their antiglycating potential is comparable to that of AG on the weight concentration basis. (120) During in vitro conditions, ethyl acetate extracts of Erigeron annuus inhibited glycation of BSA, prevented opacification of lenses and inhibited aldose reductase. (114) Extract of Empetrum nigrum L. inhibits the glycation in vitro and its antiglycating activity is correlated with the radical scavenging activity. (121) Maltol showed a significant in vitro AGE inhibiting activity as compared with AG. (113)
Polyphenolic compounds are natural phytochemicals and are common constituents of plant based foods including fruits, vegetables, cereals, seeds, nuts, chocolate, and beverages, such as coffee, tea, and wine. Their consumption is associated with many health benefits, such as prevention of cancer, (122) neurodegenerative diseases (123), cardiovascular diseases (124) and diabetes. (125) Polyphenols are classified on source of origin, biological functions, and chemical structures.
Chlorogenic acids, present in Chrysanthemum species, are free radical and metal scavengers and may interfere with glucose absorption and alter gene expression of antioxidant enzymes. (126) Cinnamic acid derivatives such as ferulic acid (3-methoxy-4-hydroxycinnamic acid) also have shown AGEs inhibiting activity. (127–129) Ellagic acid prevents the glycation-mediated β-sheet formation in hemoglobin and lysozyme that shows its antiglycating ability. (130) Quercetin is a flavonoid and belongs to the subclass flavonols. It is widely distributed in many plants: flowers, leaves, and fruits. Quercetin possesses strong anti-diabetic activity. (131) It has the ability to trap MG and glyoxal and thus, can inhibit AGEs formation. (132) Quercetin offers protection against lipid peroxidation and also has antioxidant effect in diabetes. (133) It is a strong antioxidant. (134) Thymoquinone, an active principle component of the volatile oil of Nigella sativa seeds possess several pharmacological activities including anti-diabetic effects. (135) Research in my laboratory has recently shown that thymoquinone also has good antiglycating activity. (136, 137)
Possible mechanisms of inhibition of glycation by antidiabetic plants
The complexity of Maillard reaction is the major obstacle in identifying the mechanisms of inhibition of glycation by natural products and molecules. AGEs are considered to be major pathogenic culprits for diabetes and its complication. Several mechanisms have been proposed for the inhibition of glycation by plant products and natural compounds, which target essential stages of glycation as well as their free radical scavenging activity (Fig. 4).
Figure-4.
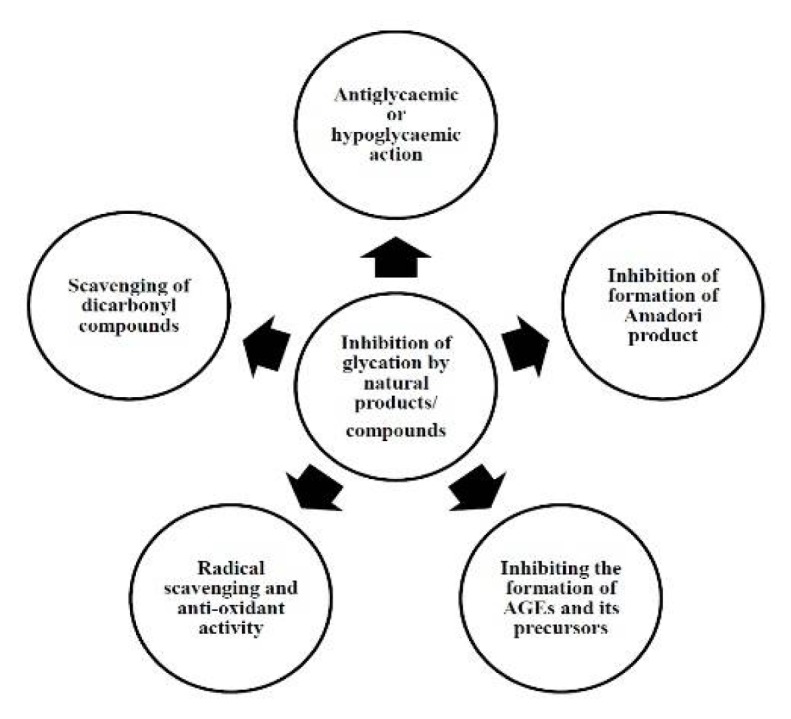
Possible mechanisms for the inhibition of glycation by natural products/compounds.
These mechanisms can correlate antiglycating activity with antidiabetic potential of plants and their products/compounds. The mechanisms include: antiglycaemic or hypoglycaemic actions of plant products and their compounds e.g. Albizia odoratissima, (138) Allium cepa; (89) inhibition of Amadori products formation e.g., Salacia chinensis, (94) etc.; inhibition of the formation of AGEs and its precursors e.g. Ilex paraguariensis (119), etc.; reduction of crosslinking e.g. green tea extract; (92) radical scavenging and anti-oxidant activity e.g. extracts of wild berries, (121) Aegle marmelos, (139) olive leaves, (140) etc.; scavenging of dicarbonyl compounds e.g. catechin and epicatechin procyanidin B2 isolated from cinnamon bark extract, (118) etc.
Current limitations of natural and synthetic inhibitors
Synthetic drugs are often associated with some limitations such as high cost, development of hypoglycaemia, gastrointestinal disturbances, liver toxicity, weakness, fatigue, shortness of breath, nausea, dizziness, lactic acidosis, and kidney toxicity etc. (141, 142) Some renal patients are not allowed to take some specific type of synthetic drugs. Sometimes, flatulence is also found to be associated with intake of synthetic drugs. Current limitations of natural inhibitors include lack of dose dependent standardization of inhibitors for their efficacy and safety, (143) poor bioavailability and poor aqueous solubility etc.
