Abstract
In the current study, we used 56 female BALB/c mice with induced dry eye syndrome to evaluate the therapeutic effects of formal saline (FS), sodium hyaluronate (SH), diclofenac sodium (DS), olopatadine (OP), retinoic acid (RA), fluoromethanole (FML), cyclosporine A (CsA), and doxycycline hyclate (DH). All subjects were kept in an evaporative ‘dry eye cabinet’ for the assessment of blink rate, tear production, tear break-up time, and impression cytology prior to (baseline) and during weeks 2, 4, and 6 of the study. The right eyes of all subjects were treated topically with 5 µL of the test agent twice daily during weeks 2 through 6. Impression cytology and tear break-up time differed between time points in all groups and differed between groups at weeks 4 and 6. Blink rate differed by time point only in the FS, FML, and DH groups. Tear production according to the phenol red cotton thread test differed by time point for all groups except RA, CsA, and DH and differed between groups only at week 6. Among the compounds tested in the present study, DS and CsA were the most effective therapeutic agents in our mouse model of dry eye syndrome; these agents likely exert their therapeutic effect through their antiinflammatory activity.
Abbreviations: CsA, cyclosporine A; DES, dry eye syndrome; DH, doxycycline hyclate; DS, diclofenac sodium; FML, fluoromethanole; FS, formal saline; OP, olopatadine; RA, retinoic acid; SH, sodium hyaluronate; TBUT, tear break up-time; PTF, precorneal tear film
Dry eye syndrome (DES) is a multifactorial disorder that is characterized by inflammation, tear film hyperosmolarity and instability, and vision impairment with a potential to induce ocular surface damage.8,44 DES is an important cause of ocular surface disturbances and is commonly encountered in women, especially postmenopausal women, likely due to their decreased androgen and estrogen levels.30,41,42 Some epidemiologic studies suggest that the prevalence of DES increases with aging.37,42 This situation is thought to result from the repressive effects on tear production of antihistaminic, diuretic, and anticholinergic drugs used to treat prevalent systemic diseases in elderly people.16 Tear production declines with aging because of the decreased functional capacities of the nictitating and lacrimal glands.5,18 DES is also prevalent in dogs, whose tear production varies according to weight, age, and size.18,19 DES is occasionally seen in cats and horses18,36 and has been reported in birds and reptiles.19 The low incidence of DES in animals other than dogs likely reflects the dearth of epidemiologic studies in other species.18,36
Tear evaporation rate, hyperosmolarity, and inflammation play key roles in the pathogenesis of DES.17,30 Decreases in tear production, cycling, and flow due to low relative humidity, high air flow, and decreased air temperature lead to tear hyperosmolarity, which in turn induces a cascade of inflammatory processes on the ocular surface.17,30
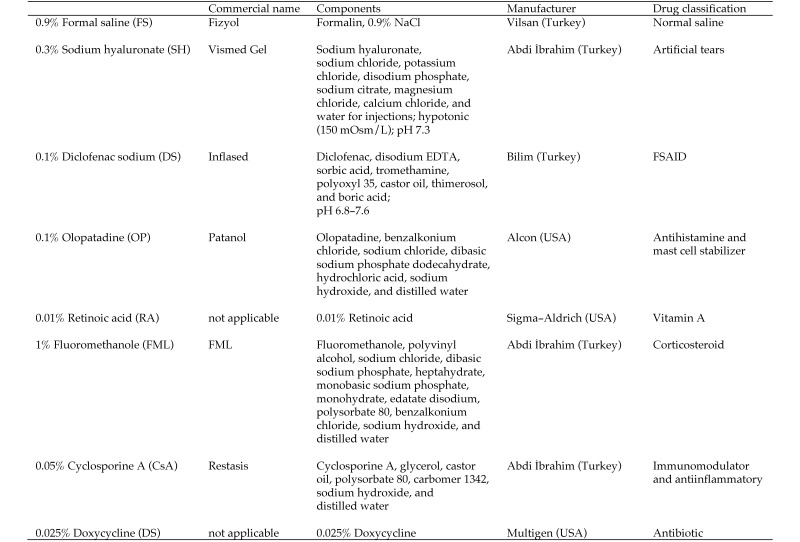
Various parameters, including blink rate, tear production rate, tear break-up time (TBUT) and impression cytology, are commonly used for the diagnosis of DES.39 The treatment of this disorder is based on alleviating its clinical signs and removing key factors in its development.16,17 To this end, artificial tears, NSAID, antihistamines and mast cell stabilizers, vitamin A, corticosteroids, immunomodulators, antiinflammatory agents, and antibiotics have often been used to treat DES in recent years.16,30,34 In the present study, we evaluated representative, commonly used agents from each of these drug groups by comparing their therapeutic efficacy on blink rate, tear production rate, TBUT, and impression cytology in a murine model of DES.
Materials and Methods
The study population comprised 56 female BALB/c mice (age, 8 wk) provided by Experimental Animal Center of Firat University. The research was initiated only after receiving official approval from Animal Experiment and Ethic Committee of Firat University (protocol no. 2011/09-119). Ethical principles were strictly applied for all experimental procedures performed on animals. Prior to the beginning of the study, all subjects were acclimated to the study environment and restraint techniques, and all tests were performed without anesthesia. All mice had unrestricted access to food and water.
The mice were allocated randomly into 8 groups of 7 animals each and were placed in a ‘dry eye cabinet’ with a temperature of 22.5 ± 0.4 °C, relative humidity of 25.1% ± 0.6%, air flow rate of 15 L/min, and air flow speed of 2.3 ± 0.5 m/s. Air turbulence within the cabinet was further accelerated by using a couple of air fans (1200 ± 250 rpm, 50 ft3/min) installed in the animal facility (Figure 1). A total of 8 agents were evaluated, and each group of mice received only one agent (Figure 2). As controls, additional mice (equal in number to those in the test groups) were unexposed to evaporative stress (normal room conditions: relative humidity, 60% to 80%; temperature, 21 to 23 °C) to detect any side effects of individual agents. In this regard, no statistically significant difference was found between the agents.
Figure 1.

Components of the murine DES model used. (1) The dry eye cabin. (2) Experimental units (a through c). (3) Mice. (4) Air compressor. (5) Tubing for air compressor. (6) Humidity regulators (a through c). (7) Flowmeter. (8) Desiccator. (9) Control board. (10) Fan. (11) Humidity and temperature monitor. (12) Movable glass plates. (13) Water bottle. (14) Feeder. (15) Air inlet holes. (16) Pneumatic tubing. (17) Flexible inhalation hoses. (18) Fan switchboard. (19) Air compressor pressure monitor. (20) Air compressor pressure gauge.
Figure 2.
The agents evaluated in the current study.
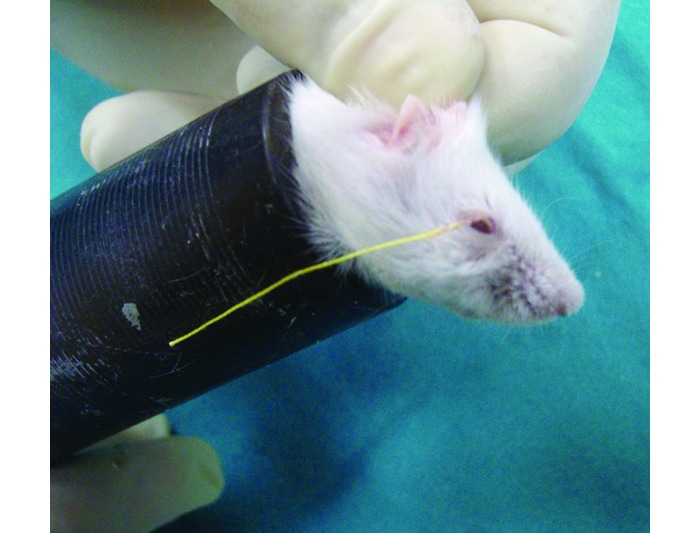
During the first 2 wk of the study, all mice were exposed to evaporative stress in the absence of any treatment. Then, the right eyes of all subjects were instilled with 5 µL of the appropriate test agent twice daily during weeks 2 through 6 while exposure to evaporative stress continued. All subjects were assessed in terms of blink rate, tear production rate, TBUT, and impression cytology prior to (baseline) study initiation and at weeks 2, 4, and 6. To determine the blink rate, both authors counted the number of blinks in 1 min, and the average of these values was recorded. Tear production rate was quantified by using phenol red cotton thread (Zone Quick; Menicon, Nagoya, Japan). Prior to testing, the tears in the lacrimal lake were removed by using absorbent paper points (Absorbent Paper Points, Sure-Endo, Korea), which were left in place for the standard time (4 s). By using forceps, the treated threads were placed in the right lower conjunctival fornix of the subjects at the lateral cantus, left in the place for 1 min (Figure 3), and then removed and the wetted length was measured according to the scale provided by the manufacturer; measurements were made under a slit-lamp biomicroscope (XL1, Shin-Nippon, Niigata, Japan) to maximize accuracy. For the TBUT, a 1-µL drop of 1% fluorescein sodium was instilled into the right eye of each subject. Once the mouse blinked, the eyelids were held open gently by the researcher's fingers, and the TBUT was assessed under a slit-lamp biomicroscope with a cobalt blue filter. The time at which breaks in the stain first became visible was recorded as the TBUT.
Figure 3.
Phenol red cotton thread test (FS group, baseline, mouse 1).
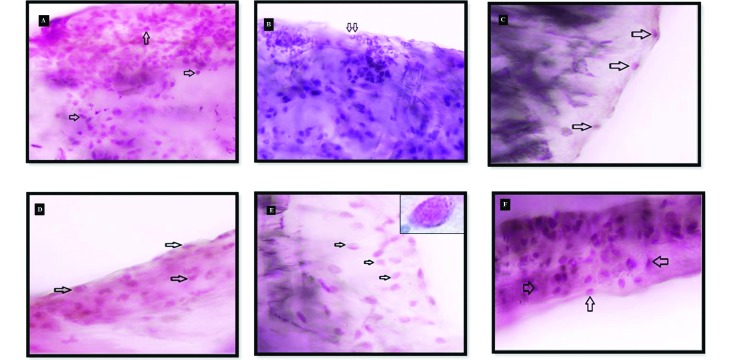
For impression cytology, a sample of conjunctival tissue from the inferior fornix of the right eye of each subject was obtained by using a strip of nitrocellulose filter paper (pore size, 0.45 µm; Nitrocellulose–Filter Paper Sandwich, Invitrogen, Grand Island, NY). The strip was placed in the inferior fornix through the lateral cantus approach and was pressed gently against the globe for 2 to 3 s to allow cells from the conjunctival surface to adhere. Each filter paper was processed as described previously31 and examined under a digital microscope at 100× magnification. For each preparation, 4 randomly selected areas were photographed (Figure 4), the goblet cells were counted, and the mean value was recorded.
Figure 4.
Cytologic samples obtained from the upper conjunctival fornix of the subjects show (A) normal goblet-cell density (arrows; FS group, baseline, mouse 2), (B and C) a significant decrease in goblet cell number compared with baseline (B: FS group, week 2, mouse 6; C: CsA group, week 2, mouse 5), (D and E) a significant increase compared with week 2 (D: CsA group, week 4, mouse 6; E: DS group, wk 4, mouse 7), and (F) a significant increase compared with weeks 2 and 4 (DS group, week 6, mouse 1). Periodic acid–Shiff and hematoxylin stain; magnification, 100×.
The agents were ranked in order of their efficacy for the treatment of DES in mice (1, most effective; 8, least beneficial) and the overall rank (sum of all scores) obtained (Table 1).
Table 1.
Ranking of DES therapeutic agents from most (1) to least (8) beneficial according to the evaluated parameters
| FS | SH | DS | OP | RA | FML | CsA | DH | |
| Blink rate | 8 | 6 | 4 | 2 | 7 | 1 | 3 | 5 |
| Phenol red cotton thread test | 8 | 4 | 2 | 1 | 5 | 6 | 3 | 7 |
| Tear break-up test | 8 | 3 | 2 | 4 | 6 | 5 | 1 | 7 |
| Impression cytology | 8 | 3 | 1 | 7 | 5 | 4 | 2 | 6 |
| Total score | 32 | 16 | 9 | 14 | 23 | 18 | 9 | 25 |
Statistical analysis was performed by using SPSS for Windows (version 13.0, IBM, Chicago, IL). Intergroup differences relative to measurement time were assessed by using the Friedman test for nonparametric and repeated measures; the Wilcoxon test was applied to determine whether significant differences between groups persisted at different measurement times. Differences within groups between time points underwent ANOVA followed by a posthoc Tukey test. A P value less than 0.05 was considered statistically significant.
Results
Impression cytology.
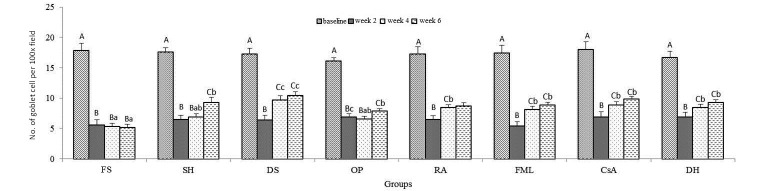
When the data were compared within groups in regard to time point, the number of goblet cells at week 2 was lower (P < 0.05) than that at baseline in all groups (Figure 5); this decrease persisted throughout the study for the FS group and until week 4 in the OP-treated mice. In all other groups, goblet cells numbers were significantly (P < 0.05) increased at week 4 (except for SH) and week 6 compared with baseline values (Figure 5). When the data were compared between groups at the same time point, goblet cell counts at weeks 4 and 6 were significantly (P < 0.05) greater in the DS group compared with FS (Figures 4 and 5).
Figure 5.
Impression cytology. Uppercase letters refer to comparisons within groups between time points; lowercase letters indicate comparisons between groups at the same time point; different letters indicate values that differ significantly (P < 0.001). Data are shown as mean values; bar, 1 SD.
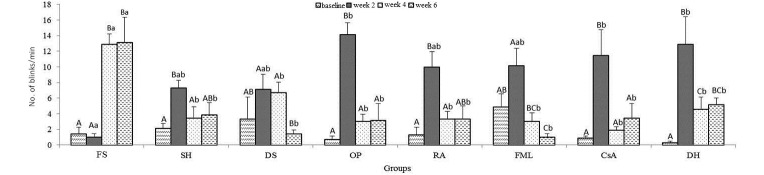
Blink rate.
When the mean data were compared within groups in regard to time point, the blink rate of the FS group was increased (P < 0.05) at weeks 4 and 6 as compared with the baseline value. The blink rates of the DS and FML groups reached their peaks at week 2 and then decreased (DS, at wk 4, P > 0.05, at wk 6, P < 0.05; FML at weeks 4 and 6, P < 0.05) thereafter (Figure 6). When the data were compared between groups at the same time point, differences emerged between FS and OP, CsA, and DH for week 2 and between FS and all other groups at weeks 4 and 6 (P < 0.05). The most significant decrease was found in FML in wk 6.
Figure 6.
Blink rate. Uppercase letters refer to comparisons within groups between time points; lowercase letters indicate comparisons between groups at the same time point; different letters indicate values that differ significantly (P < 0.001). Data are shown as mean values; bar, 1 SD.
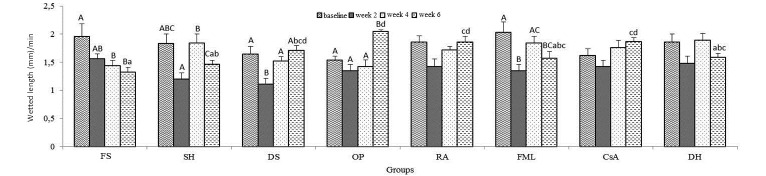
Phenol red cotton thread test.
When the mean data were compared within groups in regard to time point, the differences in the FS, SH, DS, OP, and FML groups were found to be significant (P < 0.001, Figure 7). The average tear production rate in FS group decreased over weeks. This was evident in the last 2 measurements as compared with baseline values. In comparison, tear production in the SH, FML, and DH groups was decreased at week 2, increased at week 4, and then decreased again at week 6 compared with baseline values. In the DS, OP, RA, and CsA groups, the significant decline at week 2 was followed by successive increases at weeks 4 and 6; this increase was most pronounced in the OP-treated mice (Figure 7). When the data were compared between groups at the same time point, significantly increased tear production was present at week 6 in the DS, OP, RA, and CsA groups compared with FS and in the OP, RA, and CsA groups compared with SH. However, tear production at week 6 was decreased in the OP group compared with FML and DH.
Figure 7.
The phenol red cotton thread test. Uppercase letters