Abstract
Alzheimer's disease (AD) is the most common neurodegenerative disease in the elderly population. Despite significant advancements in understanding the genetic and molecular basis of AD, the pathology still lacks treatments that can slow down or reverse the progression of cognitive deterioration. Recently, the relationship between nutrient deficiency and dementia onset has been highlighted. AD is in fact a multifactorial pathology, so that a multi-target approach using combinations of micronutrients and drugs could have beneficial effects on cognitive function in neurodegenerative brain disorders leading to synaptic degeneration. Primarily, this review examines the most recent literature regarding the effects of nutrition on the risk/progression of the disease, focusing attention mostly on antioxidants agents, polyunsaturated fatty acids and metals. Secondly, it aims to figure out if nutritional supplements might have beneficial effects on drug therapy outcome. Even if nutritional supplements showed contrasting evidence of a likely effect of decreasing the risk of AD onset that could be studied more deeply in other clinical trials, no convincing data are present about their usefulness in combination with drug therapies and their effectiveness in slowing down the disease progression.
Keywords: Acetylcholinesterase inhibitors, Alzheimer, antioxidant, metals, nutritional supplements, PUFAs.
INTRODUCTION
Alzheimer's disease (AD) represents 45-60% of dementia cases [1]. It affects an estimated 20-30 million people worldwide and more than 50% are over 85 years [2, 3]. Histopathological features in the AD brain include synaptic and neuronal loss, extracellular deposition of amyloid β (Aβ) peptide in the formation of senile plaques, and neurofibrillary tangles due to the intraneuronal hyper-phosphorylated tau protein precipitation [4]. Clinically, AD is characterized by multiple domaincognitive involvement with a progressive and irreversible deterioration. Currently, no satisfying advance in understanding the cause of this disease is available and AD still lacks disease-modifying treatments that can slow down or reverse the progression of cognitive deterioration.
The relationship between nutrient deficiency and dementia onset has been recently highlighted. Analysis of various endogenous and exogenous protective factors in the serum of AD patients detected a significant decrease in glutathione peroxidase levels, vitamin E, vitamin C, carotenoids, zinc, transferrin and albumin [5-8]. This could reflect an incorrect diet.
As suggested by recent reviews, a nutritional approach to counteract onset and progression of neurodegenerative disease might be recommended [8-9], but is still under discussion.
The purpose of this paper is to give an overall view of the role of food-derived nutrients and compounds, such as antioxidants, unsaturated fatty acids and metals on AD patients. Secondly, it aims to figure out how they influence mental health and cognition as well as therapy outcome, updating the latest findings and ideas on these topics already present in current available reviews.
ANTIOXIDANT AGENTS
The nervous system is more vulnerable to oxidative stress than other systems. The higher brain energy demand, in fact, leads to increased oxygen consumption, which in turn results in an excessive production of reactive oxygen species. Moreover, the brain is relatively poor in antioxidants and its membranes are more susceptible to free radical attack because rich in polyunsaturated fatty acids [20]. Since oxidative stress and inflammation appear to be involved in brain aging and in neurodegenerative diseases [21], it is theorized that an increased intake of antioxidants could be effective in preventing or ameliorating these changes.
The use of antioxidant agents to prevent neuronal damage or to delay its progression is currently studied at both experimental and clinical stages (Table 1). Several antioxidants are able to protect cultured neurons against Aβ toxicity, as well as against oxidative stress produced by other important factors involved in the disease pathogenesis [22]. These antioxidants include Vitamin E. Vitamin E is fat-soluble and protects cell membranes from damage by reactive oxygen species such as peroxyl-radicals during fat oxidation. Studies in animal models of neurodegenerative diseases have demonstrated a significant positive effect of vitamin E in ameliorating neurodegeneration [23, 24]. A 4-month 500 IU/kg vitamin E supplemented diet in rats was able to counteract the effects of fluid percussion injury by upregulating genes involved in resistance to oxidative stress (e.g. superoxide dismutase, Sir2) [23]. Similarly, a 4-week vitamin E supplemented diet in Tg2576 mice subjected to repetitive concussive brain injury decreased brain lipid peroxidation levels, attenuated learning deficits and did not lead to the increase of Aβ peptide deposition as in control mice [24].
Table 1.
Summary of nutritional supplements and principal effects in different study models of Alzheimer’s disease.
| Treatment | Model of Study | Time | Effects/Outcome | Refs. |
|---|---|---|---|---|
| Vitamin E | Rat | 4 months | -Upregulation of genes involved in resistance to oxidative stress counteraction of the effects of fluid percussion injury | [23] |
| Vitamin E | AD mouse model | 1 month | -Decreased brain lipid peroxidation levels-Attenuated learning deficits | [24] |
| Vitamin E + Vitamin C | Human, elderly population | 5 years follow-up | -Reduced prevalence and incidence of AD | [26] |
| Vitamin E + Vitamin C + β-carotene | Human, meta-analysis | -Lower risk of AD-Vitamin E plays the pivotal role | [28] | |
| Vitamin E ± Vitamin C | Dementia-free community | 13 years follow-up | -No delay in dementia or AD incidence | [33] |
| Vitamin E | Dementia-free community | 5.5 years follow-up | -No protective role against dementia | [34] |
| Vitamin E | Human, MCI patients | 3 years | -No affection of disease progression | [35] |
| Vitamin E | Human, AD patients | 6 months | -Cognitive status maintenance, in patients where Vit E lowered oxidative stress status-Detrimental cognitive effects, in patients where Vit E did not affect oxidative stress status | [36] |
| Fig | AD mouse model | 15 months | -Reduction of plasma Aβ(1-40) and Aβ(1-42)-Enhanced activity of antioxidant enzymes in cortex and hippocampus | [40] |
| Fig | AD mouse model | 15 months | -Improvement of cognitive and behavioral deficits | [41] |
| Resveratrol | Age-related AD mouse model | 2 months | -Increased mean life expectancy and life span-Decreased amyloid burden and reduced tau hyper phosphorylation | [45] |
| Alcohol-free wine | Human, young volunteers | 1 week | - the activity of the antioxidant enzymes is not dueto the alcohol content in wine but to the polyphenolic composition | [46] |
| Ginkbo Biloba extract (EGb 761) | Rat | 2 weeks | -Cardio protective effect-Inhibition of free radical formation | [47] |
| Ginkbo Biloba extract (EGb 761) | N2a cell line stably expressing Swedish mutant APP695 and the exon-9 deletion mutant PS1 | -Neuroprotective effects (e.g. attenuation of apoptosis and direct inhibition of Aβaggregation) | [48] | |
| Ginkbo Biloba extract (EGb 761) | Human | 6.1 years follow-up | -Not effective in reducing either the overall incidence rate of dementia or AD incidence | [49] |
| ω3 fatty acids | Mild to moderate AD patients | 6 months | -No effects in the rate of cognitive decline | [125] |
| ω3 fatty acids | Mild to moderate AD patients | 6 months | -Positive effect on weight and appetite | [68] |
| ω3 fatty acids | Mild to moderate AD patients | 6 months | -Blood mononuclear monocytes up-regulation of genes involved in inflammation and neurodegeneration | [128] |
| ω3 fatty acids | Mild to moderate AD patients | 6 months | -No clear effect on free radical-mediated formation of F2-isoprostane or cyclooxygenase-mediated formation of prostaglandin F2α | [63] |
| ω3 fatty acids | AD mouse model | 7 months | -No protection against AD development in high-risk individuals | [129] |
| ω3 fatty acids | MCI and mild to moderate AD patients | 24 weeks | -Improvement in ADAS-cog in MCI patients but not in AD patients | [65] |
| Souvenaid | Mild AD patients | 12 weeks | -Memory improvement (delayed verbal recall) | [120] |
| Souvenaid | Mild AD patients | 24 weeks | -Good tolerance-Improvement of memory performance | [118] |
| Souvenaid | Mild to moderate AD patients | 24 weeks | -No slowing down of cognitive decline-Well tolerated in combination with standard AD medication | [121] |
| Souvenaid | Mild AD patients | 24 weeks | -Preservation of the organization of brain networks | [117] |
On the other hand, clinical studies have demonstrated mixed or little evidence for a neuroprotective role of this vitamin [24, 25]. Similarly to most non-enzymatic antioxidants, vitamin E supplementation in humans has shown to be effective only in the case of an evident deficiency. Additionally, recent studies suggested that vitamin E could be more effective in protecting from neurodegenerative disease if combined with other supplementations in both animal models and humans. The Cache County study demonstrated that vitamin E supplementation together with vitamin C, was associated with reduced prevalence and incidence of AD in elderly population [26]. Similar results were obtained by Morris et al. [27]. Li et al. studied the role of dietary intakes, instead of supplements, of the three most common antioxidants (vitamin E, vitamin C, and β-carotene) on the risk of AD. From the meta-analysis, the authors concluded that antioxidants can lower the risk of AD, with vitamin E exhibiting the most pronounced protective effects [28].
Vitamin E administration proved to be effective also coupled with selegiline (a selective irreversible monoamine oxidase inhibitor type B) in patients with moderate or severe AD, slowing down the progression of the disease in comparison with placebo [29].
Vitamin E is easily tolerated, negative side effects are unusual [30] and a low-dose supplementation combined with other agents is associated with a statistically significant reduction in all-cause mortality [31]. Moreover, it would have a protective effect on the immunological response and on heart diseases as well [32].
Nevertheless, whilst many studies support the idea of a positive role of vitamin E, other clinical trials show the opposite.