Future perspectives
Based on in vitro studies and animal models, several plant products/compounds have been proposed to be effective supplements against glycation and hence, in management and prevention of its long term complications of diabetes mellitus. However, it is very necessary to investigate their clinical effects in human beings, appropriate physiological concentration of inhibitors as well as their mode of action to confirm their beneficial effects as supplementary treatments for diabetic patients. Bioavailability of polyphenols is influenced by several factors including bioaccessibilty, molecular structures, transporters, metabolizing enzymes, etc. Therefore, it is very necessary to develop new techniques such as nanotechnology and homogenization that can enhance bioavailability of natural inhibitors including polyphenols. Nanoparticles and nanoencapsulation based albumin and polyphenols been developed. (144, 145) In future it is very likely that there will be more investigations regarding the long term and acute hypoglycaemic, antiglycating and hypolipidemic effects of these homogenization, nanoparticles and nanoparticle encapsulation on type 2 diabetes.
Conclusion
Glycation is considered to be the main molecular basis of several diabetic complications. The antidiabetic potential of a possible drug can be considered to be related with inhibition of glycation. Current antidiabetic therapy is based on synthetic drugs that very often have side effects. Alternative medicines and natural therapies have stimulated new interest of research to find for more efficacious agents with lesser side effects. Plants are excellent sources of drugs and many of the modern drugs have been obtained directly or indirectly from them. Consumption of plant based foods including fruits, vegetables, etc., is associated with many health benefits, such as prevention of cancer, (122) neurodegenerative diseases, (123) cardiovascular diseases (124) and diabetes. (125) A wide range of plant extracts have been used worldwide to prevent diabetes. Some plant products/compounds can inhibit glycation and hence can prevent diabetic complications. Furthermore, naturally occurring phytochemicals with anti-diabetic and antiglycating activities are relatively nontoxic, inexpensive and available in an ingestable form. The details of the inhibitors of glycation have been discussed in this review. A summary of the antidiabetic effects of some of the synthetic and natural inhibitors is shown in Table 1 and Table 2, respectively.
Table 1.
Synthetic inhibitors with antidiabetic effects.
| S. N. | Synthetic Inhibitor | Effect | Mode of Action | Reference |
|---|---|---|---|---|
| 1 | Aspirin, Diclofenic | Antiglycating | Blockage of attachment of reducing sugar with protein | 44, 45, 46 |
| 2 | Inositol | Antiglycating | Glucose can be scavenged | 47 |
| 3 | Arginine, arginine-lysine | Antiglycating | Competitive attachment of these amino acids to glucose | 48 |
| 4 | Metformin | Inhibition of early stage of glycation | Blood sugar lowering | 40 |
| 5 | Pioglitazone, pentoxifylline | Antiglycating | Blood sugar lowering | 41 |
| 6 | Aminoguanidine, pyridoxamine | Blockage of Amadori product formation | Potent carbonyl and free radical scavengers | 49 |
| 7 | Pyridoxamine, thiamine pyrophosphate | Inhibition of AGEs formation | Dicarbonyl scavengers | 52, 53, 54, 57 |
| 8 | Buformin, carnosine | Prevents in vitro protein glycation and cross-linking | Trapping of carbonyl compounds, antioxidants | 58, 59, 60 |
| 9 | Calcium antagonists, amlodipine, kinetin, quinine | Retardation of AGEs formation | Radical scavenging properties | 61, 62, 63, 64 |
| 10 | Tenilsetam | Inhibition of Amadori product formation | Attach with sugar-derived moieties of glycated proteins | 65 |
| 11 | Pencillamine | Inhibition of Amadori product AGEs formation | Reacts with Amadori-derived fragmentation products | 66 |
| 12 | N-phenacylthiazolium bromide, Alagebrium | Cross-link breaker | Reacts with and cleaves covalent, AGE-derived protein cross-links | 72, 73, 74 |
| 13 | TRC4186 | Cross-link breaker | Potent free radical scavenging activity, reduction in accumulation of AGEs | 75, 146 |
Table 2.
Natural inhibitors with antidiabetic effects.
| S.N. | Natural inhibitor | Plant source | Effect | Mode of action | Reference |
|---|---|---|---|---|---|
| 1 | Bark and wood extract | Illicium religiosum | Antiglycating | Antioxidant | 88 |
| 2 | Ethyl extract of dried leaves | Allium cepa | Antihyperglycaemic and antiglycating | Antioxidant | 88, 89 |
| 3 | Hull extract | Fagopyrum esculentum | Antiglycating | Antioxidant | 88 |
| 4 | Leaf extract | Origanum officinalis | Antiglycating | Antioxidant | 88 |
| 5 | Ethyl ether extract | Allium sativum | Hypoglycaemic | Stimulation of insulin secretion | 90 |
| 6 | Allicin | Allium sativum | Antidiabetic | Hypoglycemic | 91 |
| 7 | Leaf gel extract | Aloe vera | Hypoglycaemic | Reduction in blood sugar, improvement in plasma insulin | 93 |
| 8 | Extract of seeds | Acacia arabica | Hypoglycaemic | Stimulation of insulin secretion from beta cells | 94 |
| 9 | Beta vulgarocides | Beta vulgaris | Antiglycating | Antihyperglycaemic, Enhanced glucose tolerance | 97 |
| 10 | Methanolic extract | Phallanthus amarus | Reduce blood sugar level | Antioxidant | 101 |
| 11 | Dried leaf powder | Gymnema sylvestris | Antihyperglycaemic | Decrease in the activity of gluconeogenic enzymes | 110, 111 |
| 12 | Caffeic acid | Ilex paraguariensis | Antiglycating | AGEs inhibition by acting on Methylglyoxal | 119 |
| 13 | Extract | Empetrum nigrum L. | Antiglycating | Radical scavenger | 121 |
| 14 | Chlorogenic acids | Chrysanthemum sp. | Interfere with glucose absorption and alter gene expression of antioxidant enzymes | Free radical and metal scavenging activity | 126 |
| 15 | Cinnamic acid derivatives such as ferulic acid | Cimicifuga heracleifolia | Antiglycating | AGEs inhibitor | 127, 128, 129 |
| 16 | Ellagic acid | Numerous fruits and vegetables | Antiglycating | Inhibition of CML through a dicarbonyl trap | 130 |
| 17 | Quercetin | Many plants | Antidiabetic and Antiglycating | Antioxidant, traps MG and Glyoxal | 131, 132, 133 |
| 18 | Thymoquinone | Nigella sativa | Antiglycating | Antioxidant | 136, 137 |
Abbreviations
- AGEs
advanced glycation end products
- ROS
reactive oxygen species
- GSH
gluthathione
- MG
methyglyoxal
- RNS
reactive nitrogen species
- AG
aminoguanidine
- ARIs
Aldose reductase inhibitors.
