Abstract
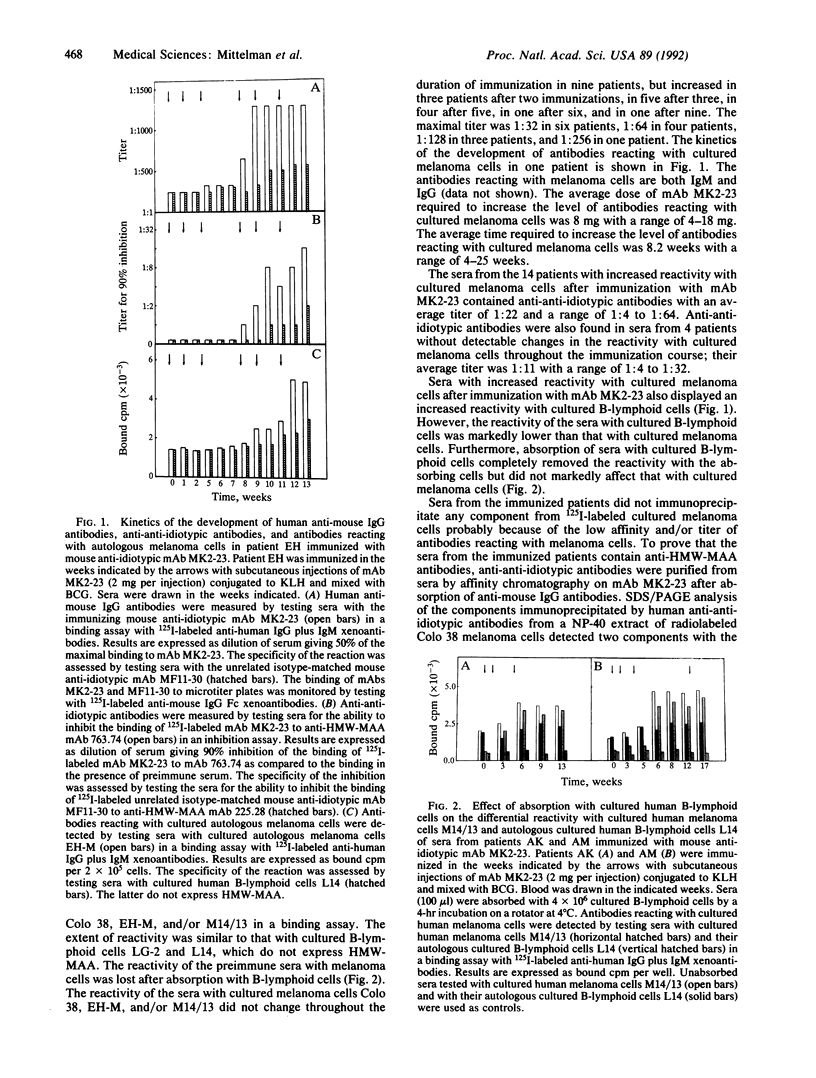
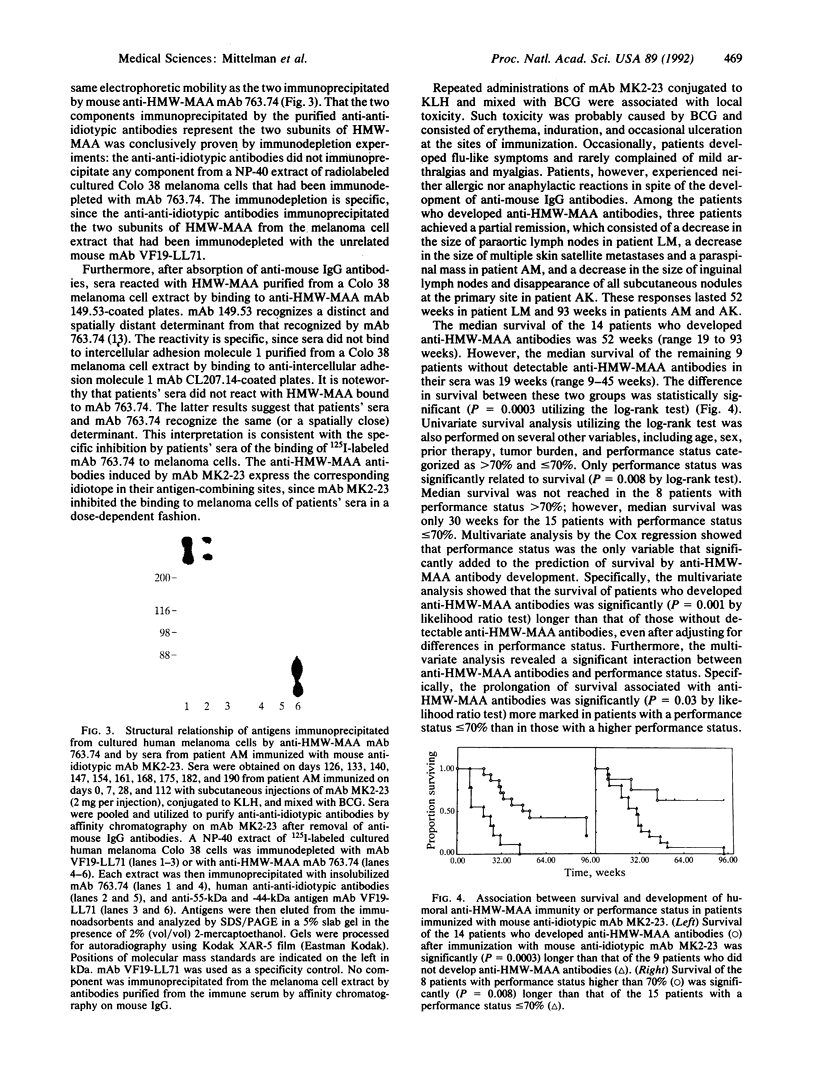
Twenty-five patients with stage IV melanoma were immunized with the mouse anti-idiotypic monoclonal antibody (mAb) MK2-23 (2 mg per injection), which bears the internal image of the determinant defined by anti-HMW-MAA mAb 763.74. Two patients were inevaluable, since they did not complete 4 weeks of therapy. Only 14 patients developed antibodies that were shown by serological and immunochemical assays to recognize the same or spatially close determinant as the anti-HMW-MAA mAb 763.74 and to express the idiotope defined by mAb MK2-23 in their antigen-combining sites. Side effects that are likely to be caused by bacillus Calmette-Guérin present in the immunogen consisted of erythema, induration, and ulceration at the sites of the injections. Occasionally, patients complained of flu-like symptoms, arthralgias, and myalgias. Three of the patients who developed anti-HMW-MAA antibodies achieved a partial response. It consisted of a decrease in the size of metastatic lesions and lasted 52 weeks in 1 patient and 93 weeks in the other 2 patients. Survival of the 14 patients who developed anti-HMW-MAA antibodies was significantly (P = 0.0003) longer than that of the 9 patients without detectable humoral anti-HMW-MAA immunity development. In the multivariate analysis, such an association between development of anti-HMW-MAA antibodies and survival prolongation was still significant (P = 0.001) after adjustment for difference in performance status, the only confounding factor found to be significantly related to survival. Lastly, a significant (P = 0.03 by likelihood ratio test) interaction between anti-HMW-MAA antibodies and patients' performance status was found, since the prolongation of survival associated with anti-HMW-MAA antibodies was more marked in patients with a performance status of less than or equal to 70% than in those with a higher one. These results suggest that anti-idiotypic mAb MK2-23 may represent a useful immunogen to implement active specific immunotherapy in patients with melanoma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bumol T. F., Walker L. E., Reisfeld R. A. Biosynthetic studies of proteoglycans in human melanoma cells with a monoclonal antibody to a core glycoprotein of chondroitin sulfate proteoglycans. J Biol Chem. 1984 Oct 25;259(20):12733–12741. [PubMed] [Google Scholar]
- Chattopadhyay P., Kaveri S. V., Byars N., Starkey J., Ferrone S., Raychaudhuri S. Human high molecular weight-melanoma associated antigen mimicry by an anti-idiotypic antibody: characterization of the immunogenicity and the immune response to the mouse monoclonal antibody IMel-1. Cancer Res. 1991 Nov 15;51(22):6045–6051. [PubMed] [Google Scholar]
- Chen Z. J., Yang H., Ferrone S. Human high molecular weight melanoma-associated antigen mimicry by mouse antiidiotypic monoclonal antibody MK2-23. Characterization of the immunogenicity in syngeneic hosts. J Immunol. 1991 Aug 1;147(3):1082–1090. [PubMed] [Google Scholar]
- Chen Z. J., Yang H., Kageshita T., Ferrone S. Human high-molecular-weight melanoma-associated antigen mimicry by mouse antiidiotypic monoclonal antibody TK7-371. Cancer Res. 1991 Sep 15;51(18):4790–4797. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigues H. J., Lark M. W., Lara S., Hellström I., Hellström K. E., Wight T. N. The melanoma proteoglycan: restricted expression on microspikes, a specific microdomain of the cell surface. J Cell Biol. 1986 Nov;103(5):1699–1710. doi: 10.1083/jcb.103.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini P., Veglia F., Cordiali Fei P., Rehle T., Natali P. G., Ferrone S. Level of a membrane-bound high-molecular-weight melanoma-associated antigen and a cytoplasmic melanoma-associated antigen in surgically removed tissues and in sera from patients with melanoma. Cancer Res. 1984 Mar;44(3):1281–1287. [PubMed] [Google Scholar]
- Harper J. R., Reisfeld R. A. Inhibition of anchorage-independent growth of human melanoma cells by a monoclonal antibody to a chondroitin sulfate proteoglycan. J Natl Cancer Inst. 1983 Aug;71(2):259–263. [PubMed] [Google Scholar]
- Herlyn M., Koprowski H. Melanoma antigens: immunological and biological characterization and clinical significance. Annu Rev Immunol. 1988;6:283–308. doi: 10.1146/annurev.iy.06.040188.001435. [DOI] [PubMed] [Google Scholar]
- Imai K., Ng A. K., Glassy M. C., Ferrone S. ADCC of cultured human melanoma cells: analysis with monoclonal antibodies to human melanoma-associated antigens. Scand J Immunol. 1981 Oct;14(4):369–377. doi: 10.1111/j.1365-3083.1981.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Kageshita T., Nakamura T., Yamada M., Kuriya N., Arao T., Ferrone S. Differential expression of melanoma associated antigens in acral lentiginous melanoma and in nodular melanoma lesions. Cancer Res. 1991 Mar 15;51(6):1726–1732. [PubMed] [Google Scholar]
- Kennedy R. C., Zhou E. M., Lanford R. E., Chanh T. C., Bona C. A. Possible role of anti-idiotypic antibodies in the induction of tumor immunity. J Clin Invest. 1987 Nov;80(5):1217–1224. doi: 10.1172/JCI113195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama M., Kageshita T., Chen Z. J., Ferrone S. Characterization of syngeneic antiidiotypic monoclonal antibodies to murine anti-human high molecular weight melanoma-associated antigen monoclonal antibodies. J Immunol. 1989 Dec 1;143(11):3844–3852. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livingston P. O., Natoli E. J., Calves M. J., Stockert E., Oettgen H. F., Old L. J. Vaccines containing purified GM2 ganglioside elicit GM2 antibodies in melanoma patients. Proc Natl Acad Sci U S A. 1987 May;84(9):2911–2915. doi: 10.1073/pnas.84.9.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman A., Chen Z. J., Kageshita T., Yang H., Yamada M., Baskind P., Goldberg N., Puccio C., Ahmed T., Arlin Z. Active specific immunotherapy in patients with melanoma. A clinical trial with mouse antiidiotypic monoclonal antibodies elicited with syngeneic anti-high-molecular-weight-melanoma-associated antigen monoclonal antibodies. J Clin Invest. 1990 Dec;86(6):2136–2144. doi: 10.1172/JCI114952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Hellström K. E. Anti-idiotypic antibodies and the induction of specific tumor immunity. Cancer Metastasis Rev. 1987;6(4):489–502. doi: 10.1007/BF00047464. [DOI] [PubMed] [Google Scholar]
- Perosa F., Kageshita T., Ono R., Ferrone S. Serological methods to detect anti-idiotypic antibodies. Methods Enzymol. 1989;178:74–90. doi: 10.1016/0076-6879(89)78007-1. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C. Conservatism of the approximation sigma (O-E)2-E in the logrank test for survival data or tumor incidence data. Biometrics. 1973 Sep;29(3):579–584. [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Cheresh D. A. Human tumor antigens. Adv Immunol. 1987;40:323–377. doi: 10.1016/s0065-2776(08)60242-4. [DOI] [PubMed] [Google Scholar]
- Temponi M., Kageshita T., Perosa F., Ono R., Okada H., Ferrone S. Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: comparison with other methods of purification. Hybridoma. 1989 Feb;8(1):85–95. doi: 10.1089/hyb.1989.8.85. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Imai K., Natali P. G., Ferrone S. Distribution and molecular characterization of a cell-surface and a cytoplasmic antigen detectable in human melanoma cells with monoclonal antibodies. Int J Cancer. 1981 Sep 15;28(3):293–300. doi: 10.1002/ijc.2910280307. [DOI] [PubMed] [Google Scholar]