A study performed in a 616 people dementia-free community aged 65-105 in the south-eastern US where vitamin supplement use is low, showed that the use of vitamins C and/or E did not delay the incidence of dementia or AD [33]. These results are confirmed by a 5.5-year follow-up of 2969 healthy participants aged > 65, which failed to demonstrate a protective role of vitamin E against the risk of dementia [34]. The same was observed by coupling vitamin E with vitamin C [34]. Additionally, the daily administration of vitamin E (2000UI) for three years did not have any benefit in patients with mild cognitive impairment (MCI) and did not affect the risk of disease progression [35]. On the other hand, the treatment of AD patients with 800UI of vitamin E daily for six months showed interesting results: in subjects where vitamin E lowered oxidative stress, the cognitive status was maintained but not enhanced; in those in whom vitamin E did not prevent oxidative stress, detrimental effects were observed in terms of cognition [36]. So the authors concluded with the suggestion to use vitamin E only after determining its antioxidant effect in each single patient.
Eventually, recent meta-analysis have concluded that supplementation with high doses of vitamin E may not be efficient in preventing chronic degenerative diseases and could even increase mortality [37, 38].
In addition to vitamin E, other compounds present in food were recently suggested to protect against neurodegeneration through an antioxidant mechanism. Among these, the positive role of polyphenols is widely reported in the literature. Polyphenols can in fact cross the blood-brain barrier, scavenge pathological concentrations of reactive oxygen and nitrogen species, and chelate transition metal ions [39]. These chemicals can be found in many plants such as in figs and are responsible for controlling the activity of enzymes and cell receptors, with the role to protect the plant from bacterial and fungal infections and UV radiation damage, thanks to their antioxidant properties. Subash et al. have recently studied the role of figs in AD mouse - models. Fig is a classical fruit tree cultivated mostly in the Mediterranean region, and its fruits are the ones containing the highest concentrations of polyphenols, especially proanthocyanidins [40], known to have excellent radical scavenging and antioxidant properties. In their AD mouse model, Subash et al. demonstrated positive effects of a 4% fig diet, including a reduction of Aβ (1-40) and Aβ (1-42) content in plasma, an enhanced activity of antioxidant and membrane bound enzymes in cortex and hippocampus [40], and an improvement of cognitive and behavioural deficits [41].
Another high phenol-containing food and promising neuroprotective agent is berry fruit, such as blackberry, black currant, blueberry, strawberry, bilberry, and mulberry [42]. According to what Subash et al. summarized in a recent review, the enhancement in motor and cognitive behavioural performance by berries could be due to their direct effect on cell signalling with the consequent improvement of neuronal communication, antioxidant and anti-inflammatory action, calcium buffering, plasticity, stress signalling pathways and inhibition of acetylcholinesterase activity [41].
Among phenol-containing compounds, the importance of red wine in the diet is widely reported in the literature. Red wine contains lower amounts of phenols than fig or berries, but a growing body of evidence shows a putative protective role against neurodegeneration other than cardiovascular disease. Moderate to mild red wine consumption is in fact associated with a decrease risk of late onset AD [43], and resveratrol could be responsible for this beneficial effect because of its anti-inflammatory and antioxidant properties [44]. In a mouse model of age-related AD, Porquet et al. demonstrated for the first time an increase in life expectancy in mice treated with a resveratrol-supplemented diet, other than a decrease of cognitive impairment. Moreover, reduced Aβ deposition, activation of non-amyloidogenic pathway (instead of amyloidogenic) and a decrease of tau hyper phosphorylation at serine 396 (marker of disease severity) in resveratrol-treated mice hippocampus was also observed [45]. Eventually, in a recent paper, Noguer et al. demonstrated for the first time the antioxidant activity of an alcohol free red wine in humans, confirming that the activity of antioxidant enzymes (superoxide dismutase, catalase and glutathione reductase) is not due to the alcohol content in wine but to the polyphenol composition [46].
Another natural compound that was thought to be promising is Ginkgo Biloba. Ginkgo Biloba is a plant extract containing several compounds that may have positive effects on cells within the brain and the body. A major mechanism by which Ginkgo Biloba is proposed to exert its effect is by action of multiple antioxidants [47]. More recently, an in vitro study indicated that ginkgo extract has an anti-amyloid aggregation effect, suggesting another mechanism whereby Ginkgo Biloba may be beneficial in dementia prevention [48]. However, despite the positive in vitro effects, the dietary supplement of Ginkgo Biloba was found to be ineffective in reducing the development of dementia and AD in older people [49]. The trial, known as the Ginkgo Evaluation of Memory (GEM) enrolled 3069 participants aged 75 or older with normal cognition or MCI. The study excluded patients with dementia. Participants were randomly assigned to receive twice-daily doses of either 120 milligrams of Ginkgo extract or an identical-appearing placebo. During the study, 523 participants were diagnosed with dementia, 246 in the placebo group and 277 in the Ginkgo group, demonstrating that Ginkgo did not have any effect in reducing AD disease risk. However, Ginkgo did not have any adverse effects, including no evidence for increased bleeding risk in persons taking it [49].
ROLE OF SATURATED AND UNSATURATED FATTY ACIDS
Hypercholesterolemia, already known as a risk factor for atherosclerotic disease, may be a contributing factor for the development of AD [50]. The involvement of fatty acid metabolism in neurodegeneration is well established by studies investigating the role of cholesterol levels and of the ratio between saturated and polyunsaturated fatty acids (PUFAs) in the diet. A high saturated fat and cholesterol consumption increases the risk of cardiovascular disease [51]. The same feeding habit has been associated with the development of MCI [52]. On the other hand, a diet rich in PUFAs seems to protect against neurodegenerative diseases, probably because it stimulates neurogenesis [53] and reduces inflammatory response [54, 55]. It is in fact now clear that plaques per se are not sufficient to explain the occurrence and development of the disease. Even a normal senile brain can be rich in plaques and yet substantially retain its functions. The difference between a senescent and an AD brain is the presence of inflammatory activity. AD plaques are infiltrated by microglia that produce a large amount of inflammatory substances, responsible for cell dysfunction and death [56] (Table 1).
In this scenario, a properly balanced intake of ω-3 and ω-6 PUFAs could play an essential role [57]. In this respect, a significant reduction in the ratio ω-3/ω-6 PUFA seems to contribute to the onset of AD [58-60]. The ω-3 PUFAs mainly involved in this process are: α-linolenic acid (ALA, only obtainable from diet), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), that are synthesizable by the desaturation of ALA. EPA is the substrate for the production of anti-inflammatory prostaglandin E3 (PGE3); it competes with arachidonic acid (AA) for incorporation into cell membrane phospholipids, and for the active site on the COX enzymes. This competition, and the resulting formation of PGE3, could result in decreased levels of pro-inflammatory prostaglandin E2 (PGE2) [61]. DHA is largely represented in the brain and is a key player in conferring fluidity to axons and neuronal membranes; this latter effect would favour the processing of APP via the non-amyloidogenic pathway. Deficit in dietary ω-3 PUFAs appears to contribute to inflammatory signalling, apoptosis, and neural dysfunction, and it is associated with age-related cognitive decline and neurological disorders [62]. Conversely, a diet rich in saturated fats and ω-6, such as AA contained in red meat, can alter the composition of the neuronal membrane and promote inflammation [16]. The opposite effect can be obtained with a diet rich in vegetables, fruit and fish [16]. Nevertheless, recent in vivo analysis in animals and humans about PUFAs supplementation show contrasting results. DHA supplementation in a transgenic mouse model of AD led to an increased DHA brain content that was associated with a reduction in various biomarkers of AD such as amyloid plaques, and Aβ levels and with an improvement of cognitive functions [63]. The same increased DHA was also observed in cerebrospinal fluid in a recent clinical trial consisting in a 6-month supplementation [63], coupled with an inverse correlation with CSF levels of total and phosphorylated tau, and a direct correlation with soluble interleukin-1 receptor type II [63]. However, the authors did not observe any clear effects on free radical-mediated formation F2-isoprostane or cyclooxygenase-mediated formation of prostaglandin F2α in urine [64]. Eventually, a 24-week ω-3 PUFAs monotherapy improved the cognitive portion of the AD Assessment Scale in MCI subjects, but did not have any effects on mild to moderate AD patients [65].
Recently a trial involving 500 subjects studied a mixture of DHA together with choline and uridine [66]. It was observed that the association of these substances facilitated the correct synthesis of the main constituents of the neuronal membrane, in particular phosphatidylcholine, the principal constituent of soya lecithin that is used to control cholesterol. The mixture under examination promotes the synthesis of both phospholipids and proteins that make up the neuronal membrane. In treated animals, it has been seen that membranes stimulate an increase in neuronal activity, thus facilitating the development of synapses. In AD, plaque deposition in the brain destroys synapses and impairs membrane function. Synapse revitalization can therefore be a very promising way to counteract cognitive decline [66].
Regarding safety, in all reported studies, no serious adverse events from PUFAs supplementation occurred [60, 64, 65, 67], indeed, it may positively affect weight and appetite in patients with mild to moderate AD [68].