References
- 1.Zhang Q, Ames JM, Smith RD, Baynes JW, Metz TO. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J Proteome Res. 2009;8:754–69. doi: 10.1021/pr800858h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed N. Advanced glycation endproducts role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Neglia CI, Cohen HJ, Garber AR, Ellis PD, Thorpe SR, Baynes JW. NMR investigation of nonenzyatic glycosylation of protein. Model studies using RNase A. J Biol Chem. 1983;258:14279–83. [PubMed] [Google Scholar]
- 4.Baynes JW, Watkins NG, Fisher CI, Hull CJ, Patrick JS, Ahmed MU, Dunn JA, Thorpe SR. The Amadori product on protein: structure and reactions. Prog Clin Biol Res. 1989;304:43–67. [PubMed] [Google Scholar]
- 5.Reddy VP, Beyaz A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discovery Today. 2006;11:646–54. doi: 10.1016/j.drudis.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Kalousová M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51:597–604. [PubMed] [Google Scholar]
- 7.Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–6. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 8.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–16. [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Abed Y, Bucala R. N-carboxymethyllysine formation by direct addition of glyoxal to lysine during the maillard reaction. Bioorg Med Chem Lett. 1995;5:2161–2. [Google Scholar]
- 10.Wells-Knecht KJ, Brinkmann E, Baynes JW. Characterization of an imidazolium salt formed from glyoxal and N.alpha.-hippuryllysine: A model for maillard reaction crosslinks in proteins. J Org Chem. 1995;60:6246–7. [Google Scholar]
- 11.Glomb MA, Lang G. Isolation and characterization of glyoxal-arginine modifications. J Agric Food Chem. 2001;49:1493–1501. doi: 10.1021/jf001082d. [DOI] [PubMed] [Google Scholar]
- 12.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids. 2003;25:275–81. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with AGE in human lens proteins. Biochem J. 1997;324:565–70. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaraj RH, Shipanova IN, Faust FM. Protein cross-linking by the maillard reaction: Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J Biol Chem. 1996;271:19338–45. doi: 10.1074/jbc.271.32.19338. [DOI] [PubMed] [Google Scholar]
- 15.Shipanova IN, Glomb MA, Nagaraj RH. Protein modification by methylglyoxal: Chemical nature and synthetic mechanism of a major fluorescent adduct. Arch Biochem Biophys. 1997;344:29–36. doi: 10.1006/abbi.1997.0195. [DOI] [PubMed] [Google Scholar]
- 16.Henle T, Walter A, Haeßner R, Klostermeyer H. Detection and identification of a protein-bound imidazolone resulting from the reaction of arginine residues and methylglyoxal. Z Lebensm Unters Forsch. 1994;199:55–8. [Google Scholar]
- 17.Portero-Otin M, Nagaraj RH, Monnier VM. Chromatographic evidence for pyrraline formation during protein glycation in vitro and in vivo. Biochim Biophys Acta. 1995;1247:74–80. doi: 10.1016/0167-4838(94)00209-y. [DOI] [PubMed] [Google Scholar]
- 18.Dyer D, Blackledge J, Thorpe S, Baynes J. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991;266:11654–60. [PubMed] [Google Scholar]
- 19.Jono T, Nagai R, Lin X, Ahmed N, Thornalley PJ, Takeya M, Horiuchi S. N-(carboxymethyl) lysine and 3-DG-imidazolone are major AGE structures in protein modification by 3-deoxyglucosone. J Biochem. 2004;136:351–8. doi: 10.1093/jb/mvh124. [DOI] [PubMed] [Google Scholar]
- 20.Giardino I, Edelstein D, Brownlee M. Nonenzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity. A model for intracellular glycosylation in diabetes. J Clin Investig. 1994;94:110–7. doi: 10.1172/JCI117296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 22.Smit AJ, Hartog JW, Voors AA, van Veldhuisen DJ. Advanced glycation endproducts in chronic heart failure. Ann NY Acad Sci. 2008;1126:225–30. doi: 10.1196/annals.1433.038. [DOI] [PubMed] [Google Scholar]
- 23.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 24.Singh VP, Bali A, Singh N, Jaggi AS. Advanced Glycation Endproducts And Diabetic Complications. Korean J Physiol Pharmacol. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brownlee M. The pathological implications of protein glycation. Clin Invest Med. 1995;18:275–81. [PubMed] [Google Scholar]
- 26.Aldini G, Vistoli G, Stefek M, Chondrogianni N, Grune T, Sereikaite J, Sadowska-Bartosz I, Bartosz G. Molecular strategies to prevent; inhibit; and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic Res. 2013;47:93–137. doi: 10.3109/10715762.2013.792926. [DOI] [PubMed] [Google Scholar]
- 27.Stitt AW. The Mailard reaction in eye diseases. Ann N Y Acad Sci. 2005;1043:582–97. doi: 10.1196/annals.1338.066. [DOI] [PubMed] [Google Scholar]
- 28.Forbes JM, Yee LT, Thallas B, Lassila M, Candido R, Jandeleit-Dahn KA, Thomas MC, Burns WC, Deemer EK, Thorpe SR, Cooper ME, Allel TJ. Advanced glycation endprducts interventions reduce diabetes-accelerative atherosclerosis. Diabetes. 2004;53:1813–23. doi: 10.2337/diabetes.53.7.1813. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto Y, Doi T, Kato I, Shinohara H, Sakurai S, Yonekura H, Watanabe T, Myint KM, Harashima A, Takeuchi M, Takasawa S, Okamoto H, Hashimoto N, Asano M, Yamamoto H. Receptor for advance glycation enproducts is a promising target of a diabetic nephropathy. Ann N Y Acad Sci. 2005;1043:562–66. doi: 10.1196/annals.1333.064. [DOI] [PubMed] [Google Scholar]
