Abstract
Opioids are traditionally associated with pain, analgesia and drug abuse. It is now clear, however, that the opioids are central players in mood. The implications for mood disorders, particularly clinical depression, suggest a paradigm shift from the monoamine neurotransmitters to the opioids either alone or in interaction with monoamine neurons. We have a special interest in dynorphin, the last of the major endogenous opioids to be isolated and identified. Dynorphin is derived from the Greek word for power, dynamis, which hints at the expectation that the neuropeptide held for its discoverers. Yet, dynorphin and its opioid receptor subtype, kappa, has always taken a backseat to the endogenous b-endorphin and the exogenous morphine that both bind the mu opioid receptor subtype. That may be changing as the dynorphin/ kappa system has been shown to have different, often opposite, neurophysiological and behavioral influences. This includes major depressive disorder (MDD). Here, we have undertaken a review of dynorphin/ kappa neurobiology as related to behaviors, especially MDD. Highlights include the unique features of dynorphin and kappa receptors and the special relation of a plant-based agonist of the kappa receptor salvinorin A. In addition to acting as a kappa opioid agonist, we conclude that salvinorin A has a complex pharmacologic profile, with potential additional mechanisms of action. Its unique neurophysiological effects make Salvinorina A an ideal candidate for MDD treatment research.
Keywords: Agonists, animal models, anhedonia, antagonists, clinical depression, CRH, dopamine, dynorphin, kappa, neuropeptides, stress
INTRODUCTION
The most debilitating of the mood disorders is major depressive disorder (MDD). The clinical diagnosis of depression includes a collection of symptoms such as loss or gain of body weight, insomnia or excessive sleep, loss of energy, feelings of worthlessness or guilt, diminished cognitive function and suicidal thoughts.
For decades the monoamine neurotransmitters were at the center of MDD preclinical and clinical research. The emphasis was justified because drugs that enhanced activities of serotonin, norepinephrine and, to a lesser extent, dopamine relieved depressive-like behaviors in animal models and symptoms of patients. Nonetheless, it became clear that the typical and atypical anti-depressant drug therapies were relatively ineffective with a significant number of people diagnosed with clinical depression or related mood disorders. These failures have initiated the search for other possible mechanisms and therapies. The opioids have become a focus of that search in recent years.
Interest in the opioids was peaked by findings of differences in features of endogenous opioids between depressed and healthy people, and in anti-depressive effects of exogenous opioid drugs in animal models. Endogenous opioids and their receptors were discovered in brain regions richly supplied by monoamine neurons. These regions were related to the expression of mood and cemented the need for further research on opioid – MDD relations.
Of the three opioid receptor systems, much of the initial work targeted the mu (µ) receptor, or µ opioid peptide (MOP), system receptor nomenclature follows [1]. This is the receptor for the most studied endogenous and exogenous opioid substances, β-endorphin and morphine, respectively [2, 3]. Consideration of the delta (δ) receptor (DOP) and kappa (κ) opioid receptor (KOP) systems being involved in clinical depression is more recent [4, 5]. To note, there is another receptor that is sometimes included in discussions of the opioids. The homologous nociceptin, also known as orphanin FQ, receptor is a non-opioid receptor. Its role in opioid neurobiology remains unclear [6, 7].
A literature has emerged to make a case that κ agonists induce dysphoria in humans [8] and depressive-like behaviors in animal models. One result has been intense discussion for the potential of κ antagonists as novel anti-depressant medications. Our initial foray into this research area, however, was as a contrarian.
We reported [9] that salvinorin A (Salv A), a plant-derived compound, alleviated depressive-like symptoms in an animal model. Salv A is purported to be a highly selective opioid agonist of the KOP [10]. Rather than dwelling on therapeutic value of Salv A, our lab has turned our attention to the KOP and its endogenous ligand, dynorphin.
Here, we will review briefly the involvement of the KOP and dynorphin in subcortical and cortical brain regions responsible for psychopathology, including the symptoms of MDD. The inescapable conclusion will be the involvement of Dynorphin/ KOP activation in depression and other related mood disorders [11].
The final section will raise questions about whether Salv A follows the same principles as dynorphin and other synthetic agents serving as κ agonists and antagonists. We will make a case for Salv A as an atypical κ agonist with complex neurophysiological and behavioral influences. The complexity of Salv A suggests it could possess anti-depressive properties under some conditions. Salv A, we believe, deserves a second look as a treatment for depression.
NEUROBIOLOGY OF KOP
Introductory descriptions of the opioids in the central nervous system (CNS) often leave the impression that the three opioids systems are functionally similar [12]. More nuanced descriptions reveal significant differences among MOP, DOP and KOP.
Receptors for the three opioid systems are distributed in the brain differently [13]. The results are differential influences on neurophysiology and behavior. Activation of the KOP system is often found to have CNS effects that are opposite to those of DOP and, especially, MOP systems [14]. As an example, KOP activation is reported to decrease dopamine (DA) release in structures of the mesolimbic pathway while endorphin and MOP activation increase DA release in those same structures [15]. Opposite effects for KOP and MOP also have been reported in animal models for feeding, drinking, aggressive behaviors and drug seeking, as well as on seizures and body temperature [16].
The range of influences exerted by the KOP system is impressive and have been widely observed in animal models [17]. The KOP is involved in pain by its peripheral anti-nociception and centrally mediated analgesia [18-21]. The KOP regulates neuronal excitability broadly in the brain to influence normal learning, cognition, motor function, endocrine release and the experience of reward [22-24]. In some situations, KOP activation can be neuroprotective and act as anti- itch and anti-inflammatory agents [25, 26]. The KOP system also has been implicated in psychopathologies of anxiety, schizophrenia, stress and drug abuse in animal models [27-29]. Of particular relevance is involvement of KOP in MDD in humans [11].
Dynorphin and Kappa
The KOP system includes dynorphin and its κ opioid receptor. As a result, we will hereafter use the designation Dyn/KOP for the system unless specifically referring to dynorphin vis a vis the κ receptor.
Dynorphin is the term for several opioid peptides cleaved from the large prodynorphin (PDyn) precursor. The result of cleavage is dynorphin A (Dyn A) and dynorphin B (Dyn B). The aptly named big dynorphin is a precursor peptide cleaved from PDyn consisting of both Dyn A and Dyn B [23, 30]. Most Dyn A activity is accounted by its derivative, Dyn A1-13. Other Dyn A derivatives include Dyn A1-11 and Dyn A1-17, which yields Dyn A1-8 [4]. All bind the κ opioid receptor [31].
Dynorphins are released in the periphery and CNS in response to physical pain [32]. But, in animal models, dynorphins are also released in stressful situations, in situations perceived as unpleasant [33], and in response to administration of drugs of abuse [22]. Dyn is released by“dual mechanisms.” The first mechanism consists of the typical axonal release, and the second of somatodendritic release. The former allows Dyn to bind axonal autoreceptors or post-synaptic dendritic receptors. Somatodendric release of Dyn can bind pre-synaptic receptors as well as axonal receptors. Dual release enhances the plasticity and complexity of Dyn outcomes [34].
Similarly to other opioid receptors, κ is a G-protein coupled metabotropic receptor. Opioid receptors belong to the same receptor superfamily as DA, serotonin and glutamate that bind ligands and initiate signal transduction through the activation of G-proteins [35]. Nonetheless, opioids are neuropeptides that have properties unlike neurotransmitters. Whereas neurotransmitters undergo reuptake into the presynaptic neuron, neuropeptides are degraded after release into the synapse. Therefore, opioids must be constantly replenished to the releasing neuron.
Also, opioids interact with the classical neurotransmitters in unique ways. Binding of pre-synaptic κ modulates monoamine and glutamate neurotransmission [36]. For example, activation of the DYN/ KOP decreases DA levels in the nucleus accumbens (NAcc) and suppresses serotonin (5-HT) firing rates from the raphe nucleus (raphe n.) [37]. Binding of post-synaptic κ typically hyperpolarizes the cell membrane, thus inhibiting neuronal excitation [38]. Likely mechanism for neuronal excitation include inhibition of adenylyl cyclases, suppression of calcium currents, elevated intracellular calcium levels, opening of potassium channels and allowing potassium to leave the cell [39] and, ultimately, the regulation of the mitogen-activated protein (MAP) kinase second messenger cascade [4].