METALS
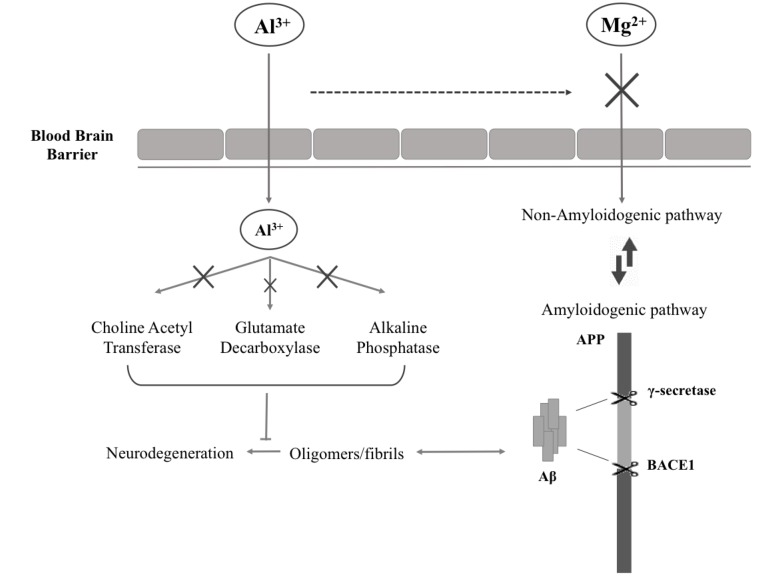
Although some authors have in the past criticized the involvement of metals in the development of neurodegenerative diseases [69], there is experimental evidence showing that dementia is associated with magnesium (Mg) insufficiency [70-73]. Mg is a metal well known for the protective action on brain tissues, although its exact role in neurodegenerative process is still not clear. Yu J. et al. in an in vitro study demonstrated that decreased total intracellular Mg level by its deprivation impairs cell viability [73]. Moreover, high extracellular Mg([Mg2+]o) stimulates the α-secretase cleavage (non-amyloidogenic) pathway by enhancing retention of Amyloid Precursor Protein (APP) on plasma membrane. The opposite effect is observed with low[Mg2+]o [73] (Fig. 1).
Fig. (1).
Al3+/Mg2+competition: mechanism of action. APP = amyloid precursor protein; Aβ = amyloid beta; BACE1 = beta secretase-1.
In vivo, Mg insufficiency could be caused either by a deficiency or by its depletion [72]. Mg deficiency is the result of a low dietetic intake and/or by a poor ability of the organism to maintain its physiological concentration. Mg depletion, in contrast, is due to the dysregulation in the mechanism controlling Mg metabolism [74, 75]. These insufficiencies could be corrected by increasing Mg intake [74]. An explanation of the phenomenon can be ascribed to aluminium (Al). Numerous studies have revealed the increased presence of Al in brain tissue obtained from autopsies of AD patients [72], indirectly related to intracellular Mg deposits in AD neurons. Al is in fact a neurotoxic metal capable of inhibiting the activity of enzymes that utilize Mg as a cofactor [71], such as choline acetyltransferase, glutamate decarboxylase and alkaline phosphatase [74]. The alteration of these enzymes leads to an injured brain, in terms of malfunction of cholinergic neurons, reduction in glutamatergic neurotransmission, formation of senile plaques and generation of neurofibrillary tangles [74] (Fig. 1). Another hypothesis involves altered serum protein content (e.g. albumin). Altered albumin, in fact, would act by binding Al with greater affinity than Mg, facilitating the transport through the blood brain barrier. This would prevent Mg brain uptake [71]. This is confirmed by the fact that Mg depletion, especially in the hippocampus, is associated with high Al incorporation into brain neurons [72].
Moreover, Mg activates the tubulin-enzyme complex involved in the maintenance of nerve tissue cells [76]. It has been suggested that in Mg-deficiency condition Al takes its place instead. This leads to tubulin inactivation and, consequently, inadequate nerve function [74].
For all his properties, Mg supplementation in the treatment of AD patients was proposed by Ozturk S. et al. [75]. The authors suggest that this metal used in conjunction with memantinecouldserve to increase memantine’s symptomatic and neuroprotective effects, via its influence on N-methil D-aspartate (NMDA) receptor [75] (see paragraph 5). Unfortunately, to our knowledge, no clinical trials have been published so far. However, Mg supplementation has been studied for other pathologies, and no specific side effects were reported [67, 77-79].
Calcium (Ca) could also play a role in the pathogenesis of dementia: experimental models of neuronal pathology are based, in fact, not only on Mg deficiency, but also on the simultaneous lack of Ca and Mg and a concomitant Al intoxication [80]. The same benefit might be played by vitamin D (vit D), by enhancing antioxidant pathways, increasing neuron growth factor production, and decreasing levels of inflammatory markers [81]. Despite in vitro studies showed promising positive effects of vit D, a clinical trial failed to demonstrate this: neither cognition nor disability changed significantly after a 8-week high dose vit D in mild to moderate AD patients [82].
Ca and vit D are often taken together, as vit D acts by increasing Ca absorption in the intestine, and both are thought to influence neuronal functioning [83]. However, a recent randomized trial aimed to examine the effect of Ca and vit D on cognitive outcome in elderly women showed no association between the treatment and the incidence of dementia, MCI or cognitive function [83].
CURRENT DRUG THERAPIES FOR MEMORY LOSS AND COGNITIVE FUNCTION
The U.S. Food and Drug Administration (FDA) has approved two types of medications in order to treat cognitive symptoms (e.g. memory loss, confusion, problems with thinking and judgment) of AD. These include acetyl cholinesterase (AchE) inhibitors and memantine (Table 2).
Table 2.
Therapy options for Alzheimer’s disease. IR = immediate release; ODT = orally disintegrating; ER = extended release; NMDA = N-methyl-D-aspartate.
| Drug | Brand Name | Approved for | Year | Dosageform |
|---|---|---|---|---|
| Cholinesterase inhibitor | ||||
| Donepezil | Aricept® | All stages | 1996 | IR tablet ODT |
| Galantamine | Razadyne® | Mild to Moderate | 2001 | IR tabletsER tablets |
| Rivastigmine | Exelon® | All stages | 2000 | IR capsulesOralsolution |
| NMDA-receptor antagonist | ||||
| Memantine | Namenda® | Moderate to severe | 2003 | IR tablets |
| Cholinesterase inhibitor/NMDA-receptor antagonist | ||||
| Donepezil and Memantine | Namzaric® | Moderate to severe | 2014 | / |
AchE inhibitors are mostly used for treating mild to moderate AD [84]. The therapeutic strategy is to increase the persistence of synaptic acetylcholine by blocking its degradation, leading to an increased activation of cholinergic receptor [85]. Thus, the increased residence of acetylcholine molecules within synapses by inhibiting AchE can at least partially counteract a deficiency in either the release of neurotransmitter or a reduction in cholinergic receptors/signaling [85], delaying the breaking down process.
The most prescribed AchE inhibitor is donepezil, better known as Aricept®, the only one approved for all stages of AD. Donepezil, unlike the other AchE inhibitors, acts also in the first stages of the pathology, when cognitive symptoms are still mild and the everyday life of the patient is not compromised. According to the latest clinical trials, the drug improves cognitive functions and daily activities (e.g. decreased ADAS-Cog total scores and Activity of Daily Living scores) through the promotion of α-secretase activity and the decreased of β-secretase activity in platelets [86]. Donepezil decreases P300 latency (parameter involved in decision-making) together with the improvement of cognitive capability, in terms of remote memory, recent memory, visual instruction, and orientation [87], and stabilize the connectivity of medial temporal regions during resting state and of brain efficiency during a cognitive demand [88]. Moreover, it improves the neuropsychiatric inventory (NPI) and Behave-AD total scores after one-year of treatment [89], it reduces caregiver burden [90] and, if taken in the morning, can even improve the sleeping state [91]. Some studies indicate that donepezil efficacy might be influenced by a genetic factor; in particular, AD patients with mutant allele (*10) in CYP2D6 gene were found to respond better to the drug than those with wild allele (*1) [92], while rs1080985 polymorphism could be accountable for poor response [93, 94]. ApoE ε4 does not seem to be associated with the efficacy of donepezil [92]. The most effective dose is 23 mg/die instead of the 10 mg/die dose approved initially by the FDA [95, 96]. Higher concentrations of the drug in plasma, in fact, improves long-term memory in patients with mild AD and imply the possible benefits for advanced stages of AD [97]. Long-term treatment with 23 mg/die dosage does not cause an elevated incidence of adverse effects, neither alone [98] nor in association with memantine [99], compared with lower dosage.