- 30.Jono T, Kimura T, Takamatsu J, Nagai R, Miyazaki K, Yuzuriha T, Kitamura T, Horiuchi S. Accumulation of Imidazolone, pentosidine and N(epsilon)-(carboxymethyl) lysine in hippocampal CA4 pyramidal neurons of aged human brain. Pathol Int. 2002;52:563–71. doi: 10.1046/j.1320-5463.2002.01390.x. [DOI] [PubMed] [Google Scholar]
- 31.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornalley PJ. Glutathione-dependent detoxification of alpha-oxoaldehydes by the glyoxalase system: involvement in disease mechanisms and antiproliferative activity of glyoxalase I inhibitors. Chem Biol Interact. 1998;111:137–51. doi: 10.1016/s0009-2797(97)00157-9. [DOI] [PubMed] [Google Scholar]
- 33.Gerhardinger C, Marion MS, Rovner A, Glomb M, Monnier VM. Novel degradation pathway of glycated amino acids into free fructosamine by a Pseudomonas sp. soil strain extract. J Biol Chem. 1995;270:218–24. doi: 10.1074/jbc.270.1.218. [DOI] [PubMed] [Google Scholar]
- 34.Szwergold BS, Howell S, Beisswenger PJ. Human fructosamine-3-kinase: purification, sequencing, substrate specificity, and evidence of activity in vivo. Diabetes. 2001;50:2139–47. doi: 10.2337/diabetes.50.9.2139. [DOI] [PubMed] [Google Scholar]
- 35.Boel E, Selmer J, Flodgaard HJ, Jensen T. Diabetic late complications: will aldose reductase inhibitors or inhibitors of advanced glycosylation endproduct formation hold promise? J. Diabetes Complicat. 1995;9:104–29. doi: 10.1016/1056-8727(94)00025-j. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Koh YH, Mizuno H, Hamaoka R, Taniguchi NJ. Overexpression of aldehyde reductase protects PC12 cells from the cytotoxicity of methylglyoxal or 3-deoxyglucosone. Biochem (Tokyo) 1998;123:353–57. doi: 10.1093/oxfordjournals.jbchem.a021944. [DOI] [PubMed] [Google Scholar]
- 37.Taha M, Naz H, Rasheed S, Ismail NH, Rahman AA, Yousuf S, Choudhary MI. Synthesis of 4-methoxybenzoylhydrazones and evaluation of their antiglycation activity. Molecules. 2014;19:1286–1301. doi: 10.3390/molecules19011286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harding JA, Ganea E. Protection against glycation and similar post-translational modifications of proteins. Biochim Biophys Acta. 2006;1764:1436–46. doi: 10.1016/j.bbapap.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Brownson C, Hipkiss AR. Carnosine reacts with a glycated protein. Free Radic Biol Med. 2000;28:1564–70. doi: 10.1016/s0891-5849(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 40.Rahbar S, Figarola JL. Novel inhibitors of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:63–79. doi: 10.1016/j.abb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Rahbar S, Natarajan R, Yerneni K, Scott S, Gonzales N, Nadler J. Evidence that pioglitazone, metformin and pentoxifylline are inhibitors of glycation. Clin Chim Acta. 2000;301:65–77. doi: 10.1016/s0009-8981(00)00327-2. [DOI] [PubMed] [Google Scholar]
- 42.Williams ME. Clinical studies of advanced glycation end product inhibitors and diabetic kidney disease. Curr Diabetes Rep. 2004;4:441–6. doi: 10.1007/s11892-004-0054-0. [DOI] [PubMed] [Google Scholar]
- 43.Thomas MC, Baynes JW, Thorpe SR, Cooper ME. The role of AGEs and AGE inhibitors in diabetic cardiovascular disease. Curr Drug Targets. 2005;6:453–74. doi: 10.2174/1389450054021873. [DOI] [PubMed] [Google Scholar]
- 44.Crompton M, Rixon KC, Harding JJ. Aspirin prevents carbamylation of soluble lens proteins and prevents cyanate-induced phase separation opacities in vitro: a possible mechanism by which aspirin could prevent cataract. Exp Eye Res. 1985;40:297–311. doi: 10.1016/0014-4835(85)90014-4. [DOI] [PubMed] [Google Scholar]
- 45.Rao GN, Lardis MP, Cotlier E. Acetylation of lens crystallins: a possible mechanism by which aspirin could prevent cataract formation. Biochem Biophys Res Commun. 1985;128:1125–32. doi: 10.1016/0006-291x(85)91057-5. [DOI] [PubMed] [Google Scholar]
- 46.Van Boekel MAM, Van den Bergh PJ, Hoenders HJ. Glycation of human serum albumin: inhibition by Diclofenac. Biochim Biophys Acta. 1992;1120:201–4. doi: 10.1016/0167-4838(92)90270-n. [DOI] [PubMed] [Google Scholar]
- 47.Ramakrishnan S, Sulochana KN, Punitham R. Two new functions of inositol in the eye lens: antioxidation and antiglycation and possible mechanisms. Indian J Biochem Biophys. 1999;36:129–33. [PubMed] [Google Scholar]
- 48.Mendez JD, Leal LI. Inhibition of in vitro pyrraline formation by L-arginine and polyamines. Biomed Pharm. 2004;58:598–604. doi: 10.1016/j.biopha.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–32. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 50.Corbett JA, Tilton RG, Chang K, Hasan KS, Ido Y, Wang JL, Sweetland A, Lancaster JR, Williamson JR, McDaniel ML. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–6. doi: 10.2337/diab.41.4.552. (1992) [DOI] [PubMed] [Google Scholar]
- 51.Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Metz TO, Alderson NL, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: a novel therapy for treatment of diabetic complications. Arch Biochem Biophys. 2003;419:41–49. doi: 10.1016/j.abb.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Voziyan PA, Metz TO, Baynes JW, Hudson BG. A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J Biol Chem. 2002;277:3397–403. doi: 10.1074/jbc.M109935200. [DOI] [PubMed] [Google Scholar]
- 54.Khalifah RG, Baynes JW, Hudson BG. Amadorins: novel post-Amadori inhibitors of advanced glycation reactions. Biochem Biophys Res Commun. 1999;257:251–8. doi: 10.1006/bbrc.1999.0371. [DOI] [PubMed] [Google Scholar]
