Abstract
Periodontal diseases, considered as inflammatory diseases have proved to have a spectrum of systemic implications. Earliest research has associated periodontal disease with common systemic aliments such as hypertension, diabetes, osteoporosis, rheumatoid arthritis to name a few. The evolution of advanced diagnostic aids let researchers make vast inroads in linking periodontal diseases to systemic diseases like Alzheimer’s disease (AD) and even Schizophrenia. Our aim was to review and critically evaluate comprehensive literature and provide knowledge to medical practitioners on these associations so as to pave way for closer interactions between medical and dental practitioners in implementing better health care.
Electronic databases such as PubMed, Google Scholar and Cochrane databases were used as source of the data for relevant studies published from 2005 up to 2015 with the following keywords, “‘Periodontal disease”, “systemic conditions”, “periodontal disease and Alzheimer’s”, “Periodontal disease and Schizophrenia”, “Periodontal disease and Psoriasis” and “Periodontal disease and erectile dysfunction”.
The evidence presented ascertains that a reasonable and modest association does exist between Periodontal disease and Alzheimer’s, Schizophrenia, Erectile dysfunction, as well as Psoriasis and thus establishes periodontal disease as a potential risk factor.
Keywords: Alzheimer’s disease, Erectile dysfunction, Periodontal disease, Psoriasis, Systemic diseases, Schizophrenia
Introduction
Mouth is the gateway to the body but not a very pristine one. It is filled with bacteria; in fact, it is believed that there are more bacteria in the mouth than people on earth.
“Oral health” is connected to “overall health”. Extensive literature over the past decade shows that periodontal disease, considered an infectious and chronic inflammatory disease has been considered to be a potential risk in cardiovascular and respiratory diseases, diabetes, as well as has implications in adverse pregnancy outcomes, osteoporosis to name some. (1, 2)
Periodontitis being a chronic oral infection is associated with numerous bacterial species organized in biofilms posing a perpetual threat to the defense mechanisms triggering inflammatory and immune responses with release of inflammatory mediators as an elevated C-reactive protein, prostaglandin E2 (PGE2)with an increase in cytokines with pro-inflammatory action such as interleukin(IL-1β),and tumor necrosis factor-alpha(TNF-α).These responses are not limited to periodontitis but also can be observed in systemic conditions like diabetes, obesity and arthritis. (3, 4, 5)
A number of possible mechanisms in the literature have emphasized a bi-directional relationship between periodontal diseases with a host of systemic conditions ranging from cardiovascular diseases to respiratory diseases establishing a causal relationship. Among these, much credibility was awarded to the theory of three mechanisms put forward by Thoden van Velzen et al, (6) who corroborated that the systemic effects of oral diseases could be attributed to metastatic infections, spread of bacterial toxins and injury mediated by immunological means.
The strength of association was given a further impetus with Van Dyke (7) reinforcing the above mentioned concept by emphasizing that periodontal disease; an infectious disease can cause inflammation with the resultant bacteremia which has the tendency to metastasize with the production of inflammatory mediators and activation of adaptive immunity having far reaching consequences on the systemic health. But with recent studies trying to associate periodontal disease with a plethora of new systemic conditions like Alzheimer’s, Schizophrenia; there is a lacuna in establishing a link to correlate the mechanisms responsible for the pathogenesis of these diseases.
There is paucity in literature in collaborating whether any relationship exists or to consider periodontal disease a risk factor and finally to establish a causal association between periodontal disease and these novel systemic associations.
So, the present paper is intended to provide a comprehensive review of literature establishing a possible link between periodontal disease and the associated conditions and provide an insight to the medical fraternity for a timely intervention in such conditions.
Methodology
Our intended research question was “Is there any relationship between periodontal disease and systemic diseases, specifically Alzheimer’s disease, schizophrenia, psoriasis and erectile dysfunction?”
Our question utilized the keywords with MeSH relevance such as, “periodontal disease”, “systemic conditions”, “Periodontal disease and Alzheimer’s”, “Periodontal disease and schizophrenia”, “Periodontal disease and psoriasis” and “Periodontal disease and erectile dysfunction”.
Extensive literature search was performed limited to English language from the following well known data bases; PubMed, Google Scholar and Cochrane library databases along with the relevant citations in the period from 2005 to May, 2015. The search included only human studies. Considering this as non-systematic review, extensive literature has been included based from large cross-sectional, cohort as well as randomized control trials (RCT) with intervention, but a selection bias of evidence might be present.
Perio systemic associations have drawn a lot of attention from researchers with numerous studies trying to establish and validate the various implications of periodontal disease on the systemic health and vice versa. Establishing a proper definition of periodontitis helps us to consolidate the findings of these studies and provides us enough evidence to associate periodontal disease with these novel associations. Studies which closely met the clinical case definition proposed by the Center for Disease Control (CDC) in association with American Academy of Periodontology (AAP), which defines periodontitis as ≥2 interproximal sites with CAL ≥4mm (not on the same tooth) and ≥2 interproximal sites with PD ≥5mm (not on the same tooth) with radiographic evidence of alveolar bone loss were included. (8) Certain studies which did not strictly comply with the above criteria but have included surrogate measures such as probing pocket depth (PPD)as part of indices e.g. Community periodontal index of treatment needs (CPITN) to define exposure of chronic periodontitis, were still considered as there is paucity of studies considering such novel associations.
Studies which have relied exclusively on parameters such as “tooth loss” have been excluded. Though tooth loss as a consequence of periodontal disease is considered a true end point, tangible outcomes associating periodontal disease and these above mentioned novel associations when any intervention was outlined would be evident only through parameters such as CAL and PPD.
Periodontal disease and schizophrenia
Schizophrenia is a mental disorder characterized by abnormal thinking, perceptual disturbances, and diminished or exaggerated emotional expression.(9) Poor periodontal health in schizophrenic patients can be attributed to the mental condition in these patients which contributes to bad oral hygiene maintenance. Only a few studies as summarized in Table 1 have co related the role of periodontal disease in the etiopathogenesis of schizophrenia and the implications of schizophrenia on the progression of periodontal disease. (10–15)
Table 1.
Details of the related publications associating periodontal disease and schizophrenia found in databases from 2005 to 2015.
| References | Study design | Participants | Periodontal diagnosis | Outcome |
|---|---|---|---|---|
| Gurbuz O et al, 201110 | Cross-sectional | 330 patients examined, 179 were males and 151 females. | CPI (Community Periodontal Index) method. | Poor periodontal health was seen in Schizophrenia patients |
| Arnaiz et al, 201111 | Cross-sectional | 66 patients with Schizophrenia and 66 controls | CPITN(Community periodontal index of treatment needs) | Patients with Schizophrenia displayed poor oral health compared with controls |
| Kebede B et al, 201212 | Cross-sectional | 240 participants | CPI (Community Periodontal Index) method. | Oral health reflected by the CPI scores was poor in psychiatric patients |
| Eltas A et al, 201313 | Cross-sectional | 53 participants | Plaque index, probing pocket depth(PPD), clinical attachment loss(CAL) | High risk of periodontal disease among patients with schizophrenia |
| Shetty S et al, 201414 | Cross-sectional | 250, 140 men and 110 females | Gingival index, Plaque index, Probing pocket depth | Positive association between CPD and Schizophrenia |
| Morales-Chavez MC et al, 201415 | Cross-sectional | 65 psychiatric pts, 39 are Schizophrenic | Gingival Index (GI) and Ramfjord Periodontal Disease Index. | Psychiatric patients are more frequently involved with oral lesions |
Most of these studies co-relating periodontal diseases with schizophrenia are observational studies which have assessed the oral health in hospitalized patients. The strength of association would give merit to studies in patients with a better state of psychological health with a better oral hygiene care. One such study by Arnaiz et al (11) evaluated the oral health of a group of schizophrenic outpatients with a control group without psychiatric illness. The findings suggest that schizophrenic patients had a poor periodontal health as suggested by the CPITN scores compared to the controls suggesting that mental health contributing to a poor oral hygiene as well as the inflammatory process paving way for the etiopathogenesis.
Many of these studies were focused on the mental health in Schizophrenic patients contributing to poor oral hygiene, but a very few investigated the effects of the antipsychotic medication used in Schizophrenia on periodontal status. One among these was a study by Eltas et al (13) that focused on the primary question of whether the severity of periodontal disease might be attributed to the medication. The results of their study emphasized the fact that there was a very high risk of periodontal disease associated with the medication. This was indeed helpful in incorporating preventive dental health programs in tandem with the medical fraternity to address these issues.
A recent cross-sectional epidemiological study, by Shetty et al (14) tried to correlate and hypothetically put forward the missing link between periodontal disease and schizophrenia. As per their methodology, about 250 schizophrenic patients were recruited from the National Institute of Mental Health and Neural Sciences (NIMHANS), Bangalore and after assessment of the periodontal status, the results though not definitive, revealed a poor periodontal status in schizophrenic patients. The authors concluded that there might be a poor oral hygiene attributable to the mental condition in these patients but the presence of cytokine activity which is common in both the conditions might indeed prove an association. Another important pathogenic mechanism highlighted by the author is attributed to IL-1β and IL-6, the key cytokines in periodontal disease that have shown to affect the neurotransmitter mechanism. They modulate the dopaminergic metabolism and enhance dopamine survival, thereby inhibiting glutamate release eventually leading to hypo function of N-Methyl-D-Aspartate (NMDA) glutamate receptors leading to schizophrenia. (15)
One of the studies which emphasized on the likely role of periodontal disease in the etiopathogenesis of schizophrenia was a study by Fawzi et al, (16) which revealed increased salivary levels of P.gingivalis in schizophrenic patients compared to the controls and a further observation co relating the severity of the psychopathic symptoms in schizophrenia with the quantity of P.gingivalis levels.
Summating the evidence from all these studies, we proposed a hypothetic model establishing a bidirectional link between periodontal disease and schizophrenia. According to the model, inadequate plaque control due to mental illness, xerostomia resulting from the use of antipsychotic medication coupled with poor access to good dental care due to financial reasons in schizophrenic patients contributes to poor periodontal health.
Periodontal disease on the other front considered to be a chronic inflammatory disease leads to an increased levels of P.gingivalis as well as elevated cytokine levels such as interleukins which can modulate the dopaminergic metabolism leading to the development of schizophrenia. Hence this hypothetic model explains the poor periodontal health in schizophrenic patients and also elucidates the cytokine theory as a result of periodontal inflammatory burden responsible for the development of schizophrenia. (Figure 1)
Figure 1.
Hypothetic Model Depicting the Bi-Directional Link between Periodontal Disease and Schizophrenia
Periodontal disease and psoriasis
Psoriasis has been defined as a chronic dermatological disease characterized by epithelial hyperplasia presenting clinically as cutaneous erythematous papules and plaques covered by whitish scales commonly observed on the extensor-dorsal cutaneous surfaces.(17) Relevant studies correlating periodontal disease and psoriasis are summated in table 2.(18–26)
Table 2.
Details of the related publications associating periodontal disease and psoriasis found in databases from 2005 to 2015.
| References | Study design | Participants | Periodontal diagnosis | Outcome |
|---|---|---|---|---|
| Preus HR et al, 201018 | Case-control | 155 Psoriasis and 155 controls | Alveolar bone loss on bite-wing x rays | Psoriatic patients had more bone loss and loss of teeth compared to controls |
| Rahul et al, 201119 | Cross-sectional | 100 patients, 50 with CP and 50 with psoriasis | Probing pocket depth(PPD), clinical attachment loss (CAL)and alveolar bone loss | Prevalence of periodontitis is higher in psoriasis subjects as compared to controls |
| Keller JJ et al, 201220 | Cohort | 1,15, 365 patients with CP and 1,15, 365 patients without CP | Probing pocket depth and clinical attachment loss | Increased risk for psoriasis among patients with CPD |
| Nakib S et al, 2013 21 | Cohort | 60,457 women | Self-reported alveolar bone loss, loss of teeth | Periodontal bone loss may increase risk of subsequent psoriasis |
| Fadel HT et al 2013 22 | Case-control | 89 pts with psoriasis an d 54 without psoriasis | Probing pocket depth, Bleeding on probing and alveolar bone level | No difference in periodontal profiles, though psoriatic pt’s had few teeth remaining. |
| Lazaridou E et al, 2013 23 | Case-control | 100 patients with CP and 100 age and gender-matched controls | Probing pocket depth, and other indices | Periodontitis may be associated with psoriasis |
| Rasa Skudutyte et al, 2014 24 | Cross-sectional | 60 patients with psoriasis and 120 healthy controls | Probing pocket depth(PPD), clinical attachment los s(CAL)and alveolar bone loss | Periodontitis more common in moderate/ severe psoriasis compared to controls |
| Antal M et al, 2014 25 | Case-control | 82 psoriasis patients and 89 controls | Bleeding on probing, clinical attachment level and probing depth | Risk of severe periodontal disease in psoriasis was six times higher in smokers than in nonsmokers. |
| Sharma et al, 2015 26 | Case-control | 33 psoriasis patients and 35 healthy controls | Probing pocket depth(PPD), clinical attachment loss (CAL)and alveolar bone loss | Psoriasis patients had poor periodontal status compared to controls |
Preus et al, (18) in a blinded case-control study investigated the prevalence of periodontal disease in psoriasis patients compared to controls. They concluded that psoriasis patients experience more bone loss when compared to gender and age matched controls, possibly a first of its kind study investigating the co morbidity between both the diseases. In a cross-sectional study, Rahul et al (19) investigated the periodontal status in psoriasis patients and tried to prove whether any association exists between them. Within the limits of their study, results have shown a significantly higher gingival, plaque index scores with an increased PPD and tooth loss compared to controls. Furthermore, their microbiological analysis showed significantly greater number of A.actinomycetemcomitans and P.gingivalis positive samples. The investigators have postulated that the association between periodontal disease and psoriasis is indeed valid.
There have been a few studies in literature which have correlated the periodontal status as an independent risk factor for increased incidence of psoriasis, but the main drawback of these studies was the presence of confounding factors. (20, 21)
To establish the strength of association with simultaneous adjustment of confounding factors, Rasa Skudutyte et al, (24) in a cross-sectional study compared the prevalence of periodontitis and bone loss in psoriatic patients to controls in a Scandinavian population. Their findings suggest that psoriatic patients had higher plaque scores, bleeding on probing and a higher prevalence of moderate to severe chronic periodontitis compared to controls. The strength of the evidence associating periodontal disease and psoriasis was given the much needed impetus by Sharma et al(26) in a study which was designed to find how frequent the association between both periodontitis and psoriasis was. The results of their study exhibited poor periodontal status in psoriasis patients compared with the controls. One unique observation of their study was periodontal status of these patients corroborated with the severity of psoriasis.
Another potential mechanism explaining the pathogenesis involves the role of gram negative periodontopathic bacteria. They may either stimulate the psoriasis pathway directly or through a systemic inflammatory response which may involve the pro-inflammatory cytokine IL-17. This is supported by the high presence of IL-17 in both psoriatic and periodontitis patients. (27, 28)
A study by Ishihara et al (29) revealed that IgG levels against bacterial heat shock proteins (HSP) was higher in psoriasis patients, suggesting that some HSP found in oral bacteria may induce some of the causative factors of Psoriasis suggesting the potential role of the periodontal bacterial burden contributing to the pathogenesis in psoriasis patients.
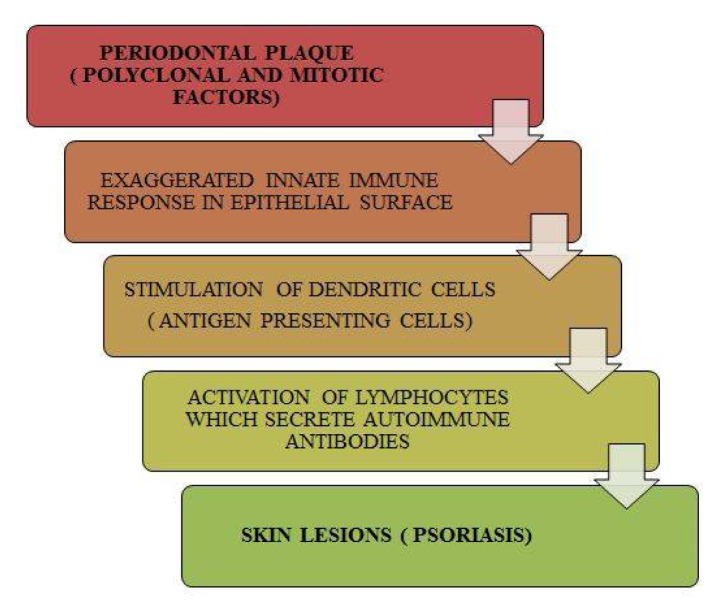
The possible hypothetic model explaining the common pathogenesis shared between periodontal disease and psoriasis can be summarized from previous studies which have highlighted role of the innate immune response being the common target in both these diseases. This has been aptly proved by an exaggerated immune response by dendritic cells (DCs) to microbial complexes in both periodontitis and psoriasis and an up regulation of toll like receptors, TLR-2 which play a crucial role in activation of T cells. (30–33) It has been proven by earlier case reports that any bursts and remissions of periodontal disease also correlate with exaggerations and remissions of psoriatic episodes, strengthening the evidence that periodontal disease can indeed act as a risk factor to psoriasis and our hypothetic model illustrates the same.(34) (Figure 2)
Figure 2.
Hypothetic Model Explaining the Role of Periodontal Disease Leading To Psoriasis
Periodontal disease and erectile dysfunction (ED)
Sexual dysfunction among males can be attributed to erectile dysfunction(ED). National institute of health has defined erectile dysfunction as the inability to attain or maintain an erection sufficient for satisfactory sexual performance. (35)
Only a handful of studies on ED have tried to establish a meaningful relationship between periodontal disease and these have been summarized in table 3. (36–42)
Table 3.
Details of the related publications associating periodontal disease and erectile dysfunction found in databases from 2005 to 2015.
| References | Study design | Participants | Periodontal diagnosis | Outcome |
|---|---|---|---|---|
| Zadik Y et al, 200936 | Cross – sectional | 305 men | Alveolar bone loss of ≥ 6 mm | ED associated with CPD |
| Sharma A et al, 201137 | Cross – sectional | 70 men | Probing pocket depth(PPD) ≥5 mm and attachment loss ≥3mm at >30% sites with radiographic evidence of bone loss | An association exists between ED and CPD, though not of statistical significance |
| Keller JJ et al, 201238 | Case – control | 32,856 patients with ED and 162,480 patients as controls | Periodontal diagnosis was based on PPD and CAL | Association exists between ED and previously diagnosed CPD |
| Eltas A et al, 201339 | Randomized control trial | 120 men with ED, 60 with Perio treatment and 60 without any treatment | Periodontal diagnosis based on plaque index, BOP, PPD and CAL | Periodontal treatment provides additional benefits in improvement of ED |
| Oguz F et al, 201340 | Randomized control trial | 80 men with ED and 82 men without ED | Periodontal diagnosis based on plaque index, BOP, PPD and CAL | CPD had a high association with ED in young adults at 30–40 years. |
| Matsumoto S et al, 201441 | Cross – sectional | 300 men | Periodontal diagnosis based on plaque index, BOP, PD and CAL | There was an association between ED and CPD |
| Uppal RS et al, 201442 | Cross – sectional | 53 men | PPD >5 mm and radiographic evidence of bone loss | ED and CPD are associated with each other |
One among these studies, Sharma et al (37) evaluated the relationship between chronic periodontitis and ED. According to their methodology, a total of 70 patients with vasculogenic ED were assessed to determine their periodontal status and were subjected for colored penile Doppler ultrasound. They concluded that patients with severe vasculogenic ED had the highest prevalence for chronic periodontitis. Though there was no statistical significance, the association between both these conditions was found to be positive. In a case-control study Keller et al (38) analyzed data from Taiwan’s National Health Insurance Research Database (NHIRD) and after adjusting other confounding factors, it was found that patients with ED had poor periodontal status compared to their respective controls. They concluded that there was indeed a significant association between ED and periodontal disease.
The strength of association would give credibility to evidence available as RCT with an intervention; one such study by Eltas et al (39) evaluated the benefits of periodontal treatment on ED. The study population comprised of ED patients who received periodontal treatment and were compared with patients who were not entitled with any treatment. The results revealed that intervention by periodontal treatment was certainly helpful in the improvement of ED.
In a RCT, Oguz et al (40) compared a study group comprised of patients with ED and controls. The periodontal status represented by PPD and CAL was extremely poor in the ED group compared with the controls. They concluded that chronic periodontitis had a positive association with ED in young patients and could be a risk factor in the development of ED in such patients.
Evidence is also available from numerous other studies which have considered population based data, interview based surveys and random inclusion of patients from outpatient departments of hospitals. These studies too associate periodontal disease and ED. (41, 42)
Strength of evidence supporting the role of periodontal disease is based on the results of Mattila and Ross,(43,44) giving credibility to the theory that periodontal pathogens enter the blood stream and travel to distant sites where they stimulate release of pro inflammatory cytokines like IL-1β and IL-6 as well as acute phase proteins leading to endothelial damage causing ED.
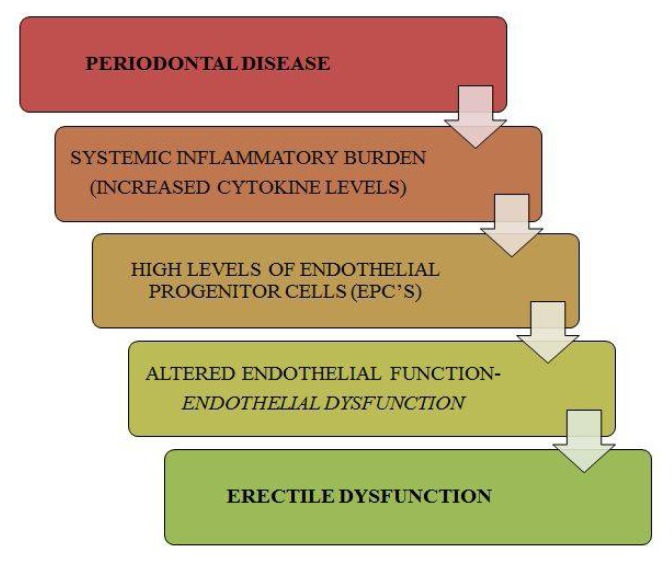
One more mechanism suggests that ED caused by systemic inflammation induced by periodontal disease might affect small vessels first, such as the penile vasculature, and later the coronary arteries. It gives strength to the concept that periodontitis might lead to ED in young males first, and later with coronary artery disease in middle-aged males. (45,46) A model was proposed linking periodontal disease with ED. As literature suggests, chronic periodontal disease contributes to an increased systemic inflammatory burden reflected by an increase in endothelial progenitor cells (EPC) which eventually cause endothelial dysfunction, a major cause of ED. It was established that successful treatment of periodontal disease decreases the count of EPC, thus establishing the fact that periodontal disease has an overt control on the functional integrity of the endothelium, disruption of which can lead to ED. To add strength to the possible correlation, it was found that endothelial dysfunction triggered by periodontal disease can cause atherosclerotic occlusion of the small penile arteries leading to the development of vasculogenic ED. (37, 43) (Figure 3)
Figure 3.
Hypothetic Model Depicting Role of Periodontal Disease in Erectile Dysfunction
Periodontal disease and Alzheimer’s disease (AD)
Alzheimer’s disease (AD), is defined as a progressive neurodegenerative disorder characterized by loss of memory and cognition, declining ability to perform activities of daily living (ADL), changes in personality and behavior, and increased use of health care resources and medical services. (47)
The spirit of association between periodontal disease and Alzheimer’s went to such bizarre heights as to lead to one reproach ‘Brush your teeth to prevent Alzheimer’s!’ Many epidemiological studies have outlined inflammatory markers such as IL-1, IL-6 and TNF-α of periodontal disease associated with cognitive decline leading to AD, these studies have relied on ‘tooth loss’ as a measure negating a cause-effect association or to consider periodontal disease as a potential risk factor in the pathogenesis of AD. (48–50) Only a handful of studies fulfilling the criteria for the threshold of periodontitis as listed in table 4 were successful in associating periodontal disease with Alzheimer’s. (51–55)
Table 4.
Details of the related publications associating periodontal disease and Alzheimer’s disease found in databases from 2005 to 2015.
| References | Study design | Participants | Periodontal diagnosis | Outcome |
|---|---|---|---|---|
| Farhad SZ et al, 2014 51 | Case-control | 80 patients, 40 with CPD and 40 without CPD | Clinical attachment loss(CAL) and probing pocket depth(PPD) | Positive association of CPD with Alzheimer’s |
| Martande SS et al, 2014 52 | Case-control | 58 individuals with AD and 60 cognitively normal adults | Gingival index (GI), plaque index (PI), probing pocket depth (PPD), clinical attachment level (CAL), and bleeding on probing(BOP) | Periodontal parameters were higher in individuals with AD and the periodontal health status deteriorates with progression of AD |
| Rolim TS et al, 2014 53 | Longitudinal intervention study | 29 patients with mild AD | Gingival index (GI), plaque index (PI), probing pocket depth (PPD), clinical attachment level (CAL) | Periodontal treatment contributed to reduced comorbidities associated with AD |
| Noble JM et al, 2014 54 | Case-cohort | 219 subjects (110 incident AD cases and 109 controls | Probing depth (PD), clinical attachment level (CAL), and alveolar bone level | Increased Serum IgG levels to periodontal micro biota are associated with risk for developing incident AD. |
| Kamer et al, 2015 55 | Cross-sectional study | 38 cognitively normal adults | Probing pocket depth (PPD), clinical attachment level (CAL) | Periodontal disease results in increased brain Aβ accumulations, leading to AD |
In a case-control study, Farhad et al (51) assessed the risk of chronic periodontitis on AD through evaluation of levels of serum TNF-α, considered a pivotal factor for the development of AD. Their results revealed a threefold increase in TNF-α level in patients with chronic periodontitis suffering from AD compared to patients having AD with a healthy periodontium. Hence, it was evident that elevated levels of TNF-α as a result of chronic periodontitis would undeniably contribute to extensive neuronal degeneration in AD.
In a unique case-cohort design, Noble et al (54) compared individuals with incident probable Alzheimer disease with controls and evaluated the serum IgG levels to periodontal bacteria among them. They concluded that patients with high serum A.naeslundii IgG antibody were at increased risk of developing incident AD. Their study was the first of its kind utilizing serological markers of periodontal disease to identify patients at risk of incident AD.
The strength of association was given a much needed impetus with advances in medical technology, as proved by Kameret al (55) in a cross-sectional study, which is the first of its kind to have co related periodontal disease and the relative deposition of brain amyloid in normal elderly subjects. After adjusting all confounding variables, they concluded that periodontal inflammatory burden increases brain amyloid deposition in areas more prone to amyloid deposition and a leading factor in development of AD.
Other mechanism that reflects the pathogenicity of periodontal disease in Alzheimer’s is the role of TNF-α. TNF-α is considered to be the most vital inflammatory cytokine responsible for regulating the cellular cascade of events in neuroinflammatory response. TNF-α exerts its influence by exacerbating gliosis, demyelination, inflammation, blood-brain-barrier deterioration and eventually leading to cell death. Thus, TNF α, an important mediator of periodontal disease plays a crucial role in the neurodegenerative disease process. (56–58)
It was also found in a study by Rai et al (52) that certain pro-inflammatory markers like MMP-8, MMP-9 and CRP were elevated in both subjects with periodontitis and dementia compared to healthy controls. These elevated marker levels lead to increase in neurodegenerative process. (58)
The collective evidence from all these studies was summed up and we proposed a hypothetical model for periodontal disease induced progression of AD.
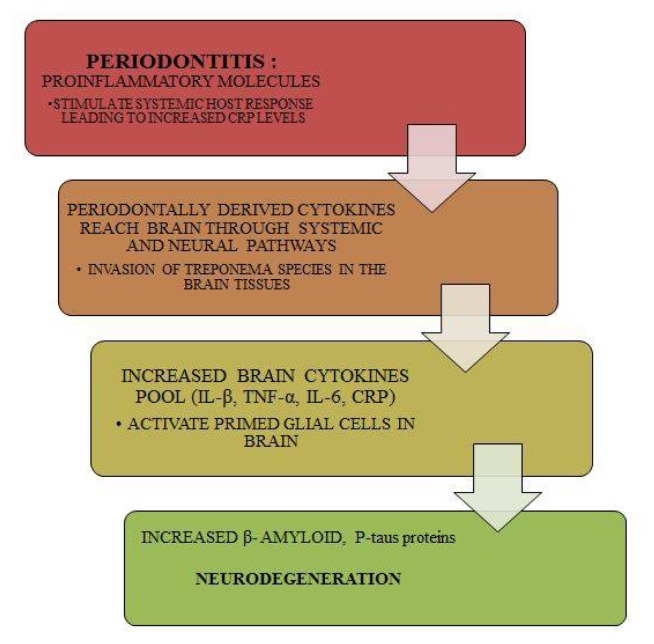
As per our model, studies in literature have proved periodontal bacteria can affect the onset and progression of AD by direct invasion of the brain tissue as demonstrated by the capabilities of T pallidum. Since T denticola belongs to the same class and evidence says that Treponema species have been found in the trigeminal ganglia, brain stem and the cortex of human brain and are found in more numbers in AD patients suggesting that they can colonize and initiate an inflammatory response. (59, 60) Apart from this, these bacteria through an indirect action can initiate the progression of AD by release of pro inflammatory cytokines and inflammatory proteins such as CRP and molecules like interleukins-1 and 6 as well as TNF-α, which could reach the brain and will lead to activation of primed glial cells leading to increased production of β-amyloidand P-taus proteins resulting in neurodegeneration and development of AD. (Figure 4)
Figure 4.
Hypothetical Model Depicting the Role of Periodontal Disease in the Progression of Alzheimer’s Disease
Limitations of the review
Most of the studies taken as a part of the review were included based on the strength of the evidence. Considering these as novel associations, the maximum strength of the evidence available in the literature were the cohort and case-control study designs. There were neither systematic reviews nor Meta-analysis to associate periodontal disease with any of these novel associations, considered as a drawback when a causal association has to be established.
The other limitation of the review is the threshold for periodontitis. Considering that most of these studies were not interventional studies, it was indeed challenging to scrutinize and include studies which have outlined adequate threshold criteria satisfying chronic periodontitis. Hence only a few studies which were closely adept to the criteria summarized by the CDC in association with American Association of Periodontology (AAP) were included as part of this review. Considering the paucity of studies, self-reported alveolar bone loss and radiographic evidence of bone loss alone too have been considered, indeed a limitation.
Implications for current clinical practitioners
Most of the medical practitioners are aware of the implications of periodontal disease on cardiovascular health and diabetes since these conditions are predominant and have well documented literature, but are unaware of the consequences of periodontal disease on other systemic conditions. However, considering the pathogenic potential of periodontal disease on Alzheimers, Schizophrenia, Psoriasis and ED highlighted by the present paper, medical practitioners can take a clue and provide proper education and guidance in collaboration with the dentists as part of integrated teaching to contribute to oral health and eventually for the overall health of the patients.
Conclusion
Numerous studies have evinced interest in establishment of a relation between periodontal disease and systemic health concerns such as Alzheimer’s, schizophrenia, psoriasis and erectile dysfunction. Though it was a complex task considering the threshold for periodontitis and other confounding variables in these studies, evidence presents a modest association indeed exists to consider periodontal disease a risk factor for these diseases.
The conclusive evidence along with the hypothetic models showcase the etiopathogentic role of periodontal disease in these systemic diseases and conditions enlightening and paving way for the dental and medical practitioners to work hand in hand to diagnose and treat the patients in establishing a good oral and general health.
References
- 1.Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Periodontol. 2013;84(4):S8–S19. doi: 10.1902/jop.2013.1340010. [DOI] [PubMed] [Google Scholar]
- 2.Arigbede AO, Babatope BO, Bamidele MK. Periodontitis and systemic diseases: A literature review. J Indian SocPeriodontol. 2012;16(4):487–491. doi: 10.4103/0972-124X.106878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11):2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 4.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 5.Koppolu P, Durvasula S, Palaparthy R, Rao M, Sagar V, Reddy SK, et al. Estimate of CRP and TNF-alpha level before and after periodontal therapy in cardiovascular disease patients. Pan Afr Med J. 2013;15:92. doi: 10.11604/pamj.2013.15.92.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thoden van Velzen SK, Abraham-Inpijn L, Moorer WR. Plaque and systemic disease: a reappraisal of the focal infection concept. J ClinPeriodontol. 1984;11(4):209–220. doi: 10.1111/j.1600-051x.1984.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Dyke TE, van Winkelhoff AJ. Infection and inflammatory mechanisms. J Periodontol. 2013;84(4):S1–S7. doi: 10.1902/jop.2013.1340018. [DOI] [PubMed] [Google Scholar]
- 8.Page RC, Eke PI. Case definitions for use in population based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 9.Fleischhacker WW, Arango C, Arteel P, Barnes TR, Carpenter W, Duckworth C, et al. Schizophrenia-Time to Commit to Policy Change. Schizophr Bull. 2014;40(3):S165–S194. doi: 10.1093/schbul/sbu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurbuz O, Alatas G, Kurt E, Dogan F, Issever H. Periodontal health and treatment needs among hospitalized chronic psychiatric patients in Istanbul, Turkey. Community Dent Health. 2011;28(1):69–74. [PubMed] [Google Scholar]
- 11.Arnaiz A, Zumarraga M, Diez-Altuna I, Uriarte JJ, Moro J, Pérez-Ansorena MA. Oral health and the symptoms of schizophrenia. Psychiatry Research. 2011;188(1):24–28. doi: 10.1016/j.psychres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Kebede B, Kemal T, Abera S. Oral health status of patients with mental disorders in Southwest Ethiopia. PLoS One. 2012;7(6):e39142. doi: 10.1371/journal.pone.0039142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eltas A, Kartalci S, Eltas SD, Dündar S, Uslu MO. An assessment of periodontal health in patients with schizophrenia and taking antipsychotic medication. Int J Dent Hyg. 2013;11(2):78–83. doi: 10.1111/j.1601-5037.2012.00558.x. [DOI] [PubMed] [Google Scholar]
- 14.Shetty S, Bose A. Schizophrenia and periodontal disease: An oro-neural connection? A cross-sectional epidemiological study. J Indian SocPeriodontol. 2014;18(1):69–73. doi: 10.4103/0972-124X.128222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales-Chavez MC, Rueda-Delgado YM, Peña-Orozco DA. Prevalence of buccodental pathologies in patients with psychiatric disorders. J ClinExp Dent. 2014;6(1):e7–e11. doi: 10.4317/jced.51147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fawzi MM, El-Amin HM, Elafandy MH. Detection and quantification of Porphyromonasgingivalis from saliva of schizophrenia patients by culture and Taqman Real-Time PCR: A Pilot Study. Life Sci J. 2011;8:65–74. [Google Scholar]
- 17.Brice DM, Danesh-Meyer MJ. Oral lesions in patients with psoriasis: clinical presentation and management. J Periodontol. 2000;71:1896–903. doi: 10.1902/jop.2000.71.12.1896. [DOI] [PubMed] [Google Scholar]
- 18.Preus HR, Khanifam P, Kolltveit K, Mørk C, Gjermo P. Periodontitis in psoriasis patients: a blinded, case-controlled study. ActaOdontolScand. 2010;68(3):165–70. doi: 10.3109/00016350903583678. [DOI] [PubMed] [Google Scholar]
- 19.Rahul K, Pradeep AR. Chronic Plaque Psoriasis and Plaque-induced Chronic Periodontitis; Is There Any Association: A Cross-sectional Study. J Periodontol Implant Dent. 2011;3(1):13–20. [Google Scholar]
- 20.Keller JJ, Lin HC. The effects of chronic periodontitis and its treatment on the subsequent risk of psoriasis. Br J Dermatol. 2012;167(6):1338–44. doi: 10.1111/j.1365-2133.2012.11126.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakib S, Han J, Li T, Joshipura K, Qureshi AA. Periodontal disease and risk of psoriasis among nurses in the United States. ActaOdontolScand. 2013;71(6):1423–1429. doi: 10.3109/00016357.2013.766360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fadel HT, Flytstrom I, Calander AM, Bergbrant IM, Heijl L, Birkhed D. Profiles of dental caries and periodontal disease in individuals with or without psoriasis. J Periodontol. 2013;84(4):477–485. doi: 10.1902/jop.2012.120119. [DOI] [PubMed] [Google Scholar]
- 23.Lazaridou E, Tsikrikoni A, Fotiadou C, Kyrmanidou E, Vakirlis E, Giannopoulou C, et al. Association of chronic plaque psoriasis and severe periodontitis: a hospital based case control study. EurAcadDermatolVenereol. 2013;27(8):967–972. doi: 10.1111/j.1468-3083.2012.04615.x. [DOI] [PubMed] [Google Scholar]
- 24.Rysstad RS, Slevolden EM, Hansen BF, Sandvik L, Preus HR. Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 2014;14:139. doi: 10.1186/1472-6831-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antal M, Braunitzer G, Mattheos N, Gyulai R, Nagy K. Smoking as a Permissive Factor of periodontal disease in psoriasis. PLoS ONE. 2014;9(3):e92333. doi: 10.1371/journal.pone.0092333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A, Raman A, Pradeep AR. Association of chronic periodontitis and psoriasis: periodontal status with severity of psoriasis. Oral Dis. 2015;21(3):31–49. doi: 10.1111/odi.12271. [DOI] [PubMed] [Google Scholar]
- 27.Ortega C, Fernandez-A S, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J LeukocBiol. 2009;86:435–43. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]
- 28.Behfarnia P, Birang R, Andalib AR, Asadi S. Comparative Evaluation of IFNγ, IL4 and IL17 Cytokines in Healthy Gingiva and Moderate to Advanced Chronic Periodontitis. Dent Res J (Isfahan) 2010;7:45–50. [PMC free article] [PubMed] [Google Scholar]
- 29.Ishihara K, Ando T, Kosugi M, Kato T, Morimoto M, Yamane G, et al. Relationships between the onset of pustulosispalmaris et plantaris, periodontitis and bacterial heat shock proteins. Oral MicrobiolImmunol. 2000;15:232–37. doi: 10.1034/j.1399-302x.2000.150404.x. [DOI] [PubMed] [Google Scholar]
- 30.Sabat R, Philipp S, Hoflich C, Kreutzer S, Wallace E, Asadullah K, et al. Immunopathogenesis of psoriasis. ExpDermatol. 2007;16(10):779–98. doi: 10.1111/j.1600-0625.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 31.Cutler CW, Jotwani R. Dendritic cells at the oral mucosal interface. J Dent Res. 2006;85:678–89. doi: 10.1177/154405910608500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonasgingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 33.Hurst J, Von Landenberg P. Toll-like receptors and autoimmunity. Autoimmune Rev. 2008;7:204–08. doi: 10.1016/j.autrev.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Yamada J, Amar S, Petrungaro P. Psoriasis associated periodontitis: A Case Report. J Periodontol. 1992;63:854–57. doi: 10.1902/jop.1992.63.10.854. [DOI] [PubMed] [Google Scholar]
- 35.NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 36.Zadik Y, Bechor R, Galor S, Justo D, Heruti RJ. Erectile dysfunction might be associated with chronic periodontal disease: Two ends of the cardiovascular spectrum. J Sex Med. 2009;6:1111–16. doi: 10.1111/j.1743-6109.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- 37.Sharma A, Pradeep AR, Raju PA. Association between chronic periodontitis and vasculogenic erectile dysfunction. J Periodontol. 2011;82:1665–69. doi: 10.1902/jop.2011.110049. [DOI] [PubMed] [Google Scholar]
- 38.Keller JJ, Chung S-D, Lin H-C. A nationwide population-based study on the association between chronic periodontitis and erectile dysfunction. J ClinPeriodontol. 2012;39:507–12. doi: 10.1111/j.1600-051X.2012.01879.x. [DOI] [PubMed] [Google Scholar]
- 39.Eltas A, Oguz F, Uslu MO, Akdemir E. The effect of periodontal treatment in improving erectile dysfunction: a randomized controlled trial. J ClinPeriodontol. 2013;40(2):148–54. doi: 10.1111/jcpe.12039. [DOI] [PubMed] [Google Scholar]
- 40.Oguz F, Eltas A, Beytur A, Akdemir E, Uslu MO, Günes A. Is there a relationship between chronic periodontitis and erectile dysfunction? J Sex Med. 2013;10:838–43. doi: 10.1111/j.1743-6109.2012.02974.x. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto S, Matsuda M, Takekawa M, Okada M, Hashizume K, Wada N, et al. Association of ED with chronic periodontal disease. Int J Impot Res. 2014;26(1):13–15. doi: 10.1038/ijir.2013.30. [DOI] [PubMed] [Google Scholar]
- 42.Uppal RS, Bhandari R, Singh K. Association between erectile dysfunction and chronic periodontitis: a clinical study. Indian J Dent Res. 2014;25(4):430–33. doi: 10.4103/0970-9290.142516. [DOI] [PubMed] [Google Scholar]
- 43.Mattila KJ, Valle MS, Nieminen MS, Valtonen VV, Hietaniemi KL. Dental infections and coronary atherosclerosis. Atherosclerosis. 1993;103:205–11. doi: 10.1016/0021-9150(93)90263-t. [DOI] [PubMed] [Google Scholar]
- 44.Ross R. Atherosclerosis - An inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 45.Zadik Y, Bechor R, Galor S, Justo D, Heruti RJ. Erectile dysfunction might be associated with chronic periodontal disease: Two ends of the cardiovascular spectrum. J Sex Med. 2009;6:1111–16. doi: 10.1111/j.1743-6109.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Tse HF, Yiu KH, Jia N, Chen H, SheungWai Li L, et al. Increased levels of circulating endothelial progenitor cells in subjects with moderate to severe chronic periodontitis. J ClinPeriodontol. 2009;36:933–39. doi: 10.1111/j.1600-051X.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin T, Feldman H, Fillit H, Sano M, Schmitt F, Aisen P. Dependence as a unifying construct in defining Alzheimer’s disease severity. Alzheimers Dement. 2010;6:482–89. doi: 10.1016/j.jalz.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein PS, Desrosier M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun Study. J Am Dent Assoc. 2007;138:1314–22. doi: 10.14219/jada.archive.2007.0046. [DOI] [PubMed] [Google Scholar]
- 49.Stein PS, Scheff S, Dawson DR. Alzheimer’s disease and periodontal disease: Mechanisms underlying a potential bi-directional relationship. Grand Rounds in Oral Systemic Medicine. 2006;3:14–24. [Google Scholar]
- 50.Gatz M, Mortimer JA, Fratiglioni L. Potentially modifiable risk factors for dementia in identical twins. Alzheimer Dement. 2006;2:110–17. doi: 10.1016/j.jalz.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Farhad SZ, Amini S, Khalilian A. The effect of chronic periodontitis on serum levels of tumor necrosis factor-alpha in Alzheimer disease. Dent Res J (Isfahan) 2014;11(5):549–52. [PMC free article] [PubMed] [Google Scholar]
- 52.Martande SS, Pradeep AR, Singh SP, Kumari M, Suke DK, Raju AP, et al. Periodontal health condition in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2014;29(6):498–02. doi: 10.1177/1533317514549650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.RolimTde S, Fabri GM, Nitrini R. Evaluation of patients with Alzheimer’s disease before and after dental treatment. ArqNeuropsiquiatr. 2014;72(12):919–24. doi: 10.1590/0004-282X20140140. [DOI] [PubMed] [Google Scholar]
- 54.Noble JM, Scarmeas N, Celenti RS, Elkind MSV, Wright CB, Schupf N, et al. Serum IgG Antibody Levels to Periodontal Microbiota Are Associated with Incident Alzheimer Disease. In: Amar S, editor. PLoS ONE. 12. Vol. 9. 2014. p. e114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamer AR, Pirragliad E, Tsui W, Rusinek H, Vallabhajosula S, Mosconi L, et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol Aging. 2015;36(2):627–33. doi: 10.1016/j.neurobiolaging.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal. 2010;22:977–83. doi: 10.1016/j.cellsig.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J NeuroimmunePharmacol. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 58.Rai B, Kaur J, Anand SC. Possible relationship between periodontitis and dementia in a North Indian old age population: a pilot study. Gerodontology. 2012;29:e200–5. doi: 10.1111/j.1741-2358.2010.00441.x. [DOI] [PubMed] [Google Scholar]
- 59.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dementia. 2008;4:242–50. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral MicrobiolImmunol. 2002;17:113–18. doi: 10.1046/j.0902-0055.2001.00100.x. [DOI] [PubMed] [Google Scholar]