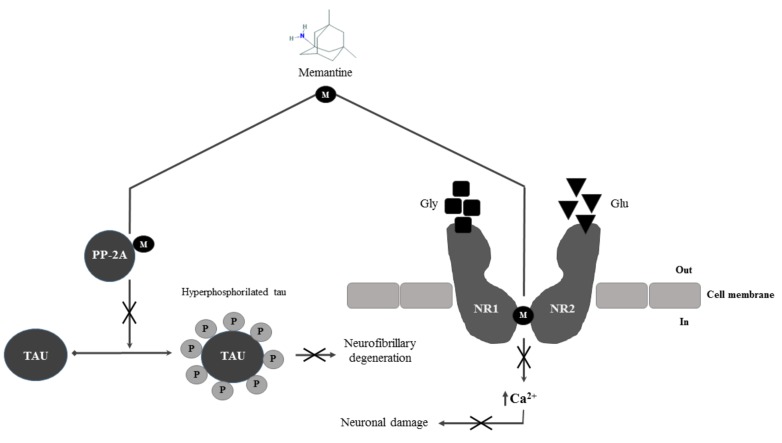
Other than AchE inhibitors, in 2003 FDA accepted thelow to moderate affinity uncompetitive N-methyl-d-aspartate (NMDA) receptor antagonistmemantine [100, 101] with similar effects on the management of disease progression (Fig. 2). Memantine is currently approved for moderate to severe AD [100, 102], even though more recent clinical trials have shown positive effects in terms of efficacyin mild to moderate AD patients [102-104]. NMDA receptors are heteromeric ligand-gated ion channelsphysiologically activated by glycine and glutamate [105,106], highly permeable to Ca2+ and voltage-dependent blocked by endogenous Mg. Under resting conditions, Mg blocks the ion channel. Post-synaptic depolarization forces Mg to unbind, leading to Ca2+ influx. In AD, NMDA receptor is continuously stimulated, leading to a continuous Ca2+ influx, the principal cause of cognitive deficit and neuronal loss [101]. Memantine’s principal mechanism of action is the blockade of current flow through NMDA-receptors channels [106, 107]. Recent studies suggest that memantine may work also by reducing the activity of phosphatase A2 (PA2) [108]. PA2 activity is in fact compromised in AD brain and it is one of the main causes of the abnormal hyperphosphorylation of tau protein and neurofibrillary degeneration [108].
Fig. (2).
Memantine’s mechanism of action. M = memantine; PP-2A = protein phosphatase 2A; Gly = glycine; Glu = glutamate; NR1 =NMDA Receptor subunit 1; NR2 = NMDA Receptor subunit 2.
The use of combination therapy (AchE inhibitors with memantine) for the treatment of moderate to severe AD has been recently investigated, but results show uncertain efficacy [84]. The combination therapy results in significantly better outcome than donepezil alone in terms of cognition, activities of daily living, global outcome, behavior and tolerability in moderate to severe AD [109-111]; moreover, it reduces agitation/aggression, irritability, as well as appetite eating disturbances [112]. However, in mild to moderate AD memantine does not exert any advantage on patients already under an AchE inhibitors regimen [113, 114].
ASSOCIATION BETWEEN ALZHEIMER DRUG THERAPY AND NUTRACEUTICAL SUPPLEMENTATION
Several clinical trials have recently tried to figure out if the drug therapy outcome would beameliorated by coupling the therapy with micro and macronutrients supplementation. Studying the literature, contrasting results were found (Table 3).
Table 3.
Molecular formula and structure of the principle compounds cited in the text.
| Name | Molecular Formula | Structure |
|---|---|---|
| Antioxidants | ||
| Vitamin E | C29H50O2 |
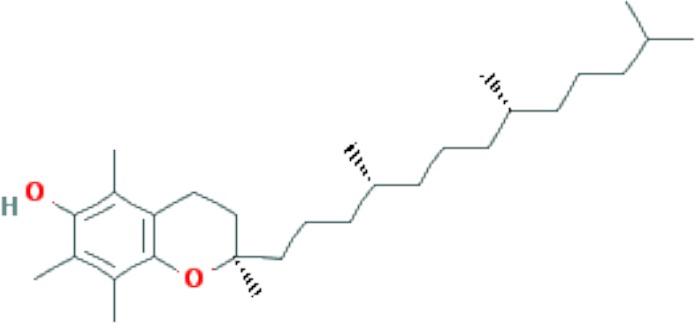
|
| Vitamin C | C6H8O6 |
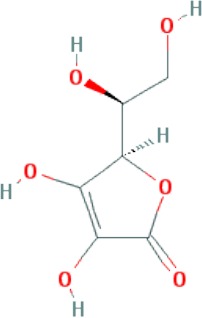
|
| β-carotene | C40H56 |
|
| Resveratrol | C14H12O3 |
|
| Proanthocyanidins | C31H28O12 |
|
| Unsaturated fatty acids | ||
| Linolenic acid (ALA) | C18H30O2 |
|
| Eicosapentaenoic acid (EPA) | C20H30O2 |
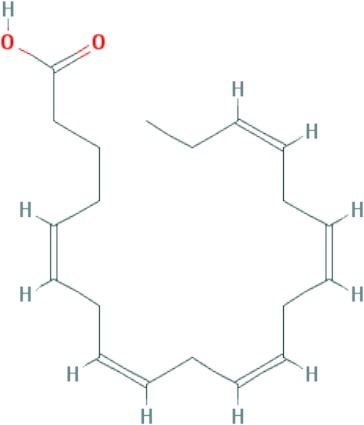
|
| Docosahexaenoic acid (DHA) | C22H32O2 |
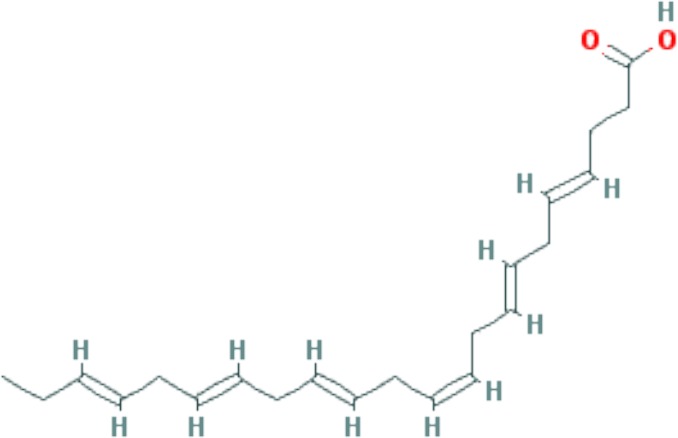
|
| Drugs | ||
| Donepezil | C24H29NO3 |
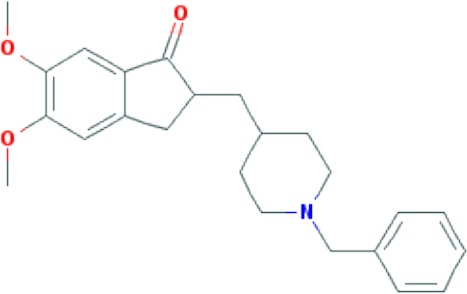
|
| Memantine | C12H21N |
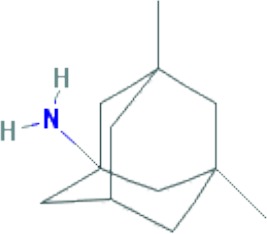
|
Souvenaid® (Nutricia N.V., Zoetermeer, The Netherlands) is the first medical nutrition product to be designed to enhance synapse formation and function in early AD, and has undergone an extensive, 12-year development program [115]. Souvenaid® is a 125-ml (125-kcal) once-daily drink [116] intended as a medical food for oral consumption aimed to address disease-specific nutrient requirements [117]. Souvenaid® contains ω-3 PUFAs (EPA and DHA), uridine (as uridine monophosphate) and choline, together with phospholipids and other cofactors [115]. Trials in drug-naïve mild-AD patients demonstrated an enhancement in memory function, an improvement on brain functional connectivity [118] (confirmed also in an animal study [119]), and a preservation of the organization of brain networks hypo-thetically counteracting the progressive network disruption over time in AD [117]. However, it did not affect Modified AD Assessment Scale-cognitive subscale and other outcome scores [120]. Despite the positive effects in naïve-drug patients, Souvenaid® did not slow cognitive decline in patients taking medications for mild-to-moderate AD [121].
The same negative outcome in improving the slowdown of the disease combining therapy with nutrients supplementation was found in vitamin E treated patients. In a three-year trial including 769 subjects, vitamin E (2000IU)did not slowdown the probability of progression or development of AD in donepezil MCI treated patients [30]. Similarly, a one-year follow up of AD subjects taking AchE inhibitors coupled with vitamin E and C supplementation did not show any effects in the pathology clinical course compared with control group [122]. Interestingly, the TEAM-AD VA randomized trial integrated the AchE inhibitors and vitamin E treatment with memantine. There were no significant differences in the groups receiving memantine alone or memantine plus vitamin E, suggesting a benefit of vitamin E in AD by slowing functional decline [123].
More promising are the results obtained with AD therapy combined with either folic acid [124] or PUFAs [125], which showed slight amelioration in cognitive function, especially in mild AD [125]. Anyway, they are all studies that need to be confirmed with other research trials.
Several authors also took into consideration the use ofa multi-target therapy. Cornelli et al. observed a significant improvement in MMSE II score in moderate AD patients treated concurrently with donepezil ± formula F, a formula containing the most common antioxidant (carnosine, coenzyme Q10, vitamin E, vitamin C, beta-carotene, selenium, L-cysteine, Ginkgo Biloba and vitamins B) for two months [126], compared with a group treated with donepezil and placebo [126]. Sun et al. did not obtain the same positive results using a multivitamin (vitamins B6 and B12 and folic acid) approach for 26 weeks, in mild-to-moderate AD patients already taking AchE inhibitors [127].
CONCLUSIONS
In this mini-review different approaches to counteract AD onset and progression were examined. Currently, there is no cure for AD, but drug and non-drug treatments may help with both cognitive and behavioral symptoms (e.g. memory loss and confusion), at least for a limited time. AD is a multifactorial pathology, so that a multi-target approach using combinations of micronutrients and drugs could have beneficial effects on cognitive function in neurodegenerative brain disorders leading to synaptic degeneration, instead of a single target therapy. From the literature analysis, we ended up with the conclusion that nutritional supplementation could in part attenuate AD risk but, although animal studies seem to be promising, human trials results are contrasting. Moreover, to our knowledge, there is no sufficient evidence to consider nutritional supplementation a factor that can help to slow down the disease progression either alone or coupled with drug therapies, especially in mild-to-moderate stage. Therefore, more clinical trials are needed, and a personalized approach should be taken into consideration.
ACKNOWLEDGEMENTS
We would like to thank Prof. Leandro Provinciali, Prof. Mauro Silvestrini, Dr. Simona Luzzi and Dr. Jacopo Sabbatinelli for their clinical support.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Vignini A., Nanetti L., Moroni C., Tanase L., Bartolini M., Luzzi S., Provinciali L., Mazzanti L. Modifications of Platelet from Alzheimer Disease Patients: A Possible Relation between Membrane Properties and NO Metabolites. 2007 doi: 10.1016/j.neurobiolaging.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D.J. Defining Molecular Targets to Prevent Alzheimer Disease. 2005. [DOI] [PubMed]
- 3.Puglielli L., Tanzi R.E., Kovacs D.M. Alzheimer’s disease: the cholesterol connection. Nat. Neurosci. 2003;6(4):345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 4.Vignini A., Giulietti A., Nanetti L., Raffaelli F., Giusti L., Mazzanti L., Provinciali L. Alzheimer’s disease and diabetes: new insights and unifying therapies. Curr. Diabetes Rev. 2013;9(3):218–227. doi: 10.2174/1573399811309030003. [DOI] [PubMed] [Google Scholar]
- 5.Lopes da Silva S., Vellas B., Elemans S., Luchsinger J., Kamphuis P., Yaffe K., Sijben J., Groenendijk M., Stijnen T. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimers Dement. 2014;10(4):485–502. doi: 10.1016/j.jalz.2013.05.1771. [DOI] [PubMed] [Google Scholar]
- 6.Lopes da Silva S., Elemans S., Kamphuis P., Sijben J., Groenendijk M. Plasma Nutrient Status of Alzheimer’s Disease Patients Compared to Cognitive Intact Elderly Controls: A Systematic Review and Meta-Analysis. Alzheimers Dement. 2012;8(4):216. doi: 10.1016/j.jalz.2012.05.2004. [DOI] [PubMed] [Google Scholar]
- 7.Jeandel C., Nicolas M.B., Dubois F., Nabet-Belleville F., Penin F., Cuny G. Lipid peroxidation and free radical scavengers in Alzheimer’s disease. Gerontology. 1989;35(5-6):275–282. doi: 10.1159/000213037. [DOI] [PubMed] [Google Scholar]
- 8.Olde Rikkert M.G., Verhey F.R., Sijben J.W., Bouwman F.H., Dautzenberg P.L., Lansink M., Sipers W.M., van Asselt D.Z., van Hees A.M., Stevens M., Vellas B., Scheltens P. Differences in nutritional status between very mild Alzheimer’s disease patients and healthy controls. J. Alzheimers Dis. 2014;41(1):261–271. doi: 10.3233/JAD-131892. [DOI] [PubMed] [Google Scholar]
- 9.Davinelli S., Calabrese V., Zella D., Scapagnini G. Epigenetic nutraceutical diets in Alzheimer’s disease. J. Nutr. Health Aging. 2014;18(9):800–805. doi: 10.1007/s12603-014-0552-y. [DOI] [PubMed] [Google Scholar]
- 10.Han J-Y., Han S-H. Primary prevention of Alzheimer’s disease: is it an attainable goal? J. Korean Med. Sci. 2014;29(7):886–892. doi: 10.3346/jkms.2014.29.7.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa S. Nutritional management of older adults with cognitive decline and dementia. Geriatr. Gerontol. Int. 2014;14(Suppl. 2):17–22. doi: 10.1111/ggi.12252. [DOI] [PubMed] [Google Scholar]
- 12.Polidori M.C., Nelles G. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease - challenges and perspectives. Curr. Pharm. Des. 2014;20(18):3083–3092. doi: 10.2174/13816128113196660706. [DOI] [PubMed] [Google Scholar]
- 13.Rijpma A., Meulenbroek O., Olde Rikkert M.G. Cholinesterase inhibitors and add-on nutritional supplements in Alzheimer’s disease: a systematic review of randomized controlled trials. Ageing Res. Rev. 2014;16:105–112. doi: 10.1016/j.arr.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Grodstein F., O’Brien J., Kang J.H., Dushkes R., Cook N.R., Okereke O., Manson J.E., Glynn R.J., Buring J.E., Gaziano M., Sesso H.D. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann. Intern. Med. 2013;159(12):806–814. doi: 10.7326/0003-4819-159-12-201312170-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mi W., van Wijk N., Cansev M., Sijben J.W., Kamphuis P.J. Nutritional approaches in the risk reduction and management of Alzheimer’s disease. Nutrition. 2013;29(9):1080–1089. doi: 10.1016/j.nut.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Morris M.C. The role of nutrition in Alzheimer’s disease: epidemiological evidence. Eur. J. Neurol. 2009;16(Suppl. 1):1–7. doi: 10.1111/j.1468-1331.2009.02735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah R. The role of nutrition and diet in Alzheimer disease: a systematic review. J. Am. Med. Dir. Assoc. 2013;14(6):398–402. doi: 10.1016/j.jamda.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Parletta N., Milte C.M., Meyer B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013;24(5):725–743. doi: 10.1016/j.jnutbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Mohajeri M.H., Troesch B., Weber P. Inadequate supply of vitamins and DHA in the elderly: implications for brain aging and Alzheimer-type dementia. Nutrition. 2015;31(2):261–275. doi: 10.1016/j.nut.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Gadoth N., Göbel H.H., editors. Oxidative Stress and Free Radical Damage in Neurology. Totowa, NJ: Humana Press; 2011. [DOI] [Google Scholar]
- 21.Casadesus G., Shukitt-Hale B., Joseph J.A. Qualitative versus quantitative caloric intake: are they equivalent paths to successful aging? Neurobiol. Aging. 2002;23(5):747–769. doi: 10.1016/S0197-4580(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 22.Doré S., Bastianetto S., Kar S., Quirion R. Protective and Rescuing Abilities of IGF-I and Some Putative Free Radical Scavengers against Beta-Amyloid-Inducing Toxicity in Neurons. 1999. [DOI] [PubMed]
- 23.Aiguo Wu, Zhe Ying, Gomez-Pinilla F., Vitamin E. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil. Neural Repair. 2010;24(3):290–298. doi: 10.1177/1545968309348318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conte V., Uryu K., Fujimoto S., Yao Y., Rokach J., Longhi L., Trojanowski J.Q., Lee V.M., McIntosh T.K., Praticò D., Vitamin E. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J. Neurochem. 2004;90(3):758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- 25.Ienco E.C., LoGerfo A., Carlesi C., Orsucci D., Ricci G., Mancuso M., Siciliano G. Oxidative stress treatment for clinical trials in neurodegenerative diseases. J. Alzheimers Dis. 2011;24(Suppl. 2):111–126. doi: 10.3233/JAD-2011-110164. [DOI] [PubMed] [Google Scholar]
- 26.Zandi P.P., Anthony J.C., Khachaturian A.S., Stone S.V., Gustafson D., Tschanz J.T., Norton M.C., Welsh-Bohmer K.A., Breitner J.C., Cache County Study Group Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch. Neurol. 2004;61(1):82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Morris M.C., Beckett L.A., Scherr P.A., Hebert L.E., Bennett D.A., Field T.S., Evans D.A., Vitamin E., Vitamin C. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1998;12(3):121–126. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Li F.J., Shen L., Ji H.F. Dietary intakes of vitamin E, vitamin C, and β-carotene and risk of Alzheimer’s disease: a meta-analysis. J. Alzheimers Dis. 2012;31(2):253–258. doi: 10.3233/JAD-2012-120349. [DOI] [PubMed] [Google Scholar]
- 29.Farlow M.R., Miller M.L., Pejovic V. Treatment options in Alzheimer’s disease: maximizing benefit, managing expectations. Dement. Geriatr. Cogn. Disord. 2008;25(5):408–422. doi: 10.1159/000122962. [DOI] [PubMed] [Google Scholar]
- 30.Petersen R.C., Thomas R.G., Grundman M., Bennett D., Doody R., Ferris S., Galasko D., Jin S., Kaye J., Levey A., Pfeiffer E., Sano M., van Dyck C.H., Thal L.J., Alzheimer’s Disease Cooperative Study Group Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 31.Jiang S., Pan Z., Li H., Li F., Song Y., Qiu Y. Meta-analysis: low-dose intake of vitamin E combined with other vitamins or minerals may decrease all-cause mortality. J. Nutr. Sci. Vitaminol. (Tokyo) 2014;60(3):194–205. doi: 10.3177/jnsv.60.194. [DOI] [PubMed] [Google Scholar]
- 32.de la Fuente M., Ferrández M.D., Burgos M.S., Soler A., Prieto A., Miquel J. Immune function in aged women is improved by ingestion of vitamins C and E. Can. J. Physiol. Pharmacol. 1998;76(4):373–380. doi: 10.1139/y98-038. [DOI] [PubMed] [Google Scholar]
- 33.Fillenbaum G.G., Kuchibhatla M.N., Hanlon J.T., Artz M.B., Pieper C.F., Schmader K.E., Dysken M.W., Gray S.L. Dementia and Alzheimer’s disease in community-dwelling elders taking vitamin C and/or vitamin E. Ann. Pharmacother. 2005;39(12):2009–2014. doi: 10.1345/aph.1G280. [DOI] [PubMed] [Google Scholar]
- 34.Gray S.L., Anderson M.L., Crane P.K., Breitner J.C., McCormick W., Bowen J.D., Teri L., Larson E. Antioxidant vitamin supplement use and risk of dementia or Alzheimer’s disease in older adults. J. Am. Geriatr. Soc. 2008;56(2):291–295. doi: 10.1111/j.1532-5415.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 35.Bennett D., Doody R., Ph D., Ferris S., Galasko D., Jin S., Kaye J., Levey A., Pfeiffer E., Sano M., Dyck C. H., Van, Thal L. J., Cooperative D., Group S. New England Journal. 2005:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 36.Lloret A., Badía M-C., Mora N.J., Pallardó F.V., Alonso M-D., Viña J., Vitamin E. Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. J. Alzheimers Dis. 2009;17(1):143–149. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- 37.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 38.Miller E.R., III, Pastor-Barriuso R., Dalal D., Riemersma R.A., Appel L.J., Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 39.Aquilano K., Baldelli S., Rotilio G., Ciriolo M.R. Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem. Res. 2008;33(12):2416–2426. doi: 10.1007/s11064-008-9697-6. [DOI] [PubMed] [Google Scholar]
- 40.Subash S., Essa M. M., Al-Asmi A., Al-Adawi S., Vaishnav R. Chronic Dietary Supplementation of 4% Figs on the Modificationof Oxidative Stress in Alzheimer’s Disease Transgenic MouseModel. Biomed Res. Int. 2014;2014:546357. doi: 10.1155/2014/546357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subash S., Essa M.M., Braidy N., Al-Jabri A., Vaishnav R., Al-Adawi S., Al-Asmi A., Guillemin G.J. Consumption of fig fruits grown in Oman can improve memory, anxiety, and learning skills in a transgenic mice model of Alzheimer’s disease. Nutr. Neurosci. 2014;••• doi: 10.1179/1476830514Y.0000000131. [DOI] [PubMed] [Google Scholar]
- 42.Subash S., Essa M.M., Al-Adawi S., Memon M.A., Manivasagam T., Akbar M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regen. Res. 2014;9(16):1557–1566. doi: 10.4103/1673-5374.139483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vingtdeux V., Dreses-Werringloer U., Zhao H., Davies P., Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl. 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastianetto S., Ménard C., Quirion R. Neuroprotective Action of Resveratrol. Biochim. Biophys. Acta. 2014 doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Porquet D., Casadesús G., Bayod S., Vicente A., Canudas A.M., Vilaplana J., Pelegrí C., Sanfeliu C., Camins A., Pallàs M., del Valle J. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age (Dordr.) 2013;35(5):1851–1865. doi: 10.1007/s11357-012-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noguer M.A., Cerezo A.B., Donoso Navarro E., Garcia-Parrilla M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol. Res. 2012;65(6):609–614. doi: 10.1016/j.phrs.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Pietri S., Maurelli E., Drieu K., Culcasi M. Cardioprotective and anti-oxidant effects of the terpenoid constituents of Ginkgo biloba extract (EGb 761). J. Mol. Cell. Cardiol. 1997;29(2):733–742. doi: 10.1006/jmcc.1996.0316. [DOI] [PubMed] [Google Scholar]
- 48.Luo Y., Smith J. V, Paramasivam V., Burdick A., Curry K. J., Buford J. P., Khan I., Netzer W. J., Xu H., Butko P. Inhibition of Amyloid-Beta Aggregation and Caspase-3 Activation by the Ginkgo Biloba Extract EGb761. Natl. Acad. Sci. U. S. A. 2002;99(19):12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeKosky S.T., Williamson J.D., Fitzpatrick A.L., Kronmal R.A., Ives D.G., Saxton J.A., Lopez O.L., Burke G., Carlson M.C., Fried L.P., Kuller L.H., Robbins J.A., Tracy R.P., Woolard N.F., Dunn L., Snitz B.E., Nahin R.L., Furberg C.D., Ginkgo Evaluation of Memory (GEM) Study Investigators Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300(19):2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blom K., Emmelot-Vonk M.H., Koek H.L. The influence of vascular risk factors on cognitive decline in patients with dementia: a systematic review. Maturitas. 2013;76(2):113–117. doi: 10.1016/j.maturitas.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Yamagishi K., Iso H., Yatsuya H., Tanabe N., Date C., Kikuchi S., Yamamoto A., Inaba Y., Tamakoshi A., JACC Study Group Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am. J. Clin. Nutr. 2010;92(4):759–765. doi: 10.3945/ajcn.2009.29146. [DOI] [PubMed] [Google Scholar]
- 52.Kalmijn S., van Boxtel M. P. J., Ocké M., Verschuren W. M. M., Kromhout D., Launer L. J. Dietary Intake of Fatty Acids and Fish in Relation to Cognitive Performance at Middle Age. Neurology. 2004;62(2):275–280. doi: 10.1212/01.WNL.0000103860.75218.A5. [DOI] [PubMed] [Google Scholar]
- 53.Valente T., Hidalgo J., Bolea I., Ramirez B., Anglés N., Reguant J., Morelló J.R., Gutiérrez C., Boada M., Unzeta M. A diet enriched in polyphenols and polyunsaturated fatty acids, LMN diet, induces neurogenesis in the subventricular zone and hippocampus of adult mouse brain. J. Alzheimers Dis. 2009;18(4):849–865. doi: 10.3233/JAD-2009-1188. [DOI] [PubMed] [Google Scholar]
- 54.Paterniti I., Impellizzeri D., Di Paola R., Esposito E., Gladman S., Yip P., Priestley J.V., Michael-Titus A.T., Cuzzocrea S. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: in-vivo and in-vitro studies. J. Neuroinflammation. 2014;11:6. doi: 10.1186/1742-2094-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang C-Y., Kuan Y-H., Li J-R., Chen W-Y., Ou Y-C., Pan H-C., Liao S-L., Raung S-L., Chang C-J., Chen C-J. Docosahexaenoic acid reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. J. Nutr. Biochem. 2013;24(12):2127–2137. doi: 10.1016/j.jnutbio.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Cai Z., Hussain M.D., Yan L-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014;124(5):307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 57.van der Beek E.M., Kamphuis P.J. The potential role of nutritional components in the management of Alzheimer’s Disease. Eur. J. Pharmacol. 2008;585(1):197–207. doi: 10.1016/j.ejphar.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 58.Lebbadi M., Julien C., Phivilay A., Tremblay C., Emond V., Kang J.X., Calon F. Endogenous conversion of omega-6 into omega-3 fatty acids improves neuropathology in an animal model of Alzheimer’s disease. J. Alzheimers Dis. 2011;27(4):853–869. doi: 10.3233/JAD-2011-111010. [DOI] [PubMed] [Google Scholar]
- 59.Corsinovi L., Biasi F., Poli G., Leonarduzzi G., Isaia G. Dietary lipids and their oxidized products in Alzheimer’s disease. Mol. Nutr. Food Res. 2011;55(Suppl. 2):S161–S172. doi: 10.1002/mnfr.201100208. [DOI] [PubMed] [Google Scholar]
- 60.Grundy T., Toben C., Jaehne E.J., Corrigan F., Baune B.T. Long-term omega-3 supplementation modulates behavior, hippocampal fatty acid concentration, neuronal progenitor proliferation and central TNF-α expression in 7 month old unchallenged mice. Front. Cell. Neurosci. 2014;8:399. doi: 10.3389/fncel.2014.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freemantle E., Vandal M., Tremblay-Mercier J., Tremblay S., Blachère J-C., Bégin M.E., Brenna J.T., Windust A., Cunnane S.C. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75(3):213–220. doi: 10.1016/j.plefa.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 62.Babenko N.A., Semenova Y.A. Effects of long-term fish oil-enriched diet on the sphingolipid metabolism in brain of old rats. Exp. Gerontol. 2010;45(5):375–380. doi: 10.1016/j.exger.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Freund Levi Y., Vedin I., Cederholm T., Basun H., Faxén Irving G., Eriksdotter M., Hjorth E., Schultzberg M., Vessby B., Wahlund L-O., Salem N., Jr, Palmblad J. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer’s disease: the OmegAD study. J. Intern. Med. 2014;275(4):428–436. doi: 10.1111/joim.12166. [DOI] [PubMed] [Google Scholar]
- 64.Freund-Levi Y., Vedin I., Hjorth E., Basun H., Faxén Irving G., Schultzberg M., Eriksdotter M., Palmblad J., Vessby B., Wahlund L-O., Cederholm T., Basu S. Effects of supplementation with omega-3 fatty acids on oxidative stress and inflammation in patients with Alzheimer’s disease: the OmegAD study. J. Alzheimers Dis. 2014;42(3):823–831. doi: 10.3233/JAD-132042. [DOI] [PubMed] [Google Scholar]
- 65.Chiu C-C., Su K-P., Cheng T-C., Liu H-C., Chang C-J., Dewey M.E., Stewart R., Huang S-Y. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(6):1538–1544. doi: 10.1016/j.pnpbp.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 66.Wurtman R.J., Cansev M., Sakamoto T., Ulus I.H. Use of Phosphatide Precursors to Promote Synaptogenesis. 2009;29:59–87. doi: 10.1146/annurev-nutr-080508-141059. [DOI] [PubMed] [Google Scholar]
- 67.Huss M., Völp A., Stauss-Grabo M. Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems - an observational cohort study. Lipids Health Dis. 2010;9:105. doi: 10.1186/1476-511X-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Irving G.F., Freund-Levi Y., Eriksdotter-Jönhagen M., Basun H., Brismar K., Hjorth E., Palmblad J., Vessby B., Vedin I., Wahlund L-O., Cederholm T. Omega-3 fatty acid supplementation effects on weight and appetite in patients with Alzheimer’s disease: the omega-3 Alzheimer’s disease study. J. Am. Geriatr. Soc. 2009;57(1):11–17. doi: 10.1111/j.1532-5415.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- 69.Ahlskog J.E., Waring S.C., Kurland L.T., Petersen R.C., Moyer T.P., Harmsen W.S., Maraganore D.M., O’Brien P.C., Esteban-Santillan C., Bush V. Guamanian neurodegenerative disease: investigation of the calcium metabolism/heavy metal hypothesis. Neurology. 1995;45(7):1340–1344. doi: 10.1212/WNL.45.7.1340. [DOI] [PubMed] [Google Scholar]
- 70.Andrási E., Igaz S., Molnár Z., Makó S. Disturbances of magnesium concentrations in various brain areas in Alzheimer’s disease. Magnes. Res. 2000;13(3):189–196. [PubMed] [Google Scholar]
- 71.Glick J.L. Dementias: the role of magnesium deficiency and an hypothesis concerning the pathogenesis of Alzheimer’s disease. Med. Hypotheses. 1990;31(3):211–225. doi: 10.1016/0306-9877(90)90095-V. [DOI] [PubMed] [Google Scholar]
- 72.Durlach J. Magnesium depletion and pathogenesis of Alzheimer’s disease. Magnes. Res. 1990;3(3):217–218. [PubMed] [Google Scholar]
- 73.Yu J., Sun M., Chen Z., Lu J., Liu Y., Zhou L., Xu X., Fan D., Chui D. Magnesium modulates amyloid-beta protein precursor trafficking and processing. J. Alzheimers Dis. 2010;20(4):1091–1106. doi: 10.3233/JAD-2010-091444. [DOI] [PubMed] [Google Scholar]
- 74.Foster H.D. How Aluminum Causes Alzheimer’ S Disease : The Implications for Prevention and Treatment of Foster’ S Multiple Antagonist Hypothesis. J. Orthomol. Med. 2000;15(1):21–51. [Google Scholar]
- 75.Ozturk S., Cillier A.E. Magnesium supplementation in the treatment of dementia patients. Med. Hypotheses. 2006;67(5):1223–1225. doi: 10.1016/j.mehy.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 76.Kentaro Yomogida S. Y.-Y. R.C.-C Chang., editor. Neurodegenerative Diseases – Processes, Prevention, Protection and Monitoring. InTech. 2011 [Google Scholar]
- 77.Mortazavi M., Moeinzadeh F., Saadatnia M., Shahidi S., McGee J.C., Minagar A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: a double-blind, randomized, placebo-controlled trial. Eur. Neurol. 2013;69(5):309–316. doi: 10.1159/000346427. [DOI] [PubMed] [Google Scholar]
- 78.Carrió M.L., Ventura J.L., Javierre C., Rodríguez-Castro D., Farrero E., Torrado H., Badia M.B., Granados J. Does post-cardiac surgery magnesium supplementation improve outcome? Magnes. Res. 2012;25(4):159–167. doi: 10.1684/mrh.2012.0324. [DOI] [PubMed] [Google Scholar]
- 79.Zorbas Y.G., Kakuris K.K., Federenko Y.F., Deogenov V.A. Utilization of magnesium during hypokinesia and magnesium supplementation in healthy subjects. Nutrition. 2010;26(11-12):1134–1138. doi: 10.1016/j.nut.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 80.Durlach J., Bac P., Durlach V., Durlach A., Bara M., Guiet-Bara A. Are age-related neurodegenerative diseases linked with various types of magnesium depletion? Magnes. Res. 1997;10(4):339–353. [PubMed] [Google Scholar]
- 81.Grant W.B. Does vitamin D reduce the risk of dementia? J. Alzheimers Dis. 2009;17(1):151–159. doi: 10.3233/JAD-2009-1024. [DOI] [PubMed] [Google Scholar]
- 82.Stein M.S., Scherer S.C., Ladd K.S., Harrison L.C. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer’s disease. J. Alzheimers Dis. 2011;26(3):477–484. doi: 10.3233/JAD-2011-110149. [DOI] [PubMed] [Google Scholar]
- 83.Rossom R.C., Espeland M.A., Manson J.E., Dysken M.W., Johnson K.C., Lane D.S., LeBlanc E.S., Lederle F.A., Masaki K.H., Margolis K.L. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J. Am. Geriatr. Soc. 2012;60(12):2197–2205. doi: 10.1111/jgs.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zemek F., Drtinova L., Nepovimova E., Sepsova V., Korabecny J., Klimes J., Kuca K. Outcomes of Alzheimer 'S DiseaseTherapy with Acetylcholinesterase Inhibitors and Memantine. 2014:759–774. doi: 10.1517/14740338.2014.914168. [DOI] [PubMed] [Google Scholar]
- 85.Pope C., Karanth S., Liu J. Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005;19(3):433–446. doi: 10.1016/j.etap.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 86.Dong G.S., Li X., Jiang Q.H., Yang H.Q. [Effects of donepezil treatment on platelets α and β secretase activities in Alzheimer’s disease patients]. Zhonghua Yi Xue Za Zhi. 2011;91(47):3341–3345. [PubMed] [Google Scholar]
- 87.Chang Y-S., Chen H-L., Hsu C-Y., Tang S-H., Liu C-K. Parallel improvement of cognitive functions and P300 latency following donepezil treatment in patients with Alzheimer’s disease: a case-control study. J. Clin. Neurophysiol. 2014;31(1):81–85. doi: 10.1097/01.wnp.0000436899.48243.5e. [DOI] [PubMed] [Google Scholar]
- 88.Solé-Padullés C., Bartrés-Faz D., Lladó A., Bosch B., Peña-Gómez C., Castellví M., Rami L., Bargalló N., Sánchez-Valle R., Molinuevo J.L. Donepezil treatment stabilizes functional connectivity during resting state and brain activity during memory encoding in Alzheimer’s disease. J. Clin. Psychopharmacol. 2013;33(2):199–205. doi: 10.1097/JCP.0b013e3182825bfd. [DOI] [PubMed] [Google Scholar]
- 89.Cumbo E., Ligori L.D. Differential effects of current specific treatments on behavioral and psychological symptoms in patients with Alzheimer’s disease: a 12-month, randomized, open-label trial. J. Alzheimers Dis. 2014;39(3):477–485. doi: 10.3233/JAD-131190. [DOI] [PubMed] [Google Scholar]
- 90.Carrasco M.M., Agüera L., Gil P., Moríñigo A., Leon T. Safety and effectiveness of donepezil on behavioral symptoms in patients with Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2011;25(4):333–340. doi: 10.1097/WAD.0b013e318212ab7a. [DOI] [PubMed] [Google Scholar]
- 91.Song H.R., Woo Y.S., Wang H-R., Jun T-Y., Bahk W-M. Effect of the timing of acetylcholinesterase inhibitor ingestion on sleep. Int. Clin. Psychopharmacol. 2013;28(6):346–348. doi: 10.1097/YIC.0b013e328364f58d. [DOI] [PubMed] [Google Scholar]
- 92.Zhong Y., Zheng X., Miao Y., Wan L., Yan H., Wang B. Effect of CYP2D6*10 and APOE Polymorphisms on the Efficacyof Donepezil in Patients with Alzheimer’s Disease. Am. J. Med.Sci. 2013;345(3):222–226. doi: 10.1097/MAJ.0b013e318255a8f9. [DOI] [PubMed] [Google Scholar]
- 93.Pilotto A., Franceschi M., D’Onofrio G., Bizzarro A., Mangialasche F., Cascavilla L., Paris F., Matera M.G., Pilotto A., Daniele A., Mecocci P., Masullo C., Dallapiccola B., Seripa D. Effect of a CYP2D6 polymorphism on the efficacy of donepezil in patients with Alzheimer disease. Neurology. 2009;73(10):761–767. doi: 10.1212/WNL.0b013e3181b6bbe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Albani D., Martinelli Boneschi F., Biella G., Giacalone G., Lupoli S., Clerici F., Benussi L., Ghidoni R., Galimberti D., Squitti R., Mariani S., Confaloni A., Bruno G., Mariani C., Scarpini E., Binetti G., Magnani G., Franceschi M., Forloni G. Replication study to confirm the role of CYP2D6 polymorphism rs1080985 on donepezil efficacy in Alzheimer’s disease patients. J. Alzheimers Dis. 2012;30(4):745–749. doi: 10.3233/JAD-2012-112123. [DOI] [PubMed] [Google Scholar]
- 95.Sabbagh M., Cummings J., Christensen D., Doody R., Farlow M., Liu L., Mackell J., Fain R. Evaluating the cognitive effects of donepezil 23 mg/d in moderate and severe Alzheimer’s disease: analysis of effects of baseline features on treatment response. BMC Geriatr. 2013;13:56. doi: 10.1186/1471-2318-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Christensen D.D. Higher-dose (23 mg/day) donepezil formulation for the treatment of patients with moderate-to-severe Alzheimer’s disease. Postgrad. Med. 2012;124(6):110–116. doi: 10.3810/pgm.2012.11.2589. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y-H., Chen C-H., Chou M-C., Li C-H., Liu C-K., Chen S-H. Concentration of donepezil to the cognitive response in Alzheimer disease. J. Clin. Psychopharmacol. 2013;33(3):351–355. doi: 10.1097/JCP.0b013e31828b5087. [DOI] [PubMed] [Google Scholar]
- 98.Tariot P., Salloway S., Yardley J., Mackell J., Moline M. Long-term safety and tolerability of donepezil 23 mg in patients with moderate to severe Alzheimer’s disease. BMC Res. Notes. 2012;5:283. doi: 10.1186/1756-0500-5-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doody R.S., Geldmacher D.S., Farlow M.R., Sun Y., Moline M., Mackell J. Efficacy and safety of donepezil 23 mg versus donepezil 10 mg for moderate-to-severe Alzheimer’s disease: a subgroup analysis in patients already taking or not taking concomitant memantine. Dement. Geriatr. Cogn. Disord. 2012;33(2-3):164–173. doi: 10.1159/000338236. [DOI] [PubMed] [Google Scholar]
- 100.Lo D., Grossberg G.T. Use of memantine for the treatment of dementia. Expert Rev. Neurother. 2011;11(10):1359–1370. doi: 10.1586/ern.11.132. [DOI] [PubMed] [Google Scholar]
- 101.Lipton S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 2006;5(2):160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 102.Bakchine S., Loft H. Memantine treatment in patients with mild to moderate Alzheimer’s disease: results of a randomised, double-blind, placebo-controlled 6-month study. J. Alzheimers Dis. 2008;13(1):97–107. doi: 10.3233/jad-2008-13110. [DOI] [PubMed] [Google Scholar]
- 103.Peskind E.R., Potkin S.G., Pomara N., Ott B.R., Graham S.M., Olin J.T., McDonald S. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am. J. Geriatr. Psychiatry. 2006;14(8):704–715. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 104.Zhang N., Wei C., Du H., Shi F-D., Cheng Y. The Effect of Memantine on Cognitive Function and Behavioral and Psychological Symptoms in Mild-to-Moderate Alzheimer’s Disease Patients. Dement. Geriatr. Cogn. Disord. 2015;40(1-2):85–93. doi: 10.1159/000430808. [DOI] [PubMed] [Google Scholar]
- 105.Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. The Time Course of Glutamate in the Synaptic Cleft. Science. 1992;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 106.Johnson J.W., Kotermanski S.E. Mechanism of action of memantine. Curr. Opin. Pharmacol. 2006;6(1):61–67. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Lipton S.A., Chen H-S. Paradigm shift in neuroprotective drug development: clinically tolerated NMDA receptor inhibition by memantine. Cell Death Differ. 2004;11(1):18–20. doi: 10.1038/sj.cdd.4401344. [DOI] [PubMed] [Google Scholar]
- 108.Li L., Sengupta A., Haque N., Grundke-Iqbal I., Iqbal K. Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett. 2004;566(1-3):261–269. doi: 10.1016/j.febslet.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 109.Tariot P.N., Farlow M.R., Grossberg G.T., Graham S.M., McDonald S., Gergel I., Memantine Study Group Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 110.Atri A., Hendrix S.B., Pejović V., Hofbauer R.K., Edwards J., Molinuevo J.L., Graham S.M. Cumulative, additive benefits of memantine-donepezil combination over component monotherapies in moderate to severe Alzheimer’s dementia: a pooled area under the curve analysis. Alzheimers Res. Ther. 2015;7(1):28. doi: 10.1186/s13195-015-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matsunaga S., Kishi T., Iwata N. Combination therapy with cholinesterase inhibitors and memantine for Alzheimer’s disease: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2015;18(5):pyu115. doi: 10.1093/ijnp/pyu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Creţu O., Szalontay A. S., Chiriţă R., Chiriţă V. . Rev. Medicochirurgicala a Soc. Medici s i Nat. din Iasi. 2008;112(3):641–645. [PubMed] [Google Scholar]
- 113.Porsteinsson A.P., Grossberg G.T., Mintzer J., Olin J.T., Memantine MEM-MD-12 Study Group Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr. Alzheimer Res. 2008;5(1):83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 114.Modrego P.J., Fayed N., Errea J.M., Rios C., Pina M.A., Sarasa M. Memantine versus donepezil in mild to moderate Alzheimer’s disease: a randomized trial with magnetic resonance spectroscopy. Eur. J. Neurol. 2010;17(3):405–412. doi: 10.1111/j.1468-1331.2009.02816.x. [DOI] [PubMed] [Google Scholar]
- 115.Ritchie C.W., Bajwa J., Coleman G., Hope K., Jones R.W., Lawton M., Marven M., Passmore P. Souvenaid®: a new approach to management of early Alzheimer’s disease. J. Nutr. Health Aging. 2014;18(3):291–299. doi: 10.1007/s12603-013-0411-2. [DOI] [PubMed] [Google Scholar]
- 116.Thaipisuttikul P., Galvin J.E. Use of medical foods and nutritional approaches in the treatment of Alzheimer’s disease. Clin. Pract. (Lond.) 2012;9(2):199–209. doi: 10.2217/cpr.12.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Waal H., Stam C.J., Lansbergen M.M., Wieggers R.L., Kamphuis P.J., Scheltens P., Maestú F., van Straaten E.C. The effect of souvenaid on functional brain network organisation in patients with mild Alzheimer’s disease: a randomised controlled study. PLoS One. 2014;9(1):e86558. doi: 10.1371/journal.pone.0086558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scheltens P., Twisk J.W., Blesa R., Scarpini E., von Arnim C.A., Bongers A., Harrison J., Swinkels S.H., Stam C.J., de Waal H., Wurtman R.J., Wieggers R.L., Vellas B., Kamphuis P.J. Efficacy of Souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. J. Alzheimers Dis. 2012;31(1):225–236. doi: 10.3233/JAD-2012-121189. [DOI] [PubMed] [Google Scholar]
- 119.Cansev M., van Wijk N., Turkyilmaz M., Orhan F., Sijben J.W., Broersen L.M. Specific multi-nutrient enriched diet enhances hippocampal cholinergic transmission in aged rats. Neurobiol. Aging. 2015;36(1):344–351. doi: 10.1016/j.neurobiolaging.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 120.Scheltens P., Kamphuis P.J., Verhey F.R., Olde Rikkert M.G., Wurtman R.J., Wilkinson D., Twisk J.W., Kurz A. Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimers Dement. 2010;6(1):1–10.e1. doi: 10.1016/j.jalz.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 121.Shah R.C., Kamphuis P.J., Leurgans S., Swinkels S.H., Sadowsky C.H., Bongers A., Rappaport S.A., Quinn J.F., Wieggers R.L., Scheltens P., Bennett D.A. The S-Connect study: results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimers Res. Ther. 2013;5(6):59. doi: 10.1186/alzrt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arlt S., Müller-Thomsen T., Beisiegel U., Kontush A. Effect of one-year vitamin C- and E-supplementation on cerebrospinal fluid oxidation parameters and clinical course in Alzheimer’s disease. Neurochem. Res. 2012;37(12):2706–2714. doi: 10.1007/s11064-012-0860-8. [DOI] [PubMed] [Google Scholar]
- 123.Dysken M.W., Sano M., Asthana S., Vertrees J.E., Pallaki M., Llorente M., Love S., Schellenberg G.D., McCarten J.R., Malphurs J., Prieto S., Chen P., Loreck D.J., Trapp G., Bakshi R.S., Mintzer J.E., Heidebrink J.L., Vidal-Cardona A., Arroyo L.M., Cruz A.R., Zachariah S., Kowall N.W., Chopra M.P., Craft S., Thielke S., Turvey C.L., Woodman C., Monnell K.A., Gordon K., Tomaska J., Segal Y., Peduzzi P.N., Guarino P.D. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311(1):33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Connelly P. J., Prentice N. P., Cousland G., Bonham J. ARandomised Double-Blind Placebo-Controlled Trial of Folic AcidSupplementation of Cholinesterase Inhibitors in Alzheimer ’ SDisease. 2008:155–160. doi: 10.1002/gps.1856. [DOI] [PubMed] [Google Scholar]
- 125.Freund-Levi Y., Eriksdotter-Jönhagen M., Cederholm T., Basun H., Faxén-Irving G., Garlind A., Vedin I., Vessby B., Wahlund L-O., Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch. Neurol. 2006;63(10):1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 126.Cornelli U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegener. Dis. 2010;7(1-3):193–202. doi: 10.1159/000295663. [DOI] [PubMed] [Google Scholar]
- 127.Sun Y., Lu C-J., Chien K-L., Chen S-T., Chen R-C. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin. Ther. 2007;29(10):2204–2214. doi: 10.1016/j.clinthera.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 128.Vedin I., Cederholm T., Freund-Levi Y., Basun H., Garlind A., Irving G. F., Eriksdotter-Jönhagen M., Wahlund L. O., Dahlman I., Palmblad J. Effects of DHA-rich n-3 fatty acid supplementation on gene expression in blood mononuclear leukocytes: the OmegAD study. PloS One. 2012;7(4):1–8. doi: 10.1371/journal.pone.0035425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arendash G. W., Jensen M. T., Salem N., Hussein N., Cracchiolo J., Dickson A., Leighty R., Potter H. A diet high in omega-3 fatty acids does not improve or protect cognitive performance in Alzheimer's transgenic mice. Neuroscience. 2007;149(2):256–302. doi: 10.1016/j.neuroscience.2007.08.018. [DOI] [PubMed] [Google Scholar]