Distribution of Kappa and Dynorphin
The DYN/ KOP system is widely expressed in the mammalian brain on numerous neuronal cell types. Both Dyn-containing neurons and κ receptors, determined most often by RNA expression, are distributed in many brain regions [13, 30, 35]. However, both Dyn and κ receptors are not necessarily expressed together in the same brain regions. Distribution estimates highlight an oddity of the DYN/ KOP system. There is a mismatch between Dyn A distribution and κ-specific binding sites in certain areas of the brain [39]. Prodynorphin (PDyn) mRNA is observed at high levels in the central amygdala, various hypothalamic nuclei, olfactory tubercles, hippocampus, striatum and NAcc. In addition, PDyn-expressing cells are found scattered throughout the cortex, including the prefrontal cortex. Dense κ receptors are localized in many structures of the same regions, e.g., striatum, NAcc, olfactory tubules, hypothalamus and PFC [34]. Regions of overlap are suggestive of local DYN/ KOP circuits.
On the other hand, κ receptors are concentrated in brain regions without evidence of notable Dyn. Specifically, the basolateral amygdala, endopiriform nucleus, interpeduncular nuclei, stria terminalis and thalamus have concentrations of the κ receptor without Dyn [13]. The ventral tegmental area (VTA) and raphe n. have minimal Dyn but projection neurons have high levels of κ receptors. Regions of the hippocampal formation have differential levels of Dyn as compared to κ receptors. And, in the amygdala, κ receptors are concentrated in the basolateral region (BLA) whereas the central nucleus (CeA) contains high levels of Dyn [40]. This is intriguing because the typical flow of neural information is from BLA projections to the CeA.
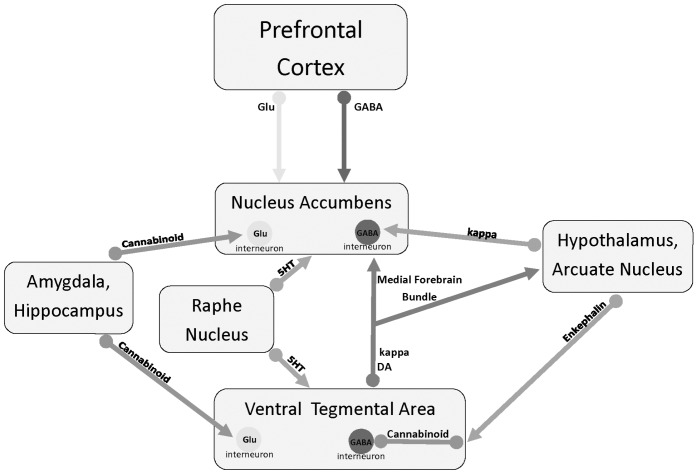
Some of the discrepancies may be explained by the inherent complexity of the DYN/ KOP system, e.g., the somatodendritic release of Dyn. Differential expression of Dyn and its receptor in the mid-brain VTA and raphe n. can be explained by transportation of κ receptors down their DA axons and 5-HT axons, respectively. Once transported, they serve as autoreceptors to modulate neurotransmitter release. The same is implied from the DYN/ KOP differences in the amygdala. Stress was reported to increase κ receptors in the BLA without affecting the levels of Dyn in the CeA [27]. Likely, increases in axonal κ receptors modulated neurotransmitter release into the CeA without changing Dyn or κ receptors in CeA. Fig. (1) illustrates the interaction of the KOP system in the brain.
Fig. (1).
Depiction of subcortical brain regions and neurotransmitter projections in anhedonia and, presumably depression. DA = dopamine, 5HT = serotonin, GABA = gamma aminobutyric acid, Glu = glutamate.
Although there is a disconnect between locations of Dyn and its receptor, the κ receptors located in the hippocampus are strategically placed to modulate GABA and glutamate neuronal activities [34]. These data suggest Dyn action may be related to Dyn interactions with other opioid (e.g., µ) or nonopioid (e.g., NMDA) receptors [39]. Still, without question, discrepancies between the distribution of κ receptor binding sites and Dyn immunoreactivity have posed a major problem for understanding the functional properties of the DYN/KOP system [33]. Before moving to our primary target in this paper, the relation of salvinorin A to MDD, the next section focuses on the Dyn/KOP system involvement in depression.
DYN/ KOP AND DEPRESSION
Keys to understanding the relation of Dyn/ KOP to MDD are the connections of the Dyn/ KOP system with the endocrine and neurotransmitter systems, especially the monoamine and the amino acid transmitters [41]. Dyn/ KOP is positioned to modulate overlapping neuronal circuits linking midbrain monoamine origins with forebrain limbic structures. The interaction of reward, stress and mood are at the level of Dyn/KOP signaling [42]. Research on the etiology of depression has focused on chronic stress and the HPA axis and on changes in neurotransmitter activities that modify the experience of pleasure. Notably, Dyn/ KOP is intimately associated with each.
Stress and Dyn/ KOP
There are many points in the brain for interaction of stress with the Dyn/ KOP system. A starting point is with the HPA endocrine axis. Disturbances to homeostasis activate synthesis and release of corticotropin releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus. Entry of CRH into the anterior pituitary produces adrenocorticotropin hormone (ACTH) release into general circulation, which triggers the adrenal cortex to increase corticosterone (CORT) levels in circulation. Of particular interest have been hypothalamic CRH neurons that project into subcortical areas to terminate on the midbrain locus coeruleus (LC) and into the limbic system. Of particular importance is the presence of CRH and CORT receptors in the hippocampus, the brain structure that has direct control of the HPA axis [43].
The interaction of neuropeptides, Dyn, and CRH play a special role in the stress response [33]. Along with CRH, Dyn is released during acute and chronic stress exposure in the hippocampus and NAcc [44,45.], and κ receptors are activated broadly in the brain following exposure to stressful stimuli [31].
Mechanisms underlying the interaction of Dyn and CRH remain elusive, including which of the two regulates the other. However, there are data suggesting exposure to a Dyn/KOP agonist suppressed hypothalamic CRH with consequent reduction in serum CORT [46]. Stress, and the consequent increase in CRH, elevates PDyn expression in the NAcc [47]. Finally, the placement of CRH neurons at the LC connects the DYN/KOP system to stress and arousal. Dynorphin and κ receptors are coexpressed within the LC on noradrenergic neurons [48].
Dynorphins are released in multiple brain regions with stress exposure. κ receptors are involved in the capacity of stress to decrease excitation of glutamate neurons in the hippocampal formation. Activation of κ receptors reduces granule neuron excitation by inhibiting glutamate release from the perforant path fibers, and reduces CA3 pyramidal neuronal activity by presynaptic inhibition of the mossy fibers [37].
Still, the relation of Dyn/ KOP to stress is observed most clearly in the LC [49, 50]. The LC is the primary source of norepinephrine (NE) neurons in the brain, and Dyn afferents make direct contact with NE neurons in the LC. Moreover, κ receptors co-localize with Dyn, CRH and glutamate in axon terminals on the LC [51]. Exposure to a stressful stimulus induces a sequence of events that engages CRH and Dyn/ KOP at the LC [49]. A hypothesis is Dyn/ KOP induces a change in NE from tonic to phasic activity [52]. One behavioral result is shifting attention to important aspects of the environment [53].
Animal behavioral studies bolster the involvement of Dyn/KOP in stress. For example, in a stress-paired odorant paradigm in which mice normally exhibit aversion, Dyn knock out (KO) mice failed to show aversion to the odorant. KO mice injected with κ antagonist nor-binaltorphimine (nor-BNI) also did not find the odorant aversive [33]. In the anxiety-sensitive Wistar Kyoto rat strain, the animals showed less defensive burying after administration of DIPPA, a κ antagonist [54]. Other short acting κ antagonists, such as LYDMPF and AZMAB attributed to a decrease in stress in an elevated plus maze [55].
However, not all stressors are equal [56], and, indeed, DYN/ KOP appears to play a different role during acute stress, sub-chronic stress, and chronic stress [57]. Although aversion paradigms show that κ agonists potentiate acute stress aversion, this was not the case in a foot shock model of stress. Mice exposed to foot shocks over four days had fewer escape failures when treated with Dyn A, and pre-treatment with nor-BNI reversed this effect [58]. Therefore, while the Dyn/ KOP may regulate responses to acute stress, its function during chronic stress is less established. This has special relevance to the chronic mild stress paradigm often used as a model of anhedonia in rodents and is associated with depression in humans. Chronic stressors are thought to be more indicative of the human experience in terms of depression. However, many depression paradigms, as we will discuss, use acute stressors, such as the forced swim test to mimic depression.
The role of Dyn/ KOP in stress is also thought to be mediated by dopaminergic activity. KO mice with κ receptors deleted from DA neurons exhibited reduced anxiety in the open field paradigm. These effects were not seen in systemic kappa KO mice, which did not differ from controls [28]. The authors interpreted these results as indicating the role of the Dyn/ KOP in stress and anxiety may be closely linked to DA pathways, which leads us into our discussion on Dyn/KOP and pleasure.