- 55.Booth AA, Khalifah RG, Hudson BG. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: comparison with aminoguanidine, Biochem Biophys Res Commun. 1996;220:113–9. doi: 10.1006/bbrc.1996.0366. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed N, Luthen R, Haussinger D, Sebekova K, Schinzel R, Voelker W, Heidland A, Thornalley PJ. Increased protein glycation in cirrhosis and therapeutic strategies to prevent it. Ann NY Acad Sci. 2005;1043:718–24. doi: 10.1196/annals.1333.083. [DOI] [PubMed] [Google Scholar]
- 57.Booth AA, Khalifah RG, Todd P, Hudson BG. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). Novel inhibition of post-Amadori glycation pathways. J Biol Chem. 1997;272:5430–37. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- 58.Kiho T, Kato T, Usui S, Hirano K. Effect of buformin and metformin on formation of advanced glycation end products by methylglyoxal. Clin Chim Acta. 2005;358:139–45. doi: 10.1016/j.cccn.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Hipkiss AR, Michaelis J, Syrris P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995;371:81–5. doi: 10.1016/0014-5793(95)00849-5. [DOI] [PubMed] [Google Scholar]
- 60.Yan H, Harding JJ. Carnosine protects against the inactivation of esterase induced by glycation and a steroid. Biochim Biophys Acta. 2005;1741:120–6. doi: 10.1016/j.bbadis.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Sobal G, Menzel EJ, Sinzinger H. Calcium antagonists as inhibitors of in vitro low density lipoprotein oxidation and glycation. Biochem Pharmacol. 2001;61:373–9. doi: 10.1016/s0006-2952(00)00548-7. [DOI] [PubMed] [Google Scholar]
- 62.Akira K, Amano M, Okajima F, Hashimoto T, Oikawa S. Inhibitory effects of amlodipine and fluvastatin on the deposition of advanced glycation end products in aortic wall of cholesterol and fructose-fed rabbits. Biol Pharm Bull. 2006;29:75–81. doi: 10.1248/bpb.29.75. [DOI] [PubMed] [Google Scholar]
- 63.Verbeke P, Siboska GE, Clark BF, Rattan SI. Kinetin inhibits protein oxidation and glycoxidation in vitro. Biochem Biophys Res Commun. 2000;276:1265–70. doi: 10.1006/bbrc.2000.3616. [DOI] [PubMed] [Google Scholar]
- 64.Jung YS, Joe BY, Cho SJ, Konishi Y. 2,3-Dimethoxy-5-methyl-1,4-benzoquinones and 2-methyl-1,4-naphthoquinones: glycation inhibitors with lipid peroxidation activity. Bioorg Med Chem Lett. 2005;15:1125–29. doi: 10.1016/j.bmcl.2004.12.029. (2005) [DOI] [PubMed] [Google Scholar]
- 65.Munch G, Taneli Y, Schraven E, Schindler U, Schinzel R, Palm D, Riederer PJ. The cognition-enhancing drug tenilsetam is an inhibitor of protein crosslinking by advanced glycosylation. Neural Park Dis Dement Sect. 1994;8:193–208. doi: 10.1007/BF02260940. [DOI] [PubMed] [Google Scholar]
- 66.Stevens A. The effectiveness of putative anticataract agents in the prevention of protein glycation. J Am Optom Assoc. 1995;66:744–49. [PubMed] [Google Scholar]
- 67.Al-Abed Y, Mitsuhashi T, Li H, Lawson JA, FitzGerald GA, Founds H, Donnelly T, Cerami A, Ulrich P, Bucala R. Inhibition of advanced glycation endproduct formation by acetaldehyde: role in the cardioprotective effect of ethanol. Proc Natl Acad Sci USA. 1999;96:2385–90. doi: 10.1073/pnas.96.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–43. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 69.Nagai R, Murray DB, Metz TO, Baynes JW. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549–59. doi: 10.2337/db11-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Susic D. Cross-link breakers as a new therapeutic approach to cardiovascular disease. Biochem Soc Trans. 2007;35:853–56. doi: 10.1042/BST0350853. [DOI] [PubMed] [Google Scholar]
- 71.Price DL, Rhett PM, Thorpe SR, Baynes JW. Chelating activity of advanced glycation end-product inhibitors. J Biol Chem. 2001;276:48967–72. doi: 10.1074/jbc.M108196200. [DOI] [PubMed] [Google Scholar]
- 72.Vasan S, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan J, Ulrich P. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382:275–78. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- 73.Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci USA. 2000;97:2809–13. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end products in diabetic nephropathy. J Am Soc Nephrol. 2003;14:254–8. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 75.Chandra KP, Shiwalkar A, Kotecha J, Thakkar P, Srivastava A, Chauthaiwale V, Sharma SK, Cross MR, Dutt C. Phase I clinical studies of the advanced glycation end-product (AGE)-breaker TRC4186: safety, tolerability and pharmacokinetics in healthy subjects. Clin Drug Investig. 2009;29:559–75. doi: 10.2165/11315260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 76.Caballero F, Gerez E, Batlle A, Vazquez E. Preventive aspirin treatment of streptozotocin induced diabetes: blockage of oxidative status and revertion of heme enzymes inhibition. Chem Biol Interact. 2000;126:215–25. doi: 10.1016/s0009-2797(00)00168-x. [DOI] [PubMed] [Google Scholar]
- 77.Liu Q, Sun L, Tan Y, Wang G, Lin X, Cai L. Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic complications. Curr Med Chem. 2009;16:113–29. doi: 10.2174/092986709787002862. [DOI] [PubMed] [Google Scholar]
- 78.Hamada Y, Odagaki Y, Sakakibara F, Naruse K, Koh N, Hotta N. Effects of an aldose reductase inhibitor on erythrocyte fructose 3-phosphate and sorbitol 3-phosphate levels in diabetic patients. Life Sci. 1995;57:23–9. doi: 10.1016/0024-3205(95)00239-3. [DOI] [PubMed] [Google Scholar]
- 79.Tsukushi S, Katsuzaki T, Aoyama I, Takayama F, Miyazaki T, Shimokata K, Niwa T. Increased erythrocyte 3-DG and AGEs in diabetic hemodialysis patients: role of the polyol pathway. Kidney Int. 1999;55:1970–6. doi: 10.1046/j.1523-1755.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 80.Miyata T, van Ypersele de Strihou C, Ueda Y, Ichimori K, Inagi R, Onogi H, Ishikawa N, Nangaku M, Kurokawa K. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol. 2002;13:2478–87. doi: 10.1097/01.asn.0000032418.67267.f2. [DOI] [PubMed] [Google Scholar]
- 81.Freedman B, Wuerth JP, Cartwright K, Bain RP, Dippe S, Hershon K, Mooradian AD, Spinowitz BS. Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II) Control Clin Trials. 1999;20:493–510. doi: 10.1016/s0197-2456(99)00024-0. [DOI] [PubMed] [Google Scholar]
- 82.Kavishankar N, Lakshmidevi S, Murthy HS. Diabetes and medicinal plants-a review. Int J Pharm Biomed Sci. 2011;2:65–80. [Google Scholar]
- 83.Coman C, Rugină OD, Socaciu C. Plants and natural compounds with antidiabetic action. Not Bot Horti Agrobo. 2012;40:314–25. [Google Scholar]
- 84.Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes care. 1989;12:553–64. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 85.Soumyanath A. Traditional Medicines in Modern Times-Anti-Diabetic Plants. CRC Press; Boca Raton, London, New York: 2006. [Google Scholar]
- 86.Peng X, Ma J, Chen F, Wang M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct. 2011;2:289–301. doi: 10.1039/c1fo10034c. [DOI] [PubMed] [Google Scholar]
- 87.Syiem D, Warji P. Hypoglycemic and antihyperglycemic effects of Aqueous extract of Ixeris gracilis DC. On normal And alloxan-induced diabetic mice. Diabetol Croat. 2011;40:89–95. [Google Scholar]
- 88.Kim HY, Kim KJ. Protein glycation inhibitory and antioxidative activities of some plant extracts in vitro. Agric Food Chem. 2003;51:1586–91. doi: 10.1021/jf020850t. [DOI] [PubMed] [Google Scholar]
- 89.Mathew PT, Augusti KT. Hypoglycaemic effects of onion, Allium cepa Linn. On diabetes mellitus- A preliminary report. Indian J Physiol Pharmacol. 1975;19:213–17. [PubMed] [Google Scholar]
- 90.Jain RC, Vyas CR. Garlic in alloxan-induced diabetic rabbits. Am J Clin Nutr. 1975;28:684–85. doi: 10.1093/ajcn/28.7.684. [DOI] [PubMed] [Google Scholar]
- 91.Sheela CG, Augusti KT. Antidiabetic effects of S-allyl cysteine sulphoxide isolated from garlic Allium sativum Linn. Indian J Exp Biol. 1992;30:523–6. [PubMed] [Google Scholar]
- 92.Rutter K, Sell DR, Fraser N, Obrenovich M, Zito M, Starke-Reed P, Monnier VM. Green tea extract suppresses the age-related increase in collagen crosslinking and fluorescent products in C57BL/6 mice. Int J Vitam Nutr Res. 2003;73:453–60. doi: 10.1024/0300-9831.73.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajasekaran S, Ravi K, Sivagnanam K, Subramanian S. Beneficial effects of aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Physiol. 2006;33:232–7. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 94.Yoshikawa M, Pongpiriyadacha Y, Kishi A, Kageura T, Wang T, Morikawa T, Matsuda H. Biological activities of Salacia chinensis originating in Thailand: the quality evaluation guided by alpha-glucosidase inhibitory activity. Yakugaku Zasshi. 2003;123:871–80. doi: 10.1248/yakushi.123.871. [DOI] [PubMed] [Google Scholar]
- 95.Karunanayake EH, Welihinda J, Sirimanne SR, Sinnadorai G. Oral hypoglycaemic activity of some medicinal plants of Sri Lanka. J Ethnopharmacol. 1984;11:223–31. doi: 10.1016/0378-8741(84)90040-0. [DOI] [PubMed] [Google Scholar]
- 96.Chattopadhyay RR. A comparative evaluation of some blood sugar lowering agents of plant origin. J Ethnopharmacol. 1999;67:367–72. doi: 10.1016/s0378-8741(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 97.Yoshikawa M, Murakami T, Kadoya M, Matsuda H, Muraoka O, Yamahara J, Murakami N. Medicinal Foodstuff. III. Sugar Beet. Hypoglycaemic Oleanolic Acid Oligoglycosides, Beta Vulgarosides I, II, III and IV, from the root of Beta vulgaris L. (Chenopodiaceae) Chem Pharm Bull (Tokyo) 1996;44:1212–17. doi: 10.1248/cpb.44.1212. [DOI] [PubMed] [Google Scholar]
- 98.Vats V, Grover JK, Rathi SS. Evaluation of anti-hyperglycemic and hypoglycemic effect of Trigonella foenum-graecum Linn, Ocimum sanctum Linn and Pterocarpus marsupium Linn in normal and alloxanized diabetic rats. J Ethnopharmacol. 2002;79:95–100. doi: 10.1016/s0378-8741(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 99.Aderibigbe AO, Emudianughe TS, Lawal BA. Antihyperglycaemic effect of Mangifera indica in rat. Phytother Res. 1999;13:504–7. doi: 10.1002/(sici)1099-1573(199909)13:6<504::aid-ptr533>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 100.Khanna P, Jain SC, Panagariya A, Dixit VP. Hypoglycemic activity of polypeptide-p from a plant source. J Nat Prod. 1981;44:648–55. doi: 10.1021/np50018a002. [DOI] [PubMed] [Google Scholar]
- 101.Raphael KR, Sabu MC, Kuttan R. Hypoglycemic effect of methanol extract of Phyllanthus amarus Schum & Thonn on alloxan induced diabetes mellitus in rats and its relation with antioxidant potential. Indian J Exp Biol. 2002;40:905–9. [PubMed] [Google Scholar]
- 102.Khan BA, Abraham A, Leelamma S. Hypoglycemic action of Murraya koeingii (curry leaf) and Brassica juncea (mustard): mechanism of action. Indian J Biochem Biophys. 1995;32:106–8. [PubMed] [Google Scholar]
- 103.Khan BA, Abraham A, Leelamma S. Biochemical response in rats to the addition of curry leaf (Murraya koeingii) and mustard seeds (Brassica juncea ) to the diet. Plant Foods Hum Nutr. 1996a;49:295–99. doi: 10.1007/BF01091978. [DOI] [PubMed] [Google Scholar]
- 104.Khan BA, Abraham A, Leelamma S. Antioxidant effects of curry leaf, Murraya koeingii and mustard seeds, Brassica juncea in rats fed with high fat diet. Indian J Exp Biol. 1997;35:148–50. [PubMed] [Google Scholar]
- 105.Khan BA, Abraham A, Leelamma S. Role of Murraya koeingii (curry leaf) and Brassica juncea (Mustard) in lipid peroxidation. Indian J Physiol Pharmacol. 1996b;40:155–58. [PubMed] [Google Scholar]
- 106.Yadav P, Sarkar S, Bhatnagar D. Lipid peroxidation and antioxidant enzymes in erythrocytes and tissues in aged diabetic rats. Indian J Exp Biol. 1997b;35:389–92. [PubMed] [Google Scholar]
- 107.Yadav P, Sarkar S, Bhatnagar D. Action of Capparis decidua against alloxan-induced oxidative stress and diabetes in rat tissues. Pharmacol Res. 1997a;36:221–28. doi: 10.1006/phrs.1997.0222. [DOI] [PubMed] [Google Scholar]
- 108.Agarwal V, Chauhan BM. A study on composition and hypolipidemic effect of dietary fibre from some plant foods. Plant Foods Hum Nutr. 1988;38:189–97. doi: 10.1007/BF01091723. [DOI] [PubMed] [Google Scholar]
- 109.Abdel-Hassan IA, Abdel-Barry JA, Tariq Mohammeda S. The hypoglycemic and antihyperglycemic effect of Citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. J Ethnopharmacol. 2000;71:325–30. doi: 10.1016/s0378-8741(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 110.Shanmugasundaram KR, Panneerselvam C, Samudram P, Shanmugasundaram ER. Enzyme changes and glucose utilization in diabetic rabbits: the effect of Gymnema sylvestre, R. Br J Ethnopharmacol. 1983;7:205–34. doi: 10.1016/0378-8741(83)90021-1. [DOI] [PubMed] [Google Scholar]
- 111.Shanmugasundaram ER, Gopinath KL, Radha Shanmugasundaram K, Rajendran VM. Possible regeneration of the islets of Langerhans in streptozotocin-diabetic rats given Gymnema sylvestre leaf extracts. J Ethnopharmacol. 1990;30:265–79. doi: 10.1016/0378-8741(90)90106-4. 1990. [DOI] [PubMed] [Google Scholar]
- 112.Ahmad H, Khan I, Wahid A. Antiglycation and antioxidation properties of Juglans regia and Calendula officinalis: Possible role in reducing diabetic complications and slowing down ageing. J Tradit Chin Med. 2012;32:411–14. doi: 10.1016/s0254-6272(13)60047-3. [DOI] [PubMed] [Google Scholar]
- 113.Kang KS, Yamabe N, Kim HY, Yokozawa T. Role of maltol in advanced glycation end products and free radicals, in vitro and in vivo studies. J Pharm Pharmacol. 2008;60:445–52. doi: 10.1211/jpp.60.4.0006. [DOI] [PubMed] [Google Scholar]
- 114.Jang DS, Yoo NH, Kim NH, Lee YM, Kim CS, Kim J, Kim JH, Kim JS. 3,5-Di-O-caffeoyl-epi-quinic acid from the leaves and stems of Erigeron annuus inhibits protein glycation, aldose reductase, and cataractogenesis. Biol Pharm Bull. 2010;3:329–33. doi: 10.1248/bpb.33.329. [DOI] [PubMed] [Google Scholar]
- 115.Hsieh CL, Yang MH, Chyau CC, Chiu CH, Wang HE, Lin YC, Chiu WT, Peng RY. Kinetic analysis on the sensitivity of glucose- or glyoxal-induced LDL glycation to the inhibitory effect of Psidium guajava extract in a physiomimic system. BioSystems. 2007;88:92–100. doi: 10.1016/j.biosystems.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 116.Ho SC, Wu SP, Lin SM, Tang YL. Comparison of anti-glycation capacities of several herbal infusions with that of green tea. Food Chem. 2010;112:768–74. [Google Scholar]
- 117.Ardestani A, Yazdanparast R. Cyperus rotundus suppresses AGE formation and protein oxidation in a model of fructose-mediated protein glycoxidation. Int J Biol Macromol. 2007;41:572–78. doi: 10.1016/j.ijbiomac.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 118.Peng X, Zheng Z, Cheung KW, Shan F, Ren GX, Chen SF, Wang M. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008;106:457–81. [Google Scholar]
- 119.Gugliucci A, Bastos DH, Schulze J, Souza MF. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia. 2009;2009;80:339–44. doi: 10.1016/j.fitote.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 120.Ahmad H, Khan I, Wahid A. Antiglycation and antioxidation properties of Juglans regia and Calendula officinalis: Possible role in reducing diabetic complications and slowing down ageing. J Tradit Chin Med. 2012;32:411–14. doi: 10.1016/s0254-6272(13)60047-3. [DOI] [PubMed] [Google Scholar]
- 121.Harris CS, Cuerrier A, Lamont E, Haddad PS, Arnason JT, Bennett SA, Johns T. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: Chemical correlates of in vitro antiglycation activity. Plant Foods Hum Nutr. 2014;69:71–7. doi: 10.1007/s11130-014-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Landis-Piwowar KR, Huo C, Chen D, Milacic V, Shi G, Chan TH, Dou QP. A Novel Prodrug of the Green Tea Polyphenol (−)-Epigallocatechin-3- Gallate as a Potential Anticancer Agent. Canser Res. 2007;67:4303–10. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 123.Mandel S, Weinreb O, Amit T, Youdim BH. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (−)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem. 2004;88:1555–69. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- 124.Vinson JA, Proch J, Bose P, Muchler S, Taffera P, Shuta D, Samman N, Agbor GA. Chocolate is a powerful ex vivo and in vivo antioxidant, an antiatherosclerotic agent in an animal model, and a significant contributor to antioxidants in the european and american diets. J Agric Food Chem. 2006;54:8071–76. doi: 10.1021/jf062175j. [DOI] [PubMed] [Google Scholar]
- 125.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab. 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fiuza SM, Gomes C, Teixeira LJ, Girão da Cruz MT, Cordeiro MN, Milhazes N, Borges F, Marques MP. Phenolic acid derivatives with potential anticancer properties-a structure-activity relationship study. Part 1: methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg Med Chem. 2004;12:3581–89. doi: 10.1016/j.bmc.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 127.Meeprom A, Sompong W, Chan CB, Adisakwattana S. Isoferulic acid, a new anti-glycation agent, inhibits fructose- and glucose-mediated protein glycation in vitro. Molecules. 2013;18:6439–54. doi: 10.3390/molecules18066439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Silván JM, Assar SH, Srey C, Dolores Del Castillo M, Ames JM. Control of the Maillard reaction by ferulic acid. Food Chem. 2011;128:208–13. doi: 10.1016/j.foodchem.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 129.Sompong W, Meeprom A, Cheng H, Adisakwattana S. A comparative study of ferulic acid on different monosaccharide-mediated protein glycation and oxidative and oxidative damage in bovine serum albumin. Molecules. 2013;18:13886–903. doi: 10.3390/molecules181113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Muthenna P, Akileshwari C, Reddy GB. Ellagic acid, a new antiglycating agent: its inhibition of Nε-(carboxymethyl)lysine. Biochem J. 2012;442:221–30. doi: 10.1042/BJ20110846. [DOI] [PubMed] [Google Scholar]
- 131.Torres-Piedra M, Ortiz-Andrade R, Villalobos-Molina R, Singh N, Medina-Franco JL, Webster SP, Binnie M, Navarrete-Vázquez G, Estrada-Soto S. A comparative study of flavonoid analogues on streptozotocin-nicotinamide induced diabetic rats: quercetin as a potential antidiabetic agent acting via 11beta-hydroxysteroid dehydrogenase type 1 inhibition. Eur J Med Chem. 2010;45:2606–12. doi: 10.1016/j.ejmech.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 132.Li X, Zheng T, Sang S, Lv L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J Agric Food Chem. 2014;2:12152–8. doi: 10.1021/jf504132x. [DOI] [PubMed] [Google Scholar]
- 133.Groot HD, Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmaco. 1998;12:249–55. doi: 10.1111/j.1472-8206.1998.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 134.Afanas’ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763–9. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- 135.El-Mahmoudy A, Shimizu Y, Shiina T, Matsuyama H, El-Sayed M, Takewaki T. Successful abrogation by thymoquinone against induction of diabetes mellitus with streptozotocin via nitric oxide inhibitory mechanism. Int Immunopharmacol. 2005;5:195–207. doi: 10.1016/j.intimp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 136.Khan MA, Anwar S, Aljarbou AN, Al-Orainy M, Aldebasi YH, Islam S, Younus H. Protective effect of thymoquinone on glucose or methylglyoxal-induced glycation of superoxide dismutase. Int J Biol Macromol. 2014;65:16–20. doi: 10.1016/j.ijbiomac.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 137.Anwar S, Khan MA, Sadaf A, Younus H. A structural study on the protection of glycation of superoxide dismutase by thymoquinone. Int J Biol Macromol. 2014;69:476–81. doi: 10.1016/j.ijbiomac.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 138.Kumar D, Kumar S, Kohli S, Arya R, Gupta J. Antidiabetic activity of methanolic bark extract of Albizia odoratissimia Benth, in alloxan induced diabetic albino mice. Asian Pac J Trop Med. 2011;4:900–3. doi: 10.1016/S1995-7645(11)60215-0. [DOI] [PubMed] [Google Scholar]
- 139.Sabu MC, Kuttan R. Antidiabetic activity of Aegle marmelos and its relationship with its antioxidant properties. Indian J Physiol Pharmacol. 2004;48:81–8. [PubMed] [Google Scholar]
- 140.Jemai H, El Feki A, Sayadi S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J Agric Food Chem. 2009;57:8798–804. doi: 10.1021/jf901280r. [DOI] [PubMed] [Google Scholar]
- 141.Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Altern Med Rev. 2002;7:45–58. [PubMed] [Google Scholar]
- 142.Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TP. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–73. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ernst E. The efficacy of herbal medicine-an overview. Fundam Clin Pharmacol. 2005;19:405–9. doi: 10.1111/j.1472-8206.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 144.Yallapu MM, Ebeling MC, Jaggi M, Chauhan SC. Plasma proteins interaction with curcumin nanoparticles: Implications in cancer therapeutics. Curr Drug Metab. 2013;14:504–15. doi: 10.2174/1389200211314040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cao H, Shi YJ, Chen XQ. Advances on the interaction between tea catechins and plasma proteins: Structure- affinity relationship, influence on antioxidant activity, and molecular docking aspects. Curr Drug Metab. 2013;14:446–50. doi: 10.2174/1389200211314040007. [DOI] [PubMed] [Google Scholar]
- 146.Dutt C, Chauthaiwale V, Mohanan A, Chandra KP, Zambad S, Gupta R, Parikh S. The Evolving Face of Heart Failure Associated with Elevated CardioMetabolic Risk Factors. In: Veselka Josef., editor. Cardiomyopathies - From Basic Research to Clinical Management. InTech; 2012. [Google Scholar]