Abstract
Glutamatergic neurotransmission, of special importance in the human brain, is implicated in key brain functions such as synaptic plasticity and memory. The excessive activation of N-methyl- D-aspartate (NMDA) receptors may result in excitotoxic neuronal damage; this process has been implicated in the pathomechanism of different neurodegenerative disorders, such as Alzheimer’s disease (AD). Memantine is an uncompetitive antagonist of NMDA receptors with a favorable pharmacokinetic profile, and is therefore clinically well tolerated. Memantine is approved for the treatment of AD, but may additionally be beneficial for other dementia forms and pain conditions. Kynurenic acid (KYNA) is an endogenous antagonist of NMDA receptors which has been demonstrated under experimental conditions to be neuroprotective. The development of a well-tolerated NMDA antagonist may offer a novel therapeutic option for the treatment of neurodegenerative disease and pain syndromes. KYNA may be a valuable candidate for future drug development.
Keywords: Dementia, glutamate, kynurenic acid, memantine, neuroprotection, NMDA.
INTRODUCTION
Glutamate is the main excitatory neurotransmitter in the human brain, and glutamate-mediated neurotransmission is of high importance in several key brain functions such as synaptic plasticity and memory formation. NMDA receptors are widely distributed in the human brain and they are of special importance in both excitatory neurotransmission and neurodegenerative processes. Under physiological resting potentials, more than 90% of the NMDA receptors are blocked by magnesium ions (Mg2+). Postsynaptic depolarization of the membranes results in the release of Mg2+ and allows NMDA activation. The voltage-dependent Mg2+ block is able to influence excitatory postsynaptic potentials, and underlies the different responses of the various subtypes of NMDA receptors. The NR2 subunit of the NMDA receptors determines the voltage dependence: the NR2A- and NR2B-containing subtypes are blocked more strongly than the NR2C or NR2D-containing subtypes [1]. The NMDA receptors are members of the ionotropic receptor family, and possess high calcium (Ca2+) permeability. The NMDA receptors also play an important role in the induction of long-term potentiation (LTP), e.g. a long-lasting increase of the synaptic strength or long-term depression, e.g. a decrease of it. LTP and long-term depression are the basis of synaptic plasticity, and are considered to be key processes in memory and learning [2].
Overactivation of the NMDA receptors results in excessive Ca2+ influx into the cells, activating various signaling pathways which may lead to neuronal damage; this process is known as excitotoxicity [3]. Excitotoxicity has been implicated in a number of pathological processes such as cerebral ischemia and neurodegenerative diseases [4, 5]. Earlier attempts to use NMDA antagonists as therapeutic agents failed despite the promising preclinical results, because they were either ineffective in the clinical setting or resulted in unacceptable side-effects, such as a cognitive impairment [6-8]. However, these results promoted a better understanding of the role of the glutamatergic neuro-transmission in physiological brain functions such as cognitive processes and memory. On the other hand, in pathological cases, where the excitatory receptors are overactivated, the inhibition of NMDA receptors may be beneficial by reestablishing the physiological glutamatergic balance, and preventing excitotoxic neuronal damage without attenuating the normal neurotransmission [9].
Memantine was the first NMDA antagonist approved for the therapy of moderate to severe Alzheimer’s disease (AD) [10, 11]. Currently no other NMDA antagonist agents are available in clinical practice, and it is still a challenge to develop effective neuroprotective drugs capable of preventing the pathological activation of NMDA receptors without impairing their physiological activity.
The kynurenine pathway (KP) of the tryptophan metabolism leads to the formation of several neuroactive molecules, including the NMDA-antagonist kynurenic acid (KYNA), which has shown promise as a neuroprotective agent in the preclinical setting. This review will focus on the neuropharmacological properties of the NMDA-antagonist memantine and KYNA, with special focus on AD, describing the similarities and future potential for drug development.
MEMANTINE
Memantine (1-amino-3,5-dimethyladamantane; Fig. (1) was first synthetized in 1968, but its NMDA-antagonistic property was discovered only in the 1980s [12, 13]. It is an uncompetitive open-channel blocker which exerts its effect by inhibiting Ca2+ influx at excessive NMDA activation, while it does not interfere with physiological activation (Fig. 2) [14]. In rats, the administration of 5-10 mg/kg memantine resulted in a plasma level of 1.0-3.2mM, while the brain levels achieved after the i.p. injection of 10 or 20mg/kg memantine were 1.2 and 2.6mM, respectively [15]. The IC50 of memantine is approximately 3µM, which is in good accordance with its therapeutic concentration range in humans [16, 17]. In AD patients, the recommended therapeutic dose is 20mg/day [11]. The administration of 5-30mg/day of memantine to humans results in cerebrospinal fluid concentrations of 0.05-0.31μM and serum concentrations of 0.025 to 0.529 μM [17, 18]. The elimination half-life of orally administered memantine in the human serum is 60–80 h [19].
Fig. (1).
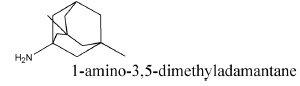
The chemical structure of memantine.
Fig. (2).
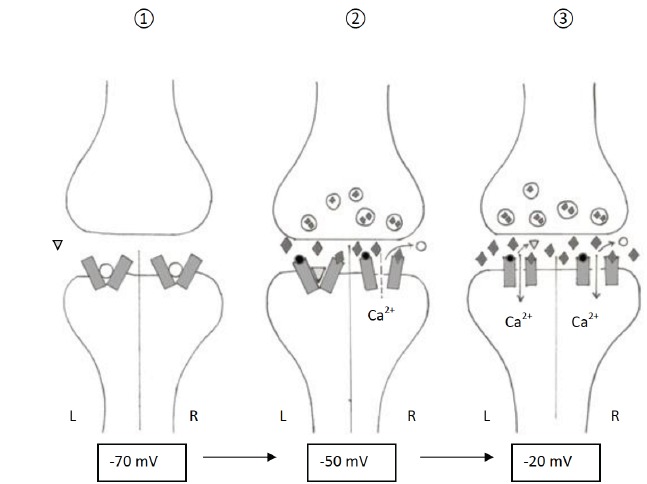
The affinity of the memantine to the NMDA receptor. ←: Resting conditions: NMDA receptors with the physiological Mg2+ block. ↑: Increased background: Left side: low to moderate affinity antagonist memantine binding to the NMDA receptor, Right side: without memantine the NMDA receptor is getting activated after the binding of glycin and glutamate. ®: Synaptic activity: Left side: after depolarization, without the memantine, the NMDA receptor is activated by the glycin and glutamate, Right side: after the depolarization the NMDA receptor becomes activated by the binding of glycin and glutamate, the Mg2+ block ceases. :memantine, : glutamate, ○:Mg2+, ●: glycin.
The experimental data indicate that memantine binds to the same channel site as Mg2+, and it does not interfere with the glutamate or glycine binding site [15]. The assumption that it shares their binding site with Mg2+ is supported by the observation that Mg2+ decreases the NMDA-antagonistic effect of memantine, and that mutations in the NR1 and NR2 subunits which are important for Mg2+ binding also influence memantine block [17, 20, 21]. Chen et al. described a slow unblocking phase of this substance from the NMDA receptors, which was inhibited by the presence of Mg2+22. Memantine has no effect on the currents evoked by kainate or quisqualate either [22]. High concentrations of this compound have been suggested to potentiate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-induced currents in neuronal cultures, but this effect was moderate and therefore its relevance is unclear [15]. Memantine displays low affinity for the NMDA receptors; the binding is strongly voltage-dependent, with fast double exponential blocking kinetics, which is strongly dependent on the agonist concentration [23, 24]. Besides NMDA antagonism, memantine has been demonstrated to influence glutamate levels. The chronic administration of memantine resulted in a decreased hippocampal glutamate level, and decreased glutamatergic neurotransmission in the frontal cortex [25, 26]. The neuroprotective effect of memantine has been confirmed by several studies, indicating that it prevents the toxic effects of NMDA and NMDA-receptor agonists in cultured cortical neurons, cultured retinal ganglion cells or the chick retina in vitro [22, 27-29].
An intriguing aspect of the glutamate antagonist memantine is its ability to improve cognitive functions. The possible explanations of this paradox effect include a decrease of synaptic “noise” induced by NMDA receptor overactivation and restoration of the physiological glutamatergic balance [15, 17]. Although NMDA receptors are necessary for some forms of LTP, the basis of the learning process, overactivation may result in impairment. In these cases, memantine may actually improve synaptic plasticity and cognition. Experimental data have indicated that it is able to prolong the duration of LTP in rats [30]. Depletion of Mg2+ results in the impairment of LTP in hippocampal slices, an effect attenuated by memantine [31]. In accordance with this, memantine also reverses the reduction of LTP in the CA1 region of the hippocampus induced by NMDA [32]. Accordingly, this drug significantly improves cognitive functions in moderate to severe AD patients and it has been approved for this indication in both the European Union and the USA [19, 33]. This effect may be partly mediated by its influence on glutamatergic neurotransmission, but it may be related in part to the counteraction of amyloid toxicity. In cultured primary cortical neurons from rats memantine was able to attenuate the tau- phosphorylation induced by Aβ1-42 [34]. In another study, memantine was able to prevent cognitive decline in triple-transgenic (3xTg-AD) mice, and the treatment also resulted in a significant reduction in the levels of insoluble amyloid beta, total tau and hyperphosphorylated tau [35].
This pharmacon might also offer a therapeutic option in other neurodegenerative disorders, such as Parkinson’s disease (PD) or Huntington’s disease (HD). In a small study, memantine slowed the progression of the neurodegenerative process in HD [36]. It also improves PD dementia and surprisingly displays beneficial symptomatic effects on the motor symptoms too [37-40]. Case reports have suggested that memantine may also improve levodopa-induced dyskinesia, but further studies are merited to confirm this [41, 42]. Another field of neurology where this drug may be beneficial is migraine. Some smaller studies have suggested that it may be able to reduce the frequency of headache [43, 44]. Further large-scale studies are awaited to confirm this observation. Neuropathic pain syndromes affect a broad range of the population, but their therapeutic management has not yet been fully resolved. Memantine effectively alleviated the development of neuropathic pain in rats and also achieved significant antinociception in an animal model of diabetic neuropathic pain [45, 46]. A clinical trial is ongoing to investigate its efficacy to prevent post-mastectomy neuropathic pain in breast cancer patients [47]. The fact that memantine is clinically well tolerated can be attributable to its favorable pharmacokinetic properties [17, 48].
KYNURENIC ACID
KYNA is one of the neuroactive metabolites synthesized in the KP of tryptophan metabolism (Fig.3). The KP produces not only the neuroprotective KYNA, but also several neurotoxic metabolites, such as quinolinic acid (QUIN) and 3-hydroxykynurenine. QUIN, an endogenous agonist of NMDA receptors, additionally induces endogenous antioxidant depletion, contributes to free radical generation and induces lipid peroxidation [49-51]. Moreover, QUIN has been confirmed to increase presynaptic glutamate release and reduce glutamate uptake by the astrocytes [52, 53]. On the other hand, KYNA is the only known broad-spectrum endogenous inhibitor affecting all ionotropic glutamate receptors. It is able to block NMDA, AMPA and kainate subtypes, but has a highest affinity for NMDA receptors which are the most permeable receptors for Ca2+ [7, 54, 55]. NMDA receptors are tetramer structures which consist of four subunits. The most prevalent forms in the brain contain NR1 and NR2 subunits. The NR1 subunit contains the glycine-binding site, whereas the NR2 subunit contains the glutamate-binding site. At low micromolar concentrations (EC50 =7.9 to 15 µM), KYNA binds with high affinity to the strychnine-insensitive glycine-binding site on the NR1 subunit of the NMDA receptors, whereas at 10-20 times higher concentrations (EC50= 200 to 500 µM) it is able to block the glutamate-binding site on the NR2 subunit as well [56, 57]. The neuroprotective effect of KYNA is mainly attributed to the prevention of glutamate excitotoxicity via antagonism of the NMDA receptors. KYNA displays antagonistic properties at the presynaptic α7 nicotinic acetylcholine receptors, inhibiting them in a noncompetitive manner, which is involved in the presynaptic regulation of glutamate release [58, 59]. Low KYNA concentrations inhibit presynaptic glutamate release, thereby contributing to its neuroprotective effect [60]. Interestingly, KYNA exerts a dose-dependent dual effect on the AMPA receptors: in the micromolar concentration range, KYNA inhibits them, whereas in low nanomolar concentrations it evokes facilitation [61, 62]. The facilitatory effect is probably associated with a positive modulatory binding site at the AMPA receptors. A recent work provided data relating to the possible molecular mechanisms, but further investigations are definitely warranted [63]. KYNA has been identified as a ligand for the previously orphan G-protein-coupled receptor too [64]. The complex molecular interactions of KYNA with the different receptors underlie its importance in the physiological processes of the central nervous system, and suggest its neuromodulatory and regulatory functions.
Fig. (3).
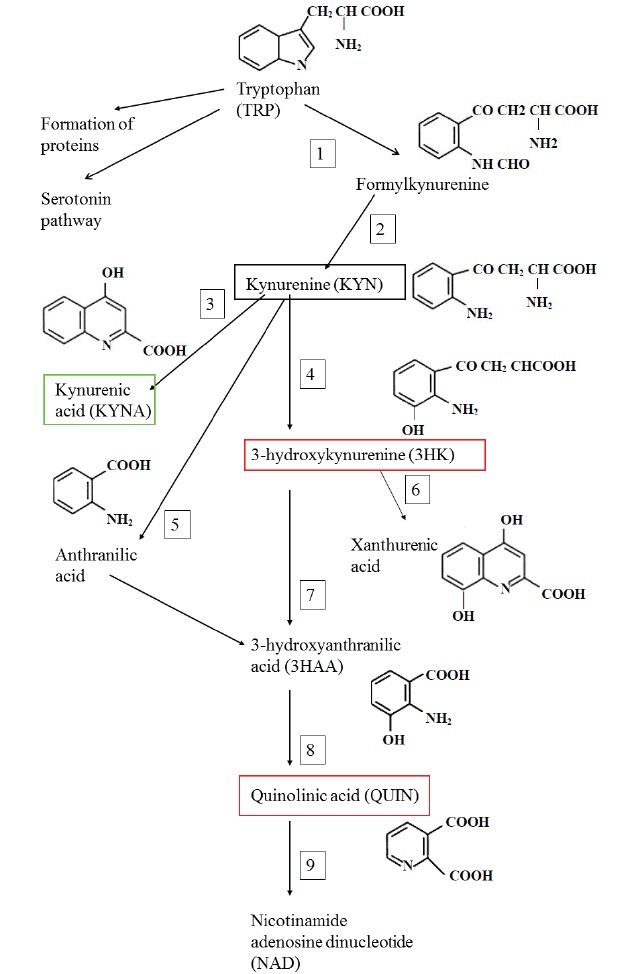
The kynurenine pathway. 1: tryptophan dioxygenase (TDO) and indoleamine-2,3-dioxygenase (IDO), 2: formamidase, 3: kynurenine aminotransferase, 4: kynurenine-3-monooxygenase (KMO), 5: kynureninase, 6: kynurenine aminotransferase, 7: kynureninase, 8: 3- hydroxyanthranilic acid dioxygenase, 9: quinolinic acid phosphoribosyltransferase.
Alterations in the delicate balance of the neurotoxic and neuroprotective compounds of the KP have been implicated in the pathomechanisms of several neurodegenerative diseases, such as AD or PD, and stroke [65, 66]. The activity of indoleamine-2,3-dioxygenase (IDO), which is responsible for the rate-limiting step of the KP, has been demonstrated to be increased in AD and stroke, reflecting an increased activation of the metabolic cascade [66, 67]. The IDO activity has been confirmed to be increased in the hippocampus of AD patients, together with an elevated immunoreactivity of the neurotoxic QUIN [68]. In human macrophages and microglia, Aβ1-42 induces IDO expression and QUIN production [69]. QUIN has been described to be co-localized with hyperphosphorylated tau in the cortex of AD patients, and it also results in tau phosphorylation in primary neuron cultures [70]. Alterations in the KP have likewise been described in PD, HD and stroke (reviews in [71, 72]).
Elevated KYNA levels have proved to be neuroprotective under different experimental conditions of neurotoxicity. The promising results in experimental studies can mainly be explained by the prevention of glutamate excitotoxicity, but other possible mechanisms have also been suggested. In an in vitro study, KYNA induced the gene expression and activity of neprilysin, an enzyme participating in the metabolism of Aβ, and this resulted in increased neuronal cell survival [73]. These data indicate that the neuroprotective effect of KYNA may be related, at least in part, to the induction of amyloid degradation. Further, KYNA has been confirmed to exert beneficial effects in PD and pain syndromes. In an experimental animal model of PD, KYNA effectively alleviated parkinsonian motor symptoms [74].
Elevation of the KYNA level in the brain is challenging, because KYNA itself can cross the blood-brain barrier only poorly; however, there are various methods to achieve this.
The first option is the administration of kynurenine, which is the prodrug of KYNA, together with probenecid, an organic aminoacid transporter inhibitor; this was able to prevent the neuronal damage induced by soluble Aβ and also significantly improved spatial memory [75]. This treatment was also neuroprotective in the 6-hydroxydopamine animal model of PD [76]. The same treatment regime exerted beneficial effects in an animal model of neuropathic pain by diminishing the allodynia [77]. Another possible option is the use of kynurenine-3-monooxygenase (KMO) inhibitors, which results in a shift of the KP toward production of the neuroprotective KYNA. A synthetic KMO inhibitor has been described that exerts beneficial effects in an animal model of AD by preventing neuronal damage and also spatial memory deficits [78]. KMO inhibition has additionally been described to improve levodopa-induced dyskinesia without diminishing the antiparkinsonian effect of simultaneously administered levodopa [79, 80].
Synthetic kynurenine derivatives may represent another therapeutic option; these molecules have proved neuro-protective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of PD [81]. A halogenated KYNA derivative, 4-chlorokynurenine, has also been confirmed to prevent cellular damage in a toxic animal model of AD [82]. A novel KYNA amide was neuroprotective in experimental models of HD [83]. The same analog demonstrated a significant neuroprotective capacity and prevented neuronal damage in hippocampal CA1 pyramids in an experimental model of global cerebral forebrain ischemia in rats [84]. An in vitro comparative electrophysiological study confirmed that this analog displays the same neuromodulatory properties as KYNA [85]. In an experimental migraine model, this compound reduced c-fos and nNOS [86, 87].
An important aspect of NMDA antagonist therapies is the possibility of cognitive side-effects. The novel KYNA analog 2-(2-N,N-dimethylaminoethylamine-1-carbonyl)-1H-quinolin-4-one hydrochloride has therefore been investigated in different behavioral paradigms to assess its side-effect profile. The results confirmed that in a dose in which it exerted its neuroprotective effect, this KYNA derivative did not give rise to any significant systemic side-effect [88]. Other studies also showed that elevation of KYNA levels in the brain did not result in further worsening of working memory function [89]. Its effects have been investigated on locomotor activity, working memory performance and long-lasting, consolidated reference memory by the means of open field, radial arm maze and Morris water maze paradigms. In these experiments, it did not impair the cognitive functions of the brain [90]. Furthermore, an electrophysiological study of the effects of this analog on the cognitive functions revealed that did not decrease LTP as might have been expected from its NMDA antagonistic properties, but rather facilitated the potentiation of field excitatory postsynaptic potentials [91]. The explanation of this somewhat paradoxical effect may be the observation that the elevation of KYNA levels results in a preferential inhibition of the extrasynaptic NMDA receptors and presynaptic nicotinic acetylcholine receptors, whereas the synaptic NMDA and AMPA receptor-mediated currents are relatively spared (reviewed in [92]). The slight facilitatory effect of the KYNA analog may possibly be related to the Janus-faced nature of KYNA, e.g. its concentration-dependent dual effect on AMPA receptors (Fig. 4).
Fig. (4).
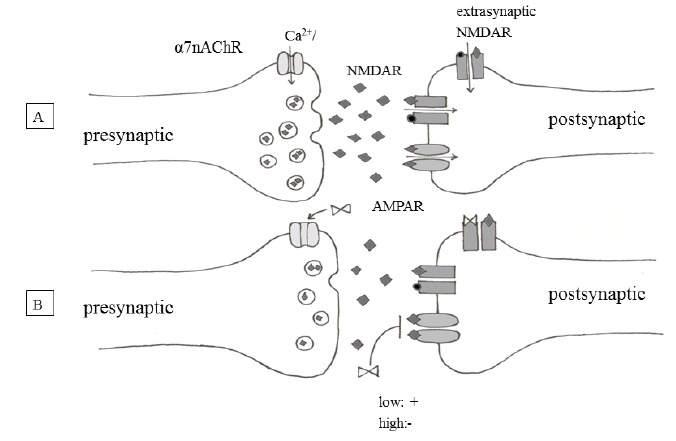
A: Normal conditions: After Ca2+ influx from the α7-nicotinic acetylcholine receptors, the glutamate is releasing and binding to its receptors (extrasynaptic NMDAR, synaptic NMDAR, AMPAR) on the postsynaptic surface of the neurons. B: With kynurenic acid: After releasing into the perisynaptic area, KYNA exerts inhibition on extrasynaptic NMDARs and α7-nicotinic acetylcholine receptors ,while sparing synaptic NMDAR and AMPA receptor-mediated currents. In some articles it is mentioned that it has a Janus-faced impact on the AMPA receptors- e.g. it exerts a concentration-dependent dual effect. ♦ :glutamate, ●: glycin, ⋈ :Kynurenic acid.
The NMDA receptors play critical roles in normal brain function (memory, synaptic communication and controlling synaptic plasticity). Consistently, their antagonists applied as potential therapeutic drugs frequently failed due to serious side-effects [7, 93]. The main parallelity between memantine and KYNA is represented by their effects on NMDA receptors, both having been hypothesized to allow their normal physiological activation while inhibiting their pathological excitotoxic overactivation [17, 94]. However, their mechanism of action is different. Memantine is a trapping open-channel blocker of these receptors, whereas KYNA binds as an inhibitor at both the strychnine-insensitive glycine-binding site at low concentrations, and at the NMDA recognition site at high concentrations, being a broad-spectrum, non-selective glutamate receptor antagonist [95]. Their overlapping therapeutic potential further reflects their similar properties and effects. Memantine is an important player in the therapy of moderate-to-severe AD; however, its beneficial effect has also been suggested in PD, HD, neuropathic pain, epilepsy, and multiple sclerosis as well [11, 15, 46, 48, 96-100]. The neuroprotective properties of KYNA might be also be beneficial in treatment of AD, PD, MS, neuropathic pain as well [75, 101-103].
Moreover the beneficial effect of the NMDA antagonist memantine and KYNA on cognitive functions exhibit marked similarities. Interestingly, memantine has recently been demonstrated to enhance the production of KYNA, which may lead in part to the beneficial therapeutic effect of this compound [104]. KYNA may contribute to the NMDA-antagonistic properties of memantine, and both compounds are also able to influence acetylcholine receptors [104]. These cholinergic receptors have recently been suggested to contribute to AD pathology by promoting amyloid accumulation in the neurons [105]. Further investigations are merited to assess the potential interactions of memantine and KYNA. On the other hand, KYNA and its analog may serve as promising candidates for the future development of well-tolerated partial NMDA antagonists. Importantly, a recent study demonstrated that orally administered KYNA did not decrease cell viability in different cell cultures, nor affect body gain or blood counts in rodents. The study confirmed that KYNA is well-tolerated in rats and mice and does not display any toxic effect. These findings suggest that oral KYNA administration would not be toxic in humans either [106]. An important aspect for future drug development is the fact, that KYNA is an endogenous compound; however, synthetic KYNA analogs or nanotechnology-based approaches may hold promise for drug development with the aim to achieve better pharmacological properties.
CONCLUSIONS
As an open-channel blocker NMDA antagonist memantine is clinically well tolerated and effective for the treatment of AD and other forms of cognitive impairment. Its potential therapeutic benefits have been suggested in other conditions too, such as migraine or neuropathic pain. KYNA and its synthetic derivatives display several similarities to memantine as concerns their mode of action, e.g. partial NMDA receptor inhibition and α7-nicotinic acetylcholine receptor inhibition. A novel KYNA analog has proved neuroprotective in different experimental settings, and does not induce any significant systemic side-effect; indeed, it improves the LTP. Further investigations are called for to assess the potential therapeutic value of KYNA derivatives with the aim of neuroprotection and cognitive improvement.
ACKNOWLEDGEMENTS
This work was supported by the project TÁMOP-4.2.2.A-11/1/KONV-2012-0052, by the Hungarian Brain Research Program (NAP, Grant No. KTIA_13_NAP-A-III/9. and KTIA_13_NAP-A-II/17.), by EUROHEADPAIN (FP7-Health 2013-Innovation; Grant No. 602633), by OTKA K105077 and by the MTA-SZTE Neuroscience Research Group of the Hungarian Academy of Sciences and the University of Szeged.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest and have received no payment in the preparation of their manuscript.
LIST OF ABBREVIATIONS
- Aβ1-42
= amyloid beta 1-42
- AD
= Alzheimer’s disease
- AMPA
= α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- Ca2+
= calcium ion
- HD
= Huntington’s disease
- IDO
= indoleamine-2,3-dioxygenase
- KMO
= kynurenine-3-monooxygenase
- KP
= kynurenine pathway
- KYNA
= kynurenic acid
- LTP
= long-term potentiation
- Mg2+
= magnesium ion
- NMDA
= N-methyl-D-aspartate
- PD
= Parkinson’s disease
- QUIN
= quinolinic acid
REFERENCES
- 1.Kuner T., Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J. Neurosci. 1996;16(11):3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke S.F., Bliss T.V. Plasticity in the human central nervous system. Brain. 2006;129(Pt 7):1659–73. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 3.Sattler R., Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 2001;24(1-3):107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- 4.Zádori D., Klivényi P., Szalárdy L., Fülöp F., Toldi J., Vécsei L. Mitochondrial disturbances, excitotoxicity, neuroinflammation and kynurenines: novel therapeutic strategies for neurodegenerative disorders. J. Neurol. Sci. 2012;322(1-2):187–191. doi: 10.1016/j.jns.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Mehta A., Prabhakar M., Kumar P., Deshmukh R., Sharma P.L. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013;698(1-3):6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Palmer G.C. Neuroprotection by NMDA receptor antagonists in a variety of neuropathologies. Curr. Drug Targets. 2001;2(3):241–271. doi: 10.2174/1389450013348335. [DOI] [PubMed] [Google Scholar]
- 7.Lipton S.A. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1(1):101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L., Yamada K., Nabeshima T., Sokabe M. alpha7 Nicotinic acetylcholine receptor as a target to rescue deficit in hippocampal LTP induction in beta-amyloid infused rats. Neuropharmacology. 2006;50(2):254–268. doi: 10.1016/j.neuropharm.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Parsons C.G., Stöffler A., Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Lipton S.A., Chen H.S. Paradigm shift in neuroprotective drug development: clinically tolerated NMDA receptor inhibition by memantine. Cell Death Differ. 2004;11(1):18–20. doi: 10.1038/sj.cdd.4401344. [DOI] [PubMed] [Google Scholar]
- 11.Witt A., Macdonald N., Kirkpatrick P. Memantine hydrochloride. Nat. Rev. Drug Discov. 2004;3(2):109–10. doi: 10.1038/nrd1311. [DOI] [PubMed] [Google Scholar]
- 12.Bormann J. Memantine is a potent blocker of N-methyl-D-aspartate (NMDA) receptor channels. Eur. J. Pharmacol. 1989;166(3):591–592. doi: 10.1016/0014-2999(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 13.Kornhuber J., Bormann J., Retz W., Hübers M., Riederer P. Memantine displaces [3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur. J. Pharmacol. 1989;166(3):589–590. doi: 10.1016/0014-2999(89)90384-1. [DOI] [PubMed] [Google Scholar]
- 14.Danysz W., Parsons C.G., Mobius H.J., Stoffler A., Quack G. Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease--a unified glutamatergic hypothesis on the mechanism of action. Neurotox. Res. 2000;2(2-3):85–97. doi: 10.1007/BF03033787. [DOI] [PubMed] [Google Scholar]
- 15.Parsons C.G., Danysz W., Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38(6):735–767. doi: 10.1016/S0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 16.Parsons C.G., Gruner R., Rozental J., Millar J., Lodge D. Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memantine (1-amino-3,5-dimethyladamantan). Neuropharmacology. 1993;32(12):1337–1350. doi: 10.1016/0028-3908(93)90029-3. [DOI] [PubMed] [Google Scholar]
- 17.Johnson J.W., Kotermanski S.E. Mechanism of action of memantine. Curr. Opin. Pharmacol. 2006;6(1):61–67. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Kornhuber J., Quack G. Cerebrospinal fluid and serum concentrations of the N-methyl-D-aspartate (NMDA) receptor antagonist memantine in man. Neurosci. Lett. 1995;195(2):137–139. doi: 10.1016/0304-3940(95)11785-U. [DOI] [PubMed] [Google Scholar]
- 19.Sonkusare S.K., Kaul C.L., Ramarao P. Dementia of Alzheimer’s disease and other neurodegenerative disorders--memantine, a new hope. Pharmacol. Res. 2005;51(1):1–17. doi: 10.1016/j.phrs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Sobolevsky A. I., Koshelev S. G., Khodorov B. I. Interaction of memantine and amantadine with agonist-unbound NMDA-receptor channels in acutely isolated rat hippocampal neurons. J. Physiol. 1998;512(Pt 1):47–60. doi: 10.1111/j.1469-7793.1998.047bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiwagi K., Masuko T., Nguyen C.D., Kuno T., Tanaka I., Igarashi K., Williams K. Channel blockers acting at N-methyl-D-aspartate receptors: differential effects of mutations in the vestibule and ion channel pore. Mol. Pharmacol. 2002;61(3):533–545. doi: 10.1124/mol.61.3.533. [DOI] [PubMed] [Google Scholar]
- 22.Chen H.S., Pellegrini J.W., Aggarwal S.K., Lei S.Z., Warach S., Jensen F.E., Lipton S.A. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 1992;12(11):4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilling K. E., Jatzke C., Parsons C. G. Agonist concentration dependency of blocking kinetics but not equilibrium block of Nmethyl-D-aspartate receptors by memantine. Neuropharmacology. 2007;53(3):415–20. doi: 10.1016/j.neuropharm.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Gilling K.E., Jatzke C., Hechenberger M., Parsons C.G. Potency, voltage-dependency, agonist concentration-dependency, blocking kinetics and partial untrapping of the uncompetitive N-methyl-D-aspartate (NMDA) channel blocker memantine at human NMDA (GluN1/GluN2A) receptors. Neuropharmacology. 2009;56(5):866–875. doi: 10.1016/j.neuropharm.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Glodzik L., King K.G., Gonen O., Liu S., De Santi S., de Leon M.J. Memantine decreases hippocampal glutamate levels: a magnetic resonance spectroscopy study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(4):1005–1012. doi: 10.1016/j.pnpbp.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Wageningen H., Jørgensen H.A., Specht K., Hugdahl K. A 1H-MR spectroscopy study of changes in glutamate and glutamine (Glx) concentrations in frontal spectra after administration of memantine. Cereb. Cortex. 2010;20(4):798–803. doi: 10.1093/cercor/bhp145. [DOI] [PubMed] [Google Scholar]
- 27.Erdö S.L., Schäfer M. Memantine is highly potent in protecting cortical cultures against excitotoxic cell death evoked by glutamate and N-methyl-D-aspartate. Eur. J. Pharmacol. 1991;198(2-3):215–217. doi: 10.1016/0014-2999(91)90625-Z. [DOI] [PubMed] [Google Scholar]
- 28.Osborne N. N., Quack G. Memantine stimulates inositol phosphates production in neurones and nullifies N-methyl-Daspartate-induced destruction of retinal neurones. Neurochem. Int. 1992;21(3):329–36. doi: 10.1016/0197-0186(92)90183-R. [DOI] [PubMed] [Google Scholar]
- 29.Weller M., Finiels-Marlier F., Paul S.M. NMDA receptor-mediated glutamate toxicity of cultured cerebellar, cortical and mesencephalic neurons: neuroprotective properties of amantadine and memantine. Brain Res. 1993;613(1):143–148. doi: 10.1016/0006-8993(93)90464-X. [DOI] [PubMed] [Google Scholar]
- 30.Barnes C.A., Danysz W., Parsons C.G. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur. J. Neurosci. 1996;8(3):565–571. doi: 10.1111/j.1460-9568.1996.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 31.Frankiewicz T., Parsons C.G. Memantine restores long term potentiation impaired by tonic N-methyl-D-aspartate (NMDA) receptor activation following reduction of Mg2+ in hippocampal slices. Neuropharmacology. 1999;38(9):1253–1259. doi: 10.1016/S0028-3908(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 32.Zajaczkowski W., Frankiewicz T., Parsons C.G., Danysz W. Uncompetitive NMDA receptor antagonists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacology. 1997;36(7):961–971. doi: 10.1016/S0028-3908(97)00070-1. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt F., Ryan M., Cooper G. A brief review of the pharmacologic and therapeutic aspects of memantine in Alzheimer’s disease. Expert Opin. Drug Metab. Toxicol. 2007;3(1):135–141. doi: 10.1517/17425255.3.1.135. [DOI] [PubMed] [Google Scholar]
- 34.Song M.S., Rauw G., Baker G.B., Kar S. Memantine protects rat cortical cultured neurons against beta-amyloid-induced toxicity by attenuating tau phosphorylation. Eur. J. Neurosci. 2008;28(10):1989–2002. doi: 10.1111/j.1460-9568.2008.06498.x. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Coria H., Green K. N., Billings L. M., Kitazawa M., Albrecht M., Rammes G., Parsons C. G., Gupta S., Banerjee P., LaFerla F. M. Memantine improves cognition and reduces Alzheimer's-like neuropathology in transgenic mice. Am. J. Pathol., 2010;176(2):870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beister A., Kraus P., Kuhn W., Dose M., Weindl A., Gerlach M. The N-methyl-D-aspartate antagonist memantine retards progression of Huntington’s disease. J. Neural Transm. Suppl. 2004;(68):117–122. doi: 10.1007/978-3-7091-0579-5_14. [DOI] [PubMed] [Google Scholar]
- 37.Aarsland D., Ballard C., Walker Z., Bostrom F., Alves G., Kossakowski K., Leroi I., Pozo-Rodriguez F., Minthon L., Londos E. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613–618. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- 38.Li W., Zhao J.H., Sun S.G., Zhang J.W., Suo A.Q., Ma M.M. [Clinical rehabilitative effect of memantine on cognitive and motor disorders in patients with Parkinson’s disease]. Zhonghua Yi Xue Za Zhi. 2011;91(5):301–303. [Clinical rehabilitative effect of memantine on cognitive and motor disorders in patients with Parkinson's disease]. [PubMed] [Google Scholar]
- 39.Leroi I., Overshott R., Byrne E.J., Daniel E., Burns A. Randomized controlled trial of memantine in dementia associated with Parkinson’s disease. Mov. Disord. 2009;24(8):1217–1221. doi: 10.1002/mds.22495. [DOI] [PubMed] [Google Scholar]
- 40.Moreau C., Delval A., Tiffreau V., Defebvre L., Dujardin K., Duhamel A., Petyt G., Hossein-Foucher C., Blum D., Sablonnière B., Schraen S., Allorge D., Destée A., Bordet R., Devos D. Memantine for axial signs in Parkinson’s disease: a randomised, double-blind, placebo-controlled pilot study. J. Neurol. Neurosurg. Psychiatry. 2013;84(5):552–555. doi: 10.1136/jnnp-2012-303182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varanese S., Howard J., Di Rocco A. NMDA antagonist memantine improves levodopa-induced dyskinesias and “on-off” phenomena in Parkinson’s disease. Mov. Disord. 2010;25(4):508–510. doi: 10.1002/mds.22917. [DOI] [PubMed] [Google Scholar]
- 42.Vidal E.I., Fukushima F.B., Valle A.P., Villas Boas P.J. Unexpected improvement in levodopa-induced dyskinesia and on-off phenomena after introduction of memantine for treatment of Parkinson’s disease dementia. J. Am. Geriatr. Soc. 2013;61(1):170–172. doi: 10.1111/jgs.12058. [DOI] [PubMed] [Google Scholar]
- 43.Bigal M., Rapoport A., Sheftell F., Tepper D., Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337–1342. doi: 10.1111/j.1526-4610.2008.01083.x. [DOI] [PubMed] [Google Scholar]
- 44.Spengos K., Theleritis C., Paparrigopoulos T. Memantine and NMDA antagonism for chronic migraine: a potentially novel therapeutic approach? Headache. 2008;48(2):284–286. doi: 10.1111/j.1526-4610.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen S.R., Samoriski G., Pan H.L. Antinociceptive effects of chronic administration of uncompetitive NMDA receptor antagonists in a rat model of diabetic neuropathic pain. Neuropharmacology. 2009;57(2):121–126. doi: 10.1016/j.neuropharm.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morel V., Etienne M., Wattiez A.S., Dupuis A., Privat A.M., Chalus M., Eschalier A., Daulhac L., Pickering G. Memantine, a promising drug for the prevention of neuropathic pain in rat. Eur. J. Pharmacol. 2013;721(1-3):382–390. doi: 10.1016/j.ejphar.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Pickering G., Morel V., Joly D., Villatte C., Roux D., Dubray C., Pereira B. Prevention of post-mastectomy neuropathic pain with memantine: study protocol for a randomized controlled trial. Trials. 2014;15:331. doi: 10.1186/1745-6215-15-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipton S.A. Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer’s disease and other neurologic disorders. J. Alzheimers Dis. 2004;6(6) Suppl.:S61–S74. doi: 10.3233/jad-2004-6s610. [DOI] [PubMed] [Google Scholar]
- 49.Behan W.M., McDonald M., Darlington L.G., Stone T.W. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. Br. J. Pharmacol. 1999;128(8):1754–1760. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Martinez E., Camacho A., Maldonado P.D., Pedraza-Chaverri J., Santamaria D., Galvan-Arzate S., Santamaria A. Effect of quinolinic acid on endogenous antioxidants in rat corpus striatum. Brain Res., 2000;858(2):436–439. doi: 10.1016/S0006-8993(99)02474-9. [DOI] [PubMed] [Google Scholar]
- 51.Rios C., Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem. Res. 1991;16(10):1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- 52.Connick J.H., Stone T.W. Quinolinic acid effects on amino acid release from the rat cerebral cortex in vitro and in vivo. Br. J. Pharmacol. 1988;93(4):868–876. doi: 10.1111/j.1476-5381.1988.tb11474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavares R.G., Tasca C.I., Santos C.E., Alves L.B., Porciúncula L.O., Emanuelli T., Souza D.O. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem. Int. 2002;40(7):621–627. doi: 10.1016/S0197-0186(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 54.Perkins M.N., Stone T.W. Actions of kynurenic acid and quinolinic acid in the rat hippocampus in vivo. Exp. Neurol. 1985;88(3):570–579. doi: 10.1016/0014-4886(85)90072-X. [DOI] [PubMed] [Google Scholar]
- 55.Swartz K.J., During M.J., Freese A., Beal M.F. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J. Neurosci. 1990;10(9):2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Birch P.J., Grossman C.J., Hayes A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988;154(1):85–87. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- 57.Kessler M., Terramani T., Lynch G., Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J. Neurochem. 1989;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 58.Hilmas C., Pereira E.F., Alkondon M., Rassoulpour A., Schwarcz R., Albuquerque E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J. Neurosci. 2001;21(19):7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marchi M., Risso F., Viola C., Cavazzani P., Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J. Neurochem. 2002;80(6):1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 60.Carpenedo R., Pittaluga A., Cozzi A., Attucci S., Galli A., Raiteri M., Moroni F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur. J. Neurosci. 2001;13(11):2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- 61.Prescott C., Weeks A. M., Staley K. J., Partin K. M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci.Lett., 2006;402(1-2):108–112. doi: 10.1016/j.neulet.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 62.Rózsa E., Robotka H., Vécsei L., Toldi J. The Janus-face kynurenic acid. J Neural Transm (Vienna) 2008;115(8):1087–1091. doi: 10.1007/s00702-008-0052-5. [DOI] [PubMed] [Google Scholar]
- 63.Csapó E., Majláth Z., Juhász Á., Roósz B., Hetényi A., Tóth G.K., Tajti J., Vécsei L., Dékány I. Determination of binding capacity and adsorption enthalpy between Human Glutamate Receptor (GluR1) peptide fragments and kynurenic acid by surface plasmon resonance experiments. Colloids Surf. B Biointerfaces. 2014;123:924–929. doi: 10.1016/j.colsurfb.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 64.Wang J., Simonavicius N., Wu X., Swaminath G., Reagan J., Tian H., Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006;281(31):22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 65.Tajti J., Majlath Z., Szok D., Csati A., Toldi J., Fulop F., Vecsei L. Novel kynurenic acid analogues in the treatment of migraine and neurodegenerative disorders: preclinical studies and pharmaceutical design. Curr. Pharm. Des. 2015;21(17):2250–2258. doi: 10.2174/1381612821666150105163055. [DOI] [PubMed] [Google Scholar]
- 66.Mo X., Pi L., Yang J., Xiang Z., Tang A. Serum indoleamine 2,3-dioxygenase and kynurenine aminotransferase enzyme activity in patients with ischemic stroke. J. Clin. Neurosci. 2014;21(3):482–486. doi: 10.1016/j.jocn.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 67.Widner B., Leblhuber F., Walli J., Tilz G.P., Demel U., Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. J Neural Transm (Vienna) 2000;107(3):343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 68.Guillemin G.J., Brew B.J., Noonan C.E., Takikawa O., Cullen K.M. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol. Appl. Neurobiol. 2005;31(4):395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 69.Guillemin G.J., Smythe G.A., Veas L.A., Takikawa O., Brew B.J. A beta 1-42 induces production of quinolinic acid by human macrophages and microglia. Neuroreport. 2003;14(18):2311–2315. doi: 10.1097/00001756-200312190-00005. [DOI] [PubMed] [Google Scholar]
- 70.Rahman A., Ting K., Cullen K.M., Braidy N., Brew B.J., Guillemin G.J. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One. 2009;4(7):e6344. doi: 10.1371/journal.pone.0006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szalardy L., Klivenyi P., Zadori D., Fulop F., Toldi J., Vecsei L. Mitochondrial disturbances, tryptophan metabolites and neurodegeneration: medicinal chemistry aspects. Curr. Med. Chem. 2012;19(13):1899–1920. doi: 10.2174/092986712800167365. [DOI] [PubMed] [Google Scholar]
- 72.Stone T.W., Forrest C.M., Stoy N., Darlington L.G. Involvement of kynurenines in Huntington’s disease and stroke-induced brain damage. J Neural Transm (Vienna) 2012;119(2):261–274. doi: 10.1007/s00702-011-0676-8. [DOI] [PubMed] [Google Scholar]
- 73.Klein C., Patte-Mensah C., Taleb O., Bourguignon J.J., Schmitt M., Bihel F., Maitre M., Mensah-Nyagan A.G. The neuroprotector kynurenic acid increases neuronal cell survival through neprilysin induction. Neuropharmacology. 2013;70:254–260. doi: 10.1016/j.neuropharm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Graham W.C., Robertson R.G., Sambrook M.A., Crossman A.R. Injection of excitatory amino acid antagonists into the medial pallidal segment of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treated primate reverses motor symptoms of parkinsonism. Life Sci. 1990;47(18):PL91–PL97. doi: 10.1016/0024-3205(90)90376-3. [DOI] [PubMed] [Google Scholar]
- 75.Carrillo-Mora P., Méndez-Cuesta L.A., Pérez-De La Cruz V., Fortoul-van Der Goes T.I., Santamaría A. Protective effect of systemic L-kynurenine and probenecid administration on behavioural and morphological alterations induced by toxic soluble amyloid beta (25-35) in rat hippocampus. Behav. Brain Res. 2010;210(2):240–250. doi: 10.1016/j.bbr.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 76.Silva-Adaya D., Pérez-De La Cruz V., Villeda-Hernández J., Carrillo-Mora P., González-Herrera I.G., García E., Colín-Barenque L., Pedraza-Chaverrí J., Santamaría A. Protective effect of L-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: implications of modulating kynurenate as a protective strategy. Neurotoxicol. Teratol. 2011;33(2):303–312. doi: 10.1016/j.ntt.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 77.Pineda-Farias J.B., Pérez-Severiano F., González-Esquivel D.F., Barragán-Iglesias P., Bravo-Hernández M., Cervantes-Durán C., Aguilera P., Ríos C., Granados-Soto V. The L-kynurenine-probenecid combination reduces neuropathic pain in rats. Eur. J. Pain. 2013;17(9):1365–1373. doi: 10.1002/j.1532-2149.2013.00305.x. [DOI] [PubMed] [Google Scholar]
- 78.Zwilling D., Huang S.Y., Sathyasaikumar K.V., Notarangelo F.M., Guidetti P., Wu H.Q., Lee J., Truong J., Andrews-Zwilling Y., Hsieh E.W., Louie J.Y., Wu T., Scearce-Levie K., Patrick C., Adame A., Giorgini F., Moussaoui S., Laue G., Rassoulpour A., Flik G., Huang Y., Muchowski J.M., Masliah E., Schwarcz R., Muchowski P.J. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145(6):863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grégoire L., Rassoulpour A., Guidetti P., Samadi P., Bédard P.J., Izzo E., Schwarcz R., Di Paolo T. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav. Brain Res. 2008;186(2):161–167. doi: 10.1016/j.bbr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Ouattara B., Belkhir S., Morissette M., Dridi M., Samadi P., Grégoire L., Meltzer L.T., Di Paolo T. Implication of NMDA receptors in the antidyskinetic activity of cabergoline, CI-1041, and Ro 61-8048 in MPTP monkeys with levodopa-induced dyskinesias. J. Mol. Neurosci. 2009;38(2):128–142. doi: 10.1007/s12031-008-9137-8. [DOI] [PubMed] [Google Scholar]
- 81.Acuña-Castroviejo D., Tapias V., López L.C., Doerrier C., Camacho E., Carrión M.D., Mora F., Espinosa A., Escames G. Protective effects of synthetic kynurenines on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Brain Res. Bull. 2011;85(3-4):133–140. doi: 10.1016/j.brainresbull.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 82.Wu H.Q., Lee S.C., Schwarcz R. Systemic administration of 4-chlorokynurenine prevents quinolinate neurotoxicity in the rat hippocampus. Eur. J. Pharmacol. 2000;390(3):267–274. doi: 10.1016/S0014-2999(00)00024-8. [DOI] [PubMed] [Google Scholar]
- 83.Zádori D., Nyiri G., Szonyi A., Szatmári I., Fülöp F., Toldi J., Freund T.F., Vécsei L., Klivényi P. Neuroprotective effects of a novel kynurenic acid analogue in a transgenic mouse model of Huntington’s disease. J Neural Transm (Vienna) 2011;118(6):865–875. doi: 10.1007/s00702-010-0573-6. [DOI] [PubMed] [Google Scholar]
- 84.Gellért L., Fuzik J., Göblös A., Sárközi K., Marosi M., Kis Z., Farkas T., Szatmári I., Fülöp F., Vécsei L., Toldi J. Neuroprotection with a new kynurenic acid analog in the four-vessel occlusion model of ischemia. Eur. J. Pharmacol. 2011;667(1-3):182–187. doi: 10.1016/j.ejphar.2011.05.069. [DOI] [PubMed] [Google Scholar]
- 85.Marosi M., Nagy D., Farkas T., Kis Z., Rózsa E., Robotka H., Fülöp F., Vécsei L., Toldi J. A novel kynurenic acid analogue: a comparison with kynurenic acid. An in vitro electrophysiological study. J Neural Transm (Vienna) 2010;117(2):183–188. doi: 10.1007/s00702-009-0346-2. [DOI] [PubMed] [Google Scholar]
- 86.Knyihar-Csillik E., Mihaly A., Krisztin-Peva B., Robotka H., Szatmari I., Fulop F., Toldi J., Csillik B., Vecsei L. The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-fos immunoreactivity in the rat caudal trigeminal nucleus: comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci. Res. 2008;61(4):429–432. doi: 10.1016/j.neures.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 87.Vamos E., Pardutz A., Varga H., Bohar Z., Tajti J., Fulop F., Toldi J., Vecsei L. l-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology, 2009;57(4):425–429. doi: 10.1016/j.neuropharm.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 88.Nagy K., Plangár I., Tuka B., Gellért L., Varga D., Demeter I., Farkas T., Kis Z., Marosi M., Zádori D., Klivényi P., Fülöp F., Szatmári I., Vécsei L., Toldi J. Synthesis and biological effects of some kynurenic acid analogs. Bioorg. Med. Chem. 2011;19(24):7590–7596. doi: 10.1016/j.bmc.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 89.Justinova Z., Mascia P., Wu H.Q., Secci M.E., Redhi G.H., Panlilio L.V., Scherma M., Barnes C., Parashos A., Zara T., Fratta W., Solinas M., Pistis M., Bergman J., Kangas B.D., Ferré S., Tanda G., Schwarcz R., Goldberg S.R. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat. Neurosci. 2013;16(11):1652–1661. doi: 10.1038/nn.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gellért L., Varga D., Ruszka M., Toldi J., Farkas T., Szatmári I., Fülöp F., Vécsei L., Kis Z. Behavioural studies with a newly developed neuroprotective KYNA-amide. J Neural Transm (Vienna) 2012;119(2):165–172. doi: 10.1007/s00702-011-0692-8. [DOI] [PubMed] [Google Scholar]
- 91.Demeter I., Nagy K., Farkas T., Kis Z., Kocsis K., Knapp L., Gellert L., Fulop F., Vecsei L., Toldi J. Paradox effects of kynurenines on LTP induction in the Wistar rat. Neurosci. Lett. 2013;553:138–141. doi: 10.1016/j.neulet.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 92.Vécsei L., Szalárdy L., Fülöp F., Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat. Rev. Drug Discov. 2013;12(1):64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 93.Li F., Tsien J.Z. Memory and the NMDA receptors. N. Engl. J. Med. 2009;361(3):302–303. doi: 10.1056/NEJMcibr0902052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winn P., Stone T.W., Latimer M., Hastings M.H., Clark A.J. A comparison of excitotoxic lesions of the basal forebrain by kainate, quinolinate, ibotenate, N-methyl-D-aspartate or quisqualate, and the effects on toxicity of 2-amino-5-phosphonovaleric acid and kynurenic acid in the rat. Br. J. Pharmacol. 1991;102(4):904–908. doi: 10.1111/j.1476-5381.1991.tb12274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szalardy L., Zadori D., Toldi J., Fulop F., Klivenyi P., Vecsei L. Manipulating kynurenic acid levels in the brain - on the edge between neuroprotection and cognitive dysfunction. Curr. Top. Med. Chem. 2012;12(16):1797–1806. doi: 10.2174/156802612803989264. [DOI] [PubMed] [Google Scholar]
- 96.Wesnes K.A., Aarsland D., Ballard C., Londos E. Memantine improves attention and episodic memory in Parkinson’s disease dementia and dementia with Lewy bodies. Int. J. Geriatr. Psychiatry. 2015;30(1):46–54. doi: 10.1002/gps.4109. [DOI] [PubMed] [Google Scholar]
- 97.Anitha M., Nandhu M.S., Anju T.R., Jes P., Paulose C.S. Targeting glutamate mediated excitotoxicity in Huntington’s disease: neural progenitors and partial glutamate antagonist--memantine. Med. Hypotheses. 2011;76(1):138–140. doi: 10.1016/j.mehy.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Milnerwood A.J., Kaufman A.M., Sepers M.D., Gladding C.M., Zhang L., Wang L., Fan J., Coquinco A., Qiao J.Y., Lee H., Wang Y.T., Cynader M., Raymond L.A. Mitigation of augmented extrasynaptic NMDAR signaling and apoptosis in cortico-striatal co-cultures from Huntington’s disease mice. Neurobiol. Dis. 2012;48(1):40–51. doi: 10.1016/j.nbd.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 99.Sulkowski G., Dąbrowska-Bouta B., Salińska E., Strużyńska L. Modulation of glutamate transport and receptor binding by glutamate receptor antagonists in EAE rat brain. PLoS One. 2014;9(11):e113954. doi: 10.1371/journal.pone.0113954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zaitsev A.V., Kim K.Kh., Vasilev D.S., Lukomskaya N.Y., Lavrentyeva V.V., Tumanova N.L., Zhuravin I.A., Magazanik L.G. N-methyl-D-aspartate receptor channel blockers prevent pentylenetetrazole-induced convulsions and morphological changes in rat brain neurons. J. Neurosci. Res. 2015;93(3):454–465. doi: 10.1002/jnr.23500. [DOI] [PubMed] [Google Scholar]
- 101.Miranda A.F., Boegman R.J., Beninger R.J., Jhamandas K. Protection against quinolinic acid-mediated excitotoxicity in nigrostriatal dopaminergic neurons by endogenous kynurenic acid. Neuroscience. 1997;78(4):967–975. doi: 10.1016/S0306-4522(96)00655-0. [DOI] [PubMed] [Google Scholar]
- 102.Heyliger S.O., Goodman C.B., Ngong J.M., Soliman K.F. The analgesic effects of tryptophan and its metabolites in the rat. Pharmacol. Res. 1998;38(4):243–250. doi: 10.1006/phrs.1998.0362. [DOI] [PubMed] [Google Scholar]
- 103.Platten M., Ho P.P., Youssef S., Fontoura P., Garren H., Hur E.M., Gupta R., Lee L.Y., Kidd B.A., Robinson W.H., Sobel R.A., Selley M.L., Steinman L. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310(5749):850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 104.Kloc R., Luchowska E., Wielosz M., Owe-Larsson B., Urbanska E.M. Memantine increases brain production of kynurenic acid via protein kinase A-dependent mechanism. Neurosci. Lett. 2008;435(2):169–173. doi: 10.1016/j.neulet.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 105.D’Andrea M.R., Nagele R.G. Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer’s disease pyramidal neurons. Curr. Pharm. Des. 2006;12(6):677–684. doi: 10.2174/138161206775474224. [DOI] [PubMed] [Google Scholar]
- 106.Turski W.A., Małaczewska J., Marciniak S., Bednarski J., Turski M.P., Jabłoński M., Siwicki A.K. On the toxicity of kynurenic acid in vivo and in vitro. Pharmacol. Rep. 2014;66(6):1127–1133. doi: 10.1016/j.pharep.2014.07.013. [DOI] [PubMed] [Google Scholar]