Pleasure and Dyn/ KOP
Dyn/ KOP is also involved in regulation of monoamine release, especially in limbic structures. Of particular relevance is DA as both DA and Dyn/ KOP expression are highest in regions implicated in the modulation of reward and mood [29].
The origins of DA neurons are cell bodies located in the substantia nigra and the VTA. The former projects via the nigrostriatal dopaminergic pathway in the medial forebrain bundle (MFB) to the striatum of the basal ganglia. The caudate nucleus and putamen that comprise the striatum are involved in motor movement as well as mood [59]. The second dopaminergic pathway is the mesolimbic (or mesocorticolimbic)DA system. DA cell bodies originate in the VTA and their axons leave via the MFB to terminate in the NAcc. The mesolimbic pathway continues onward into other subcortical structures and to the PFC.
Dyn/ KOP is found in both mesolimbic and nigrostriatal dopaminergic pathways [36]. In the dorsal striatum, Dyn/ KOP is primarily located on presynaptic dopaminergic, GABAergic, and glutamatergic afferents where it inhibits neuronal activity and neurotransmitter release [60]. Binding of Dyn to these GABAergic neurons increases GABA inhibition to dopaminergic neurons in the substantia nigra. Dopaminergic cell bodies of substantia nigra become hyperpolarized and are less likely to fire.
Dyn/ KOP also exerts inhibitory control on DA release in the ventral striatum [61]. Here it inhibits the release of glutamate and, intriguingly, GABA in the NAcc [62]. Also in the NAcc, Dyn/ KOP binding on the presynaptic terminals of mesolimbic dopaminergic neurons hyperpolarizes and inhibits DA release [4]. Another suggested mechanism is that Dyn/ KOP targets the DA transporter (DAT) that regulates DA transmission via uptake of released neurotransmitter [62].
The mesolimbic pathway has special relevance to the experience of pleasure and has been dubbed the dopamine brain reward system (BRS). That processing of rewards depends on an intact mesolimbic dopaminergic pathway is well established in animal models [63, 64] and in humans [65, 66]. Central to the BRS is the NAcc.
Unlike µ agonists, κ agonists do not produce reinforcing effects [16], and, indeed, are considered a main anti-BRS system agent [7]. Dyn/ KOP may be especially important in development of drug abuse. A genetic link suggests the association of PDyn and κ receptor polymorphisms with opioid dependence in humans [67]. In addition, the Dyn/ KOP system altered with exposure to psychostimulants in animal models. For example, κ receptor levels in the NAcc are also modified with acute or chronic administrations of the stimulant drugs [61].
It has been proposed that repeated drug use promotes dysregulation of the Dyn/ KOP system underlying behaviors observed with addiction [68]. The result is a remarkably high recidivism rate after initially successful treatment of drug abuse. Preclinical studies indicating that Dyn/ KOP antagonists can reverse the attraction to cocaine has led to suggestions of the development of new anti-addiction drugs based on Dyn/ KOP [69].
Dyn/ KOP also plays an important role in stress – induced relapse from drug abuse in recovering patients and reinstatement of drug use in animal models of addiction. Using KO mice and κ agonists and antagonists, researchers have clearly established that dynorphins acting through κ receptors encode the effects of stress. The results of stress or κ agonists are increases in the risk of drug use and reinstatement of extinguished drug preference in lab animals [37, 51].
The dopamine BRS system, under partial control by Dyn/ KOP, is a central player in drug addiction and in hedonic experiences, including depressive and anhedonic symptoms occurring after stimulant withdrawal. The anhedonic symptoms accompanying psychopathologies such as MDD are also under control of the BRS system [70]. A primary symptom of depression is anhedonia, the absence of the ability to experience pleasure [71]. We now turn attention to the literature on Dyn/ KOP relations to depression.
Depression and Dyn/ KOP
Opposite to the effects of other opioid receptor agonists, administrations of κ receptor agonists have been reported widely to be aversive. In healthy humans, this was recorded as anxiety and dysphoria, a milder form of depression [8]. Considerable evidence in animal models confirms aversive, anxiogenic and depressive-like outcomes with κ agonists and the relief from those behaviors with κ antagonists.
A sampling of those preclinical data indicates κ receptors mediate depressive-like behaviors when Dyn/ KOP activity is higher, e.g., under acute stressful conditions [7]. Performance of rats in the forced swim test (FST), also known as the Porsolt test, is changed in opposite directions after administration of a κ agonist or an antagonist [72]. Intracerebroventricular (i.c.v.) administration into the NAcc of the synthetic κ agonist U-69593 increased immobility, while i.c.v. antagonists decreased immobility. The authors concluded that the effects of κ antagonists resembled those of typical and atypical antidepressants drugs [72]. Various other κ antagonists have also decreased immobility in the FST: LY25622, GNTI, nor-BNI, and MCL 144B [73, 74].
The FST is a popular paradigm for simulating learned helplessness, a symptom of MDD. Immobility is measured in the FST under the assumption that high levels of inactivity represent a marker of depression. A rodent is dropped into an inescapable pool of water and latency and duration of immobility are recorded as a behavioral model of “despair.” The FST has been criticized for various reasons. The procedure uses the same setting to induce “depression” and to measure it, i.e., the stressor is applied at the same time as the test is performed [9]. Other critiques have focused on etiology and face validity of FST. One criticism is the FST can also be used as stressor or as a measure of anxiety [75]. In addition, the mechanism behind FST depressive symptoms and its relationship to the Dyn/KOR system is not wholly understood. There is evidence for the interplay of interferon alpha (INF-alpha) and the opioids in FST induced immobility. Treating mice with INF-alpha increased immobility in the FST, and this was reversed by treatment with naloxone, but not by treatment with nor-BNI [76].
Another model of despair, or learned helplessness, used to mimic depressive symptomology is a conditioned fear avoidance foot shock paradigm. After a series of inescapable foot shocks, the animal no longer attempts to free himself from his environment. Treatment with 10mg/kg of κ agonist U-50488 decreased depressive symptoms of animals exposed to foot shock. Treatment with κ antagonist MR22 in conjunction with U-50488 increased escape failures [77].
Thus the Dyn/KOP system has behavioral implications for depressive symptoms in animal models precipitated by stress. There is much support, but with some exceptions, for κ antagonists to remedy depressive symptoms caused by the FST. However, when another stressor, such as chronic foot shock, elicits depressive symptoms, κ agonists appear to mediate alleviation of learned helplessness. There is also evidence for the role of Dyn/KOP and stress interaction in depressed humans. Particularly, humans have greater Pdyn mRNA expression in the entorhinal cortex of the hippocampal complex than rodents. Output from the hippocampus is responsible for inhibition of the HPA axis, thus controlling the stress response. A subgroup of depressed patients experience hypercotisolemia, which may result from HPA inhibition [34].
The Dyn/KOP system also plays a role in depressive symptoms related to pleasure and anhedonia. Cocaine withdrawal in humans is accompanied by a depressive state. Animal models experience anhedonia, a marker of depression, after cocaine withdrawal. Intracerebralventricular administra-tion of the κ antagonist nor-BNI attenuated development of cocaine withdrawal-induced anhedonia-like responses. However, the effects were limited to nor-BNI exposure prior to the regimen that produces anhedonia. Administrations in the middle of the regimen were without consequence [47]. Nor-BNI did not reverse or treat depressive symptoms, but rather inhibited the manifestation of anhedonia. A recent study used changes in thresholds for intracranial self-stimulation (ICSS) as a model of depression induced by cocaine withdrawal [78]. Administration of a κ agonist (U-50488) raised the ICSS threshold of rats. The authors described the need for greater stimulation as consistent with a prodepressant effect. Of considerable interest in this experiment was the inclusion of females. Males were much more sensitive to the agonist than the females, an inclusion that is rare in this literature. These data make a strong case to consider sex differences in future studies.
Behavioral outcomes of cocaine withdrawal studies suggest Dyn/KOP is exerting its effect on withdrawal-induced depression through the BRS with κ agonist potentially creating prodepressant effects. The neuro-physiological data provide additional evidence for κ agonists being unpleasant. κ agonists reduced basal DA release in the DA mesolimbic pathways. However, chronic exposure to a κ agonist increased K+ stimulated release of extracellular DA, allowing an augmented response to stimuli that increase DA neurotransmission. Augmented response to mesolimbic stimulation may enhance rewarding properties of psychostimulants, but can also act to enhance mood. [79]. In addition, acute administration of the κ agonist U-69593 increased hippocampal derived neurotrophic factor (BDNF) expression, BDNF levels have been reported to be lower with increased depressive symptoms [80]. There is yet to be a clear verdict on the role of acute stressors and the BRS in depression. However, outcomes may be different in chronic stress paradigms of anhedonia, and that initiates our discussion of Salvinorin A.
THE SPECIAL CASE OF SALVINORIN A
Salvinorin A (Salv A) is a biologically active ingredient of salvia divinorum, a plant in the mint family, with high selectivity for the κ opioid receptor [81]. Salv A has gained notoriety from people using the drug for its hallucinogenic effects but also perhaps for inducing feelings of happiness and well being [82, 83].
Controlled experiments with human volunteers confirmed the dominant effects of Salv A are hallucinogenic. Hallucinogenic effects were observed in doses as small as 4.5μ/kg as well in a relatively large doses of 8mg. No adverse physiological effects were reported at any dose, although one study reported increase in respiration [84]. Despite hallucinogenic outcomes, general self-reports described pleasurable effects across doses; although, a study by Maqueda and colleagues [85] reported trait anxiety at doses of 0.25mg and 0.5mg. No anxiety was associated with the higher dose of 1mg. Overall, participants reported positive or silly experiences. Lack of hallucinogenic adverse effects might be related to the short duration of these effects. Hallucinogenic effects peak around 2 min and typically last 20 min or less [86, 87].
Preclinical findings include that, as with most other hallucinogens, lab animals failed to readily self- administer Salv A [88]. Indeed, results with animal models suggest that the drug reduces activity of DA in the BRS and even is aversive [88]. It is puzzling that a compound that leads to conditioned place aversion in rodents is self-administered by humans [90].
Yet, Salv A has many unique properties and has peaked the interest of biomedical researchers. Foremost is its selectivity for the κ receptor. It is intriguing as the first naturally occurring κ agonist [91]. The lipid-like Salv A molecule is a rare non-protein κ agonist and the first non-nitrogenic hallucinogenic to be identified that does not interact directly with the serotonin 5-HT2A receptor [31, 92].
These features have led to calls for development of synthetic drugs based on the Salv A scaffold that could be useful for treatment of mood disorders, addiction, chronic pain [93, 94] and the hallucinations of schizophrenia [95]. Nonetheless, much of the work has centered on Salv A as a novel approach to the neurobiological underpinnings of anxiety and mood disorder, especially MDD.
It is notable that a sizeable number of the publications indicating Salv A and other κ agonists induce anxious and depressive-like behaviors are from the Carlezon laboratory [47, 73, 96, 97]. Indeed, studies from the same group accept the anti-depressant effects without actually measuring behaviors [98, 97]. Probably the most cited paper on Salv A is an empirical study with rats by Carlezon and colleagues [99]. They administered a range of Salv A dosages from low to high (0.125-2.0 mg/ kg I.P.). Results were a dose-dependent elevation in thresholds for ICSS and increases in immobility in the FST, both markers of depressive-like behaviors in animal models [98]. At the same time, Salv A was reported to suppress DA concentrations in the NAcc, a key structure in the dopamine BRS. Subsequent studies from the same laboratory [60] used acute and chronic exposure to demonstrate that Salv A can similarly modulate dopaminergic activities in the nigrostriatal pathway. The general conclusion from their studies is Salv A inhibits DA in the mesolimbic and nigrostriatal pathways with consequences of both anxiogenic and pro-depressant effects.
There are, however, DA findings inconsistent with that conclusion. Zhang and collaborators [90] used the high doses of Salv A similar to those used by the Carlezon laboratory. Results confirmed the decrease of DA in the striatum, but they found no change in the NAcc. At the higher dosage, Salv A also induced conditioned place aversion and decreased locomotor activity. Low Salv A dosages had no effect on any of the measures [90].
After a series of experiments with rats, Braida and colleagues [100, 101] came to two important conclusions. First, Salv A (0.1 – 1 mg/ kg) reduced both anxiety-like and depressive-like behaviors while increasing extracellular DA in the NAcc. Of note, they used the same EPM and FST paradigms of the Carlezon research group to generate the opposite data and conclusions. Second, the specificity of Salv A for the κ receptor was questioned with evidence of interactions between DYN/ KOP and the cannabinoid system [100]. Thus, it is clear Salv A is modulating DA in the BRS, but behavioral results are still uncertain.
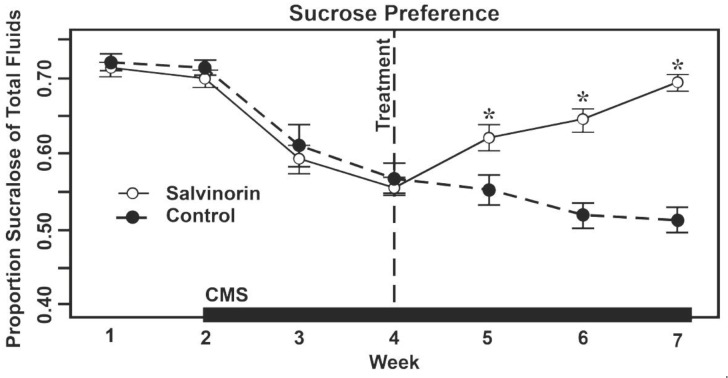
There are other behavioral data suggesting an opposing result to the findings of aversion and depression with Salv A. We reported an experiment using chronic exposures to moderate dosages (1 mg Salv A/ kg) and found an antidepressant effect [9]. The main differences with the earlier studies reporting a pro- depressive outcome was the use of a chronic mild stress paradigm (CMS) and recovery from anhedonia. Rodents typically will prefer a sucrose solution over plain water. A reduction of sucrose preference during CMS indicates anhedonia, a common symptom in MDD. A drug that restores preference for the sweet water indicates an anti-depression effect.
In our experiment, administration of Salv A reversed anhedonia after CMS exposure. Of additional notice is that animals exposed to Sal A in the absence CMS did not show anhedonic signs of depression [9]. These are similar to findings that Sal A (0.3 and 1 mg/kg) also did not alter preferences for sweet water [102]. Interestingly, their Salv A animals showed a trend toward a faster recovery in a taste aversion paradigm. The results of our study are illustrated in (Fig.2).
Fig. (2).
Anhedonia and recovery from anhedonia as measured by relative intake of sucrose flavored water or plain water in groups of rats administered either 1mg Salvinorin A/ kg bodyweight or vehicle only. Chronic mild stress (CMS) was induced by different, daily manipulations of the environment of the animals, e.g., tilting cages or a strobe light. Figure adapted from Harden et al., 2012.
We are not the only lab to be puzzled by a dogma that has deemed that Salv A or, more generally, κ agonists invariably produce depressive-like behaviors in animal models [7, 15, 103]. Olianas and colleagues [104] pointed out that established medications for treating mood disorders are κ agonists! A number of tricyclic anti-depressants (TCAs), including amitriptyline, nortriptyline, desipramine and imipramine, bind to and activate distinct opioid receptors with a preference for the κ receptor.
Importantly, the high agonist activity at κ receptors occurred with typical TCA levels in the brain. The authors proposed that the direct agonist activity at κ receptors contribute to the antidepressant actions of TCAs. That this effect extends to mianserin indicates that some atypical antidepressants also are κ agonists [104].
A recent review by Kivell et al. [93] declined to choose a side, concluding, “It is clear from these studies that there are no consistent effects for Sal A in its ability to modulate both pro- and anti-depressive behaviors.” Instead, more refined hypotheses are being entertained. Whether, for instance, Salv A promotes the development of pro-depressive or anti-depressive behaviors may depend on drug administration or other procedural variables.
A prominent suggestion is that Salv A acutely administered can have opposite outcomes to repeated administration [94]. For example, Salv A had a biphasic effect on the rewarding impact of ICSS for cocaine. The initial response to Salv A exposure was an increased threshold for ICSS, whereas a delayed effect was a decrease in ICSS threshold, an anti-depressant effect [97].
In another experiment [60] with Salv A and sensitivity to cocaine, Salv A was administered acutely or chronically to rats. Cocaine-induced locomotor activity was measured in reference to DA D1 and D2 receptor stimulation. Results depended on acute or repeated Salv A exposure. Acute Salv A exposure diminished D1 receptor activity, and chronic exposure increased D1 activity [60]. Further evidence of functional differences with single or repeated Salv A administrations came from direct dialysis assays of DA. An acute dosage of Salv A decreased extracellular DA without influencing DA reuptake in the dorsal striatum of rats. Repeated administrations, however, failed to change either extracellular levels or reuptake of DA [105]. Listos and colleagues [89] proposed the hypothesis that low doses of Salv A increases subcortical DA levels, which is reflected in an increase in locomotor activity, both suggestive of anxiolytic and anti- depressant consequences. High doses produce the opposite effect, such as decreases in DA levels and locomotor activity accompanied by aversion. Biphasic DA activity indicates the role of Salv A in DA signaling and may explain the differential antidepressant and prodepressent effects during acute and chronic administration.
While many of these contradicting effects can be explained by the use of different doses and acute versus chronic administration, other considerations have emerged. For example, the prospect has been raised of “functional selectivity” or “biased agonism” whereby different agonists acting on the same receptor can have different consequences [7, 93]. Salv A may be a prime example.
That Salv A may have broader effects than simply activating the DYN/ KOP system is suggested in a study of P-glycoproteins that serve as transporters in the blood brain barrier [41]. Behavioral effects of Salv A were partly a function of the presence of competing P-glycoproteins substrates. Notably, levels of these proteins show wide individual differences in humans.
As cited above, Braida et al. [100] had questioned the selectivity of Salv A for the κ receptor, suggesting Salv also binds the CB1 cannabinoid receptor. This was based on their findings that the behavioral effects of Salv A were blocked by a κ antagonist but also were blocked by an antagonist of CB1. Subsequent research revealed that Salv A does not bind CB1 directly [106]. However, it remains likely that Salv A indirectly influences the cannabinoid system [89].
Studies of other transmitter systems appear to raise additional questions about the kappa specificity of Salv A. There is evidence that Salv A is an allosteric modulator of the µ opioid receptor [107]. The similarity of binding profiles of Salv A and ketamine at κ and NMDA receptors suggested a possible interaction with glutamate [53, 107]. Salv A interacts with monoamine neurotransmitters at post-synaptic sites, but also at pre-synaptic sites. As a result, Salv A is positioned to inhibit or enhance the release of DA, 5-HT or NE differently in different CNS tissues [36]. It is of interest that concentrating on Dyn/ KOP in the subcortex also may have limited generalizations. Salv binds κ in the PFC and, indeed, following systemic injections the highest concentration of Salv A in the brain is in the cortex and cerebellum [36].
Drilling down further, an in vitro experiment [108] suggested that Salv A binds the D2 receptor. Salv A stimulated the incorporation of 35[S]GTP-g-S, a marker of D2 binding, by 44%. The D2- selective antagonist sulpiride completely blocked this effect. The significant conclusion was that Salv A has high affinity for the D2 receptor. “Considering that salvinorin A has been highlighted to be selective for kappa opioid receptors … the present data were surprising [109].”
Another concern in this literature is the widespread use of the synthetic drug nor-BNI, a selective antagonist of DYN/ KOP. When administered alone, nor-BNI has been demonstrated to serve as an anti-depressive agent while a κ agonist administered alone induces depression-like behaviors [109]. Use of an antagonist concurrently with Salv A is based on the logic that reversal of the effects confirms that Salv A is pro-depressive and antagonists are anti-depressive [28, 73]. Other researchers eschew Salv A altogether and employed only nor-BNI [110].
However, nor-BNI has pharmacodynamics properties that make it an uncertain choice. Most notable is the remarkably long-lasting influences of nor-BNI. A single injection can continue to be effective for weeks [5, 33]. Moreover, there are data that question the effectiveness of nor-BNI over time. For example, nor-BNI was effective in reducing signs of depression in the FST 1 day after drug exposure, but not 7 or 14 days post-exposure [80].
A similar problem may be reliance on synthetic κ agonists to mimic of the pharmacodynamics of Salv A. Evidence used to strengthen the case for dopaminergic modulation and pro-depressive effects of Salv A is to cite findings with κ agonists such as U-69593, U-50488 or R-84760. However, there is evidence that the influences of Salv A on neural activity and behavior are different from synthetic agonists [111]. For example, its potency as a κ agonist is cited often, but Salv A is less potent than, at least, some synthetic agonists [91].
Failure to appreciate sex differences in response to κ agonists, including Salv A, is another pitfall in this literature [112]. Males and females are reported to have different sensitivity to κ agonists with likely functional sex differences [111, 113]. Moreover, sensitivity of females to the drug may change with phase of the ovarian hormone cycle [20]. The neuroendocrine effects of Salv A, measured by increased prolactin release, were more robust in female than in male rhesus monkeys [114]. The same research group had earlier reported sex differences in the distribution and elimination of Salv A in monkeys [115]. Yet, few studies of Salv A have included females.
Another potential factor contributing to conflicting data on depression with Salv A is reliance on the FST paradigm [102]. A valid animal model should be reasonably analogous to the symptomology of the human disorder. The FST model fails to mimic the slow onset of depression and delayed effectiveness of antidepressant medications. The sex differences observed in patients, women being at greater risk, also is opposite to that often found in rodents [116, 117]. The same failure to simulate the sex differences in human anxiety can be leveled at the elevated plus maze [118] used by many experiments in the DYN/ KOP literature.
The chronic mild stress paradigm using anhedonia as the measure has superior face validity than the FST [119, 120]. We believe the CMS paradigm is superior to the FST and other paradigms because the gradual onset of anhedonia and its gradual recovery with anti-depressant medications more closely simulates human conditions [71]. Notably, CMS and anhedonia were used in our study with Salv A [9] that reached a conclusion opposite to that of some studies based on FST findings.
CONCLUSIONS
Our conclusion is to follow the lead of authors who find it best not to choose a side at this point on the pro- or anti-depressive features of Salv A. We acknowledge the solid evidence for Dyn and activation of the κ receptor that suggests consequences of anxious and depressive behaviors in animal models. Our contention is that the jury is undecided on the neural and behavioral functions of Salv A. It is clear that differences exist between different doses of Salv A, and acute and chronic effects show biphasic behavioral and neurophysiological outcomes. More studies should examine these biphasic relationships in a chronic stress model of depression using both Salv A and other κ agonists. Future research on Salv A in human subjects should include measures of prodepressive effects. More importantly describing Salv A simply as a potent, selective κ agonist that will follow the principles of other agonists gives short-shrift to the complexity of the biological influences of Salv A, and, indeed, to the complexity of the Dyn/ KOP system.
ACKNOWLEDGEMENTS
The authors express our gratitude to the Interdisciplinary Intercampus Research Program of the University of Missouri and the Alexander von Humboldt Foundation (Bonn, Germany) for partial funding of this research.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Alexander S.P., Benson H.E., Faccenda E., Pawson A.J., Sharman J.L., Spedding M., Peters J.A., Harmar A.J., CGTP Collaborators The concise guide to Pharmacology 2013/14: G protein-coupled receptors. Br. J. Pharmacol. 2013;170(8):1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besson A., Privat A.M., Eschalier A., Fialip J. Effects of morphine, naloxone and their interaction in the learned-helplessness paradigm in rats. Psychopharmacology (Berl.) 1996;123(1):71–78. doi: 10.1007/BF02246283. [DOI] [PubMed] [Google Scholar]
- 3.Bodnar R.J., Klein G.F. Endogeous opiates and behavior. Peptides. 2005;26:2629–2711. doi: 10.1016/j.peptides.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Aldrich J.V., McLaughlin J.P. Peptide kappa opioid receptor ligands: potential for drug development. AAPS J. 2009;11(2):312–322. doi: 10.1208/s12248-009-9105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falcon E., Maier K., Robinson S.A., Hill-Smith T.E., Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology (Berl.) 2014;100:1–10. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodnar R.J. Endogenous opiates and behavior: 2010. Peptides. 2011;32(12):2522–2552. doi: 10.1016/j.peptides.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Lutz P.E., Kieffer B.L. >Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeiffer A., Brantl V., Herz A., Emrich H.M. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233(4765):774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 9.Harden M.T., Smith S.E., Niehoff J.A., McCurdy C.R., Taylor G.T. Antidepressive effects of the κ-opioid receptor agonist salvinorin A in a rat model of anhedonia. Behav. Pharmacol. 2012;23(7):710–715. doi: 10.1097/FBP.0b013e3283586189. [DOI] [PubMed] [Google Scholar]
- 10.Chavkin C., Sud S., Jin W., Stewart J., Zjawiony J.K., Siebert D.J., Toth B.A., Hufeisen S.J., Roth B.L. Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious κ-opioid receptor agonist: structural and functional considerations. J. Pharmacol. Exp. Ther. 2004;308(3):1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- 11.Harrison C. Trial watch: opioid receptor blocker shows promise in Phase II depression trial. Nat. Rev. Drug Discov. 2013;12(6):415–415. doi: 10.1038/nrd4028. [DOI] [PubMed] [Google Scholar]
- 12.Katz N., Mazer N.A. The impact of opioids on the endocrine system. Clin. J. Pain. 2009;25:170–175. doi: 10.1097/AJP.0b013e3181850df6. [DOI] [PubMed] [Google Scholar]
- 13.Mansour A., Fox C.A., Meng F., Akil H., Watson S.J. Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol. Cell. Neurosci. 1994;5(2):124–144. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- 14.Narita M., Funada M., Suzuki T. Regulations of opioid dependence by opioid receptor types. Pharmacol. Ther. 2001;89(1):1–15. doi: 10.1016/s0163-7258(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 15.Carroll F.I., Carlezon W.A., Jr Development of κ opioid receptor antagonists. J. Med. Chem. 2013;56(6):2178–2195. doi: 10.1021/jm301783x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wee S., Koob G.F. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl.) 2010;210(2):121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavkin C. Dynorphin--still an extraordinarily potent opioid peptide. Mol. Pharmacol. 2013;83:729–736. doi: 10.1124/mol.112.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodnar R.J., Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm. Behav. 2010;58(1):72–81. doi: 10.1016/j.yhbeh.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Craft R.M. Sex differences in opioid analgesia: “from mouse to man”. Clin. J. Pain. 2003;19(3):175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Liu N.J., Chakrabarti S., Schnell S., Wessendorf M., Gintzler A.R. Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal κ- and μ-opioid receptor heterodimerization and female-specific spinal morphine antinociception. J. Neurosci. 2011;31(33):11836–11845. doi: 10.1523/JNEUROSCI.1901-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderah T.W. Delta and kappa opioid receptors as suitable drug targets for pain. Clin. J. Pain. 2010;26(Suppl. 10):S10–S15. doi: 10.1097/AJP.0b013e3181c49e3a. [DOI] [PubMed] [Google Scholar]
- 22.Bruijnzeel A.W. Kappa-opioid receptor signaling and brain reward function. Brain Res. Rev. 2009;62:127–146. doi: 10.1016/j.brainresrev.2009.09.008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuzmin A., Madjid N., Terenius L., Ogren S.O., Bakalkin G. Big dynorphin, a prodynorphin-derived peptide produces NMDA receptor-mediated effects on memory, anxiolytic-like and locomotor behavior in mice. Neuropsychopharmacology. 2006;31(9):1928–1937. doi: 10.1038/sj.npp.1300959. [DOI] [PubMed] [Google Scholar]
- 24.Leposavic G., Cover P.O., Buckingham J.C. In vivo and in vitro studies on the opioidergic control of the secretion of gonadotrophin-releasing hormone and luteinizing hormone in sexually immature and adult male rats. Neuroendocrinology. 1991;53(6):579–588. doi: 10.1159/000125777. [DOI] [PubMed] [Google Scholar]
- 25.Hauser K.F., Knapp P.E., Yakovleva T., Verbeek D.S., Bakalkin G. Dynorphins in central nervous system pathology. In: Nyberg F.J., editor. Neuropeptides in Neuroprotection and Neuroregeneration. Boca Raton, Florida: CRC Press; 2012. pp. 80–106. [Google Scholar]
- 26.Kardon A.P., Polgár E., Hachisuka J., Snyder L.M., Cameron D., Savage S., Cai X., Karnup S., Fan C.R., Hemenway G.M., Bernard C.S., Schwartz E.S., Nagase H., Schwarzer C., Watanabe M., Furuta T., Kaneko T., Koerber H.R., Todd A.J., Ross S.E. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82(3):573–586. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knoll A.T., Muschamp J.W., Sillivan S.E., Ferguson D., Dietz D.M., Meloni E.G., Carroll F.I., Nestler E.J., Konradi C., Carlezon W.A. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol.Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van’t Veer A., Carlezon W.A., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl.) 2013;229(3):435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White K.L., Roth B.L. Psychotomimetic effects of kappa opioid receptor agonists. Biol. Psychiatry. 2012;72(10):797–798. doi: 10.1016/j.biopsych.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Magnusson K. Influence of anabolic androgenic steroids on dynorphinergic pathways in rat's brain. In: Nyberg F.J., editor. Neuropeptides in Neuroprotection and Neuroregeneration. Boca Raton, Florida: CRC; 2012. pp. 149–162. [Google Scholar]
- 31.Bruchas M.R., Land B.B., Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melief E.J., Miyatake M., Carroll F.I., Béguin C., Carlezon W.A., Jr, Cohen B.M., Grimwood S., Mitch C.H., Rorick-Kehn L., Chavkin C. Duration of action of a broad range of selective κ-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol. Pharmacol. 2011;80(5):920–929. doi: 10.1124/mol.111.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Land B.B., Bruchas M.R., Lemos J.C., Xu M., Melief E.J., Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008;28(2):407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarzer C. 30 years of dynorphins – new insights on their functions in neuro psychiatric diseases. Pharmacol. Therap. 2009;123:353–370. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belzung C., Yalcin I., Griebel G., Surget A., Leman S. Neuropeptides in psychiatric diseases: an overview with a particular focus on depression and anxiety disorders. CNS Neurol. Disord. Drug Targets. 2006;5(2):135–145. doi: 10.2174/187152706776359682. [DOI] [PubMed] [Google Scholar]
- 36.Grilli M., Neri E., Zappettini S., Massa F., Bisio A., Romussi G., Marchi M., Pittaluga A. Salvinorin A exerts opposite presynaptic controls on neurotransmitter exocytosis from mouse brain nerve terminals. Neuropharmacology. 2009;57(5-6):523–530. doi: 10.1016/j.neuropharm.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Schindler A.G., Messinger D.I., Smith J.S., Shankar H., Gustin R.M., Schattauer S.S., Lemos J.C., Chavkin N.W., Hagan C.E., Neumaier J.F., Chavkin C. Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. 2012;32(49):17582–17596. doi: 10.1523/JNEUROSCI.3220-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fillingim R.B., Gear R.W. Sex differences in opioid analgesia: clinical and experimental findings. Eur. J. Pain. 2004;8(5):413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Arvidsson U., Riedl M., Chakrabarti S., Vulchanova L., Lee J.H., Nakano A.H., Lin X., Loh H.H., Law P.Y., Wessendorf M.W., et al. The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc. Natl. Acad. Sci. USA. 1995;92(11):5062–5066. doi: 10.1073/pnas.92.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bilkei-Gorzo A., Mauer D., Michel K., Zimmer A. Dynorphins regulate the strength of social memory. Neuropharmacology. 2014;77:406–413. doi: 10.1016/j.neuropharm.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Butelman E.R., Caspers M., Lovell K.M., Kreek M.J., Prisinzano T.E. Behavioral effects and central nervous system levels of the broadly available κ-agonist hallucinogen salvinorin A are affected by P-glycoprotein modulation in vivo. J. Pharmacol. Exp. Ther. 2012;341(3):802–808. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalanne L., Ayranci G., Kieffer B.L., Lutz P-E. The kappa opioid receptor: from addiction to depression, and back. Front. Psychiatry. 2014;5:170. doi: 10.3389/fpsyt.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Kloet E. R., Karst H., Joëls M. Corticosteroid hormones in the central stress response: quick-and-slow. 2008;29(2):268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin J.P., Li S., Valdez J., Chavkin T.A., Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31(6):1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirayama Y., Ishida H., Iwata M., Hazama G.I., Kawahara R., Duman R.S. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J. Neurochem. 2004;90(5):1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 46.Wittmann W., Schunk E., Rosskothen I., Gaburro S., Singewald N., Herzog H., Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34(3):775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chartoff E., Sawyer A., Rachlin A., Potter D., Pliakas A., Carlezon W.A. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62(1):167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Hasani R., McCall J.G., Foshage A.M., Bruchas M.R. Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology. 2013;38(12):2484–2497. doi: 10.1038/npp.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curtis A.L., Leiser S.C., Snyder K., Valentino R.J. Predator stress engages corticotropin- releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology. 2012;62(4):1737–1745. doi: 10.1016/j.neuropharm.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valdez G.R., Platt D.M., Rowlett J.K., Rüedi-Bettschen D., Spealman R.D. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J. Pharmacol. Exp. Ther. 2007;323(2):525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- 51.Van Bockstaele E.J., Reyes B.A., Valentino R.J. The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res. 2010;1314:162–174. doi: 10.1016/j.brainres.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreibich A., Reyes B.A., Curtis A.L., Ecke L., Chavkin C., Van Bockstaele E.J., Valentino R.J. Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J. Neurosci. 2008;28(25):6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemeth C.L., Paine T.A., Rittiner J.E., Béguin C., Carroll F.I., Roth B.L., Cohen B.M., Carlezon W.A., Jr Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology (Berl.) 2010;210(2):263–274. doi: 10.1007/s00213-010-1834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carr G.V., Lucki I. Comparison of the kappa-opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague Dawley rats. Psychopharmacology (Berl.) 2010;210(2):295–302. doi: 10.1007/s00213-010-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peters M.F., Zacco A., Gordon J., Maciag C.M., Litwin L.C., Thompson C., Schroeder P., Sygowski L.A., Piser T.M., Brugel T.A. Identification of short-acting κ-opioid receptor antagonists with anxiolytic-like activity. Eur. J. Pharmacol. 2011;661(1-3):27–34. doi: 10.1016/j.ejphar.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 56.Al-Hasani R., McCall J.G., Bruchas M.R. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front. Pharmacol. 2013;4:96. doi: 10.3389/fphar.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.del Rosario Capriles N., Cancela L.M. Motivational effects mu- and κ-opioid agonists following acute and chronic restraint stress: involvement of dopamine D(1) and D(2) receptors. Behav. Brain Res. 2002;132(2):159–169. doi: 10.1016/S0166-4328(01)00414-4. [DOI] [PubMed] [Google Scholar]
- 58.Mamiya T., Hasegawa Y., Hiramatsu M. Dynorphin a (1-13) alleviated stress-induced behavioral impairments in mice. Biol. Pharm. Bull. 2014;37(8):1269–1273. doi: 10.1248/bpb.b14-00006. [DOI] [PubMed] [Google Scholar]
- 59.Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chartoff E.H., Potter D., Damez-Werno D., Cohen B.M., Carlezon W.A., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33(11):2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kivell B., Uzelac Z., Sundaramurthy S., Rajamanickam J., Ewald A., Chefer V., Jaligam V., Bolan E., Simonson B., Annamalai B., Mannangatti P., Prisinzano T.E., Gomes I., Devi L.A., Jayanthi L.D., Sitte H.H., Ramamoorthy S., Shippenberg T.S. Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology. 2014;86:228–240. doi: 10.1016/j.neuropharm.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hjelmstad G.O., Fields H.L. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J. Neurophysiol. 2003;89(5):2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- 63.Kelley A.E., Berridge K.C. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochem. Soc. Trans. 2009;37(Pt 1):313–317. doi: 10.1042/BST0370313. [DOI] [PubMed] [Google Scholar]
- 65.Andersen M.L., Sawyer E.K., Howell L.L. Contributions of neuroimaging to understanding sex differences in cocaine abuse. Exp. Clin. Psychopharmacol. 2012;20(1):2–15. doi: 10.1037/a0025219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aron A., Fisher H., Mashek D.J., Strong G., Li H., Brown L.L. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J. Neurophysiol. 2005;94(1):327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- 67.Gerra G., Leonardi C., Cortese E., D’Amore A., Lucchini A., Strepparola G., Serio G., Farina G., Magnelli F., Zaimovic A., Mancini A., Turci M., Manfredini M., Donnini C. Human kappa opioid receptor gene (OPRK1) polymorphism is associated with opiate addiction. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144B(6):771–775. doi: 10.1002/ajmg.b.30510. [DOI] [PubMed] [Google Scholar]
- 68.Shippenberg T.S., Zapata A., Chefer V.I. Dynorphin and the pathophysiology of drug addiction. Pharmacol. Ther. 2007;116(2):306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butelman E.R., Yuferov V., Kreek M.J. κ-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35(10):587–596. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nestler E.J., Carlezon W.A., Jr The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Taylor G.T., Cabrera O., Hoffman J. The neuroendocrinology of anhedonia. In: Ritsner M.S., editor. Anhedonia: A Comprehensive Handbook. Vol. 1. London: Springer; 2014. pp. 209–244. [Google Scholar]
- 72.Mague S.D., Pliakas A.M., Todtenkopf M.S., Tomasiewicz H.C., Zhang Y., Stevens W.C., Jr, Jones R.M., Portoghese P.S., Carlezon W.A., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther. 2003;305(1):323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 73.Reindl J.D., Rowan K., Carey A.N., Peng X., Neumeyer J.L., McLaughlin J.P. Antidepressant-like effects of the novel kappa opioid antagonist MCL-144B in the forced-swim test. Pharmacology. 2008;81(3):229–235. doi: 10.1159/000112867. [DOI] [PubMed] [Google Scholar]
- 74.Rorick-Kehn L.M., Witkin J.M., Statnick M.A., Eberle E.L., McKinzie J.H., Kahl S.D., Forster B.M., Wong C.J., Li X., Crile R.S., Shaw D.B., Sahr A.E., Adams B.L., Quimby S.J., Diaz N., Jimenez A., Pedregal C., Mitch C.H., Knopp K.L., Anderson W.H., Cramer J.W., McKinzie D.L. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014;77:131–144. doi: 10.1016/j.neuropharm.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 75.Deussing J.M. Animal models of depression. Disease Models. 2006;3(4):375–383. doi: 10.1016/j.ddmod.2006.11.003.. [DOI] [Google Scholar]
- 76.Makino M., Kitano Y., Komiyama C., Hirohashi M., Takasuna K. Involvement of central opioid systems in human interferon-α induced immobility in the mouse forced swimming test. Br. J. Pharmacol. 2000;130(6):1269–1274. doi: 10.1038/sj.bjp.0703432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ukai M., Suzuki M., Mamiya T. Effects of U-50,488H, a κ-opioid receptor agonist, on the learned helplessness model of depression in mice. J Neural Transm (Vienna) 2002;109(9):1221–1225. doi: 10.1007/s00702-002-0764-x. [DOI] [PubMed] [Google Scholar]
- 78.Russell S.E., Rachlin A.B., Smith K.L., Muschamp J., Berry L., Zhao Z., Chartoff E.H. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U- 50488 in rats. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.07.042.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fuentealba J.A., Gysling K., Magendzo K., Andrés M.E. Repeated administration of the selective kappa-opioid receptor agonist U-69593 increases stimulated dopamine extracellular levels in the rat nucleus accumbens. J. Neurosci. Res. 2006;84(2):450–459. doi: 10.1002/jnr.20890. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H., Shi Y.G., Woods J.H., Watson S.J., Ko M.C. Central kappa-opioid receptor- mediated antidepressant-like effects of nor- Binaltorphimine: behavioral and BDNF mRNA expression studies. Eur. J. Pharmacol. 2007;570:89–96. doi: 10.1016/j.ejphar.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan F., Roth B.L. Salvinorin A: a novel and highly selective kappa-opioid receptor agonist. Life Sci. 2004;75(22):2615–2619. doi: 10.1016/j.lfs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 82.Baggott M.J., Erowid E., Erowid F., Galloway G.P., Mendelson J. Use patterns and self-reported effects of Salvia divinorum: an internet-based survey. Drug Alcohol Depend. 2010;111(3):250–256. doi: 10.1016/j.drugalcdep.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 83.González D., Riba J., Bouso J.C., Gómez-Jarabo G., Barbanoj M.J. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006;85(2):157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Addy P.H. Acute and post-acute behavioral and psychological effects of salvinorin A in humans. Psychopharmacology (Berl.) 2012;220(1):195–204. doi: 10.1007/s00213-011-2470-6. [DOI] [PubMed] [Google Scholar]
- 85.Maqueda A.E., Valle M., Addy P.H., Antonijoan R.M., Puntes M., Coimbra J., Ballester M.R., Garrido M., González M., Claramunt J., Barker S., Johnson M.W., Griffiths R.R., Riba J. Salvinorin-A induces intense dissociative effects, blocking external sensory perception and modulating interoception and sense of body ownership in humans. Int. J. Neuropsychopharmacol. 2015;18(12):pyv065. doi: 10.1093/ijnp/pyv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson M.W., MacLean K.A., Reissig C.J., Prisinzano T.E., Griffiths R.R. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug Alcohol Depend. 2011;115(1-2):150–155. doi: 10.1016/j.drugalcdep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ranganathan M., Schnakenberg A., Skosnik P.D., Cohen B.M., Pittman B., Sewell R.A., D’Souza D.C. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist Salvinorin A in humans. Biol. Psychiatry. 2012;72(10):871–879. doi: 10.1016/j.biopsych.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serra V., Fattore L., Scherma M., Collu R., Spano M.S., Fratta W., Fadda P. Behavioural and neurochemical assessment of salvinorin A abuse potential in the rat. Psychopharmacology (Berl.) 2015;232(1):91–100. doi: 10.1007/s00213-014-3641-z. [DOI] [PubMed] [Google Scholar]
- 89.Listos J., Merska A., Fidecka S. Pharmacological activity of salvinorin A, the major component of Salvia divinorum. Pharmacol. Rep. 2011;63(6):1305–1309. doi: 10.1016/S1734-1140(11)70694-6. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y., Butelman E.R., Schlussman S.D., Ho A., Kreek M.J. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl.) 2005;179(3):551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- 91.White K.L., Scopton A.P., Rives M.L., Bikbulatov R.V., Polepally P.R., Brown P.J., Kenakin T., Javitch J.A., Zjawiony J.K., Roth B.L. Identification of novel functionally selective κ-opioid receptor scaffolds. Mol. Pharmacol. 2014;85(1):83–90. doi: 10.1124/mol.113.089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vortherms T.A., Roth B.L. Salvinorin A: from natural product to human therapeutics. Mol. Interv. 2006;6(5):257–265. doi: 10.1124/mi.6.5.7. [DOI] [PubMed] [Google Scholar]
- 93.Kivell B.M., Ewald A.W., Prisinzano T.E. Salvinorin A analogs and other κ-opioid receptor compounds as treatments for cocaine abuse. Adv. Pharmacol. 2014;69:481–511. doi: 10.1016/B978-0-12-420118-7.00012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simonson B., Morani A.S., Ewald A.W., Walker L., Kumar N., Simpson D., Miller J.H., Prisinzano T.E., Kivell B.M. Pharmacology and anti-addiction effects of the novel κ opioid receptor agonist Mesyl Sal B, a potent and long-acting analogue of salvinorin A. Br. J. Pharmacol. 2015;172(2):515–531. doi: 10.1111/bph.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gentilucci L. Addressing the interactions between opioid ligands and their receptors: classic and nonclassic cases. In: Nyberg F.J., editor. Neuropeptides in Neuroprotection and Neuroregeneration. Boca Raton, Florida: CRC; 2012. pp. 229–243. [Google Scholar]
- 96.Todtenkopf M.S., Marcus J.F., Portoghese P.S., Carlezon W.A., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl.) 2004;172(4):463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 97.Potter D.N., Damez-Werno D., Carlezon W.A., Jr, Cohen B.M., Chartoff E.H. Repeated exposure to the κ-opioid receptor agonist salvinorin A modulates extracellular signal-regulated kinase and reward sensitivity. Biol. Psychiatry. 2011;70(8):744–753. doi: 10.1016/j.biopsych.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ebner S.R., Roitman M.F., Potter D.N., Rachlin A.B., Chartoff E.H. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl.) 2010;210(2):241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carlezon W.A., Jr, Béguin C., DiNieri J.A., Baumann M.H., Richards M.R., Todtenkopf M.S., Rothman R.B., Ma Z., Lee D.Y., Cohen B.M. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J. Pharmacol. Exp. Ther. 2006;316(1):440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 100.Braida D., Limonta V., Capurro V., Fadda P., Rubino T., Mascia P., Zani A., Gori E., Fratta W., Parolaro D., Sala M. Involvement of kappa-opioid and endocannabinoid system on Salvinorin A-induced reward. Biol. Psychiatry. 2008;63(3):286–292. doi: 10.1016/j.biopsych.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 101.Braida D., Capurro V., Zani A., Rubino T., Viganò D., Parolaro D., Sala M. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br. J. Pharmacol. 2009;157(5):844–853. doi: 10.1111/j.1476-5381.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morani A.S., Schenk S., Prisinzano T.E., Kivell B.M. A single injection of a novel kappa opioid receptor agonist salvinorin A attenuates the expression of cocaine-induced behavioral sensitization in rats. Behav. Pharmacol. 2012;23:162–170. doi: 10.1097/FBP.0b013e3283512c1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berrocoso E., Sánchez-Blázquez P., Garzón J., Mico J.A. Opiates as antidepressants. Curr. Pharm. Des. 2009;15(14):1612–1622. doi: 10.2174/138161209788168100. [DOI] [PubMed] [Google Scholar]
- 104.Olianas M.C., Dedoni S., Onali P. The atypical antidepressant mianserin exhibits agonist activity at kappa-opioid receptors. Br. J.Pharmacol. 2012;167:1329–1341. doi: 10.1111/j.1476-5381.2012.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gehrke B.J., Chefer V.I., Shippenberg T.S. Effects of acute and repeated administration of salvinorin A on dopamine function in the rat dorsal striatum. Psychopharmacology (Berl.) 2008;197(3):509–517. doi: 10.1007/s00213-007-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walentiny D.M., Vann R.E., Warner J.A., King L.S., Seltzman H.H., Navarro H.A., Twine C.E., Jr, Thomas B.F., Gilliam A.F., Gilmour B.P., Carroll F.I., Wiley J.L. Kappa opioid mediation of cannabinoid effects of the potent hallucinogen, salvinorin A, in rodents. Psychopharmacology (Berl.) 2010;210(2):275–284. doi: 10.1007/s0021301018276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rothman R.B., Murphy D.L., Xu H., Godin J.A., Dersch C.M., Partilla J.S., Tidgewell K., Schmidt M., Prisinzano T.E. Salvinorin A: allosteric interactions at the mu-opioid receptor. Pharmacol. Exper. Therap. 2007;320:801–810. doi: 10.1124/jpet.106.113167.. [DOI] [PubMed] [Google Scholar]
- 108.Seeman P., Guan H.C., Hirbec H. Dopamine D2High receptors stimulated by phencyclidines, lysergic acid diethylamide, salvinorin A, and modafinil. Synapse. 2009;63(8):698–704. doi: 10.1002/syn.20647. [DOI] [PubMed] [Google Scholar]
- 109.Knoll A.T., Carlezon W.A., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lawson K.P., Nag S., Thompson A.D., Mokha S.S. Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and antihyperalgesia. Pain. 2010;151(3):806–815. doi: 10.1016/j.pain.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y., Tang K., Inan S., Siebert D., Holzgrabe U., Lee D.Y., Huang P., Li J.G., Cowan A., Liu-Chen L.Y. Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J. Pharmacol. Exp. Ther. 2005;312(1):220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 112.Franceschelli A., Herchick S., Thelen C., Papadopoulou-Daifoti Z., Pitychoutis P.M. Sex differences in the chronic mild stress model of depression. Behav. Pharmacol. 2014;25(5-6):372–383. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 113.Rasakham K., Liu-Chen L.-Y. Sex differences in kappa opioid pharmacology. Life Sci. 2011;88:2–16. doi: 10.1016/j.lfs.2010.10.007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Butelman E.R., Mandau M., Tidgewell K., Prisinzano T.E., Yuferov V., Kreek M.J. Effects of salvinorin A, a kappa-opioid hallucinogen, on a neuroendocrine biomarker assay in nonhuman primates with high kappa-receptor homology to humans. J. Pharmacol. Exp. Ther. 2007;320(1):300–306. doi: 10.1124/jpet.106.112417. [DOI] [PubMed] [Google Scholar]
- 115.Schmidt M.D., Schmidt M.S., Butelman E.R., Harding W.W., Tidgewell K., Murry D.J., Kreek M.J., Prisinzano T.E. Pharmacokinetics of the plant-derived kappa-opioid hallucinogen salvinorin A in nonhuman primates. Synapse. 2005;58(3):208–210. doi: 10.1002/syn.20191. [DOI] [PubMed] [Google Scholar]
- 116.Martínez-Mota L., Ulloa R.E., Herrera-Pérez J., Chavira R., Fernández-Guasti A. Sex and age differences in the impact of the forced swimming test on the levels of steroid hormones. Physiol. Behav. 2011;104(5):900–905. doi: 10.1016/j.physbeh.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 117.Sun M.K., Alkon D.L. Differential gender-related vulnerability to depression induction and converging antidepressant responses in rats. J. Pharmacol. Exp. Ther. 2006;316(2):926–932. doi: 10.1124/jpet.105.093948. [DOI] [PubMed] [Google Scholar]
- 118.Taylor G.T., Boggiano J., Cabrera O., Kroutil K. Steroidal influences on anxiety disorders in childhood and their animal models. Curr. Top. Steroid Res. 2011;8:47–64. [Google Scholar]
- 119.Hill M.N., Hellemans K.G., Verma P., Gorzalka B.B., Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci. Biobehav. Rev. 2012;36(9):2085–2117. doi: 10.1016/j.neubiorev.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl.) 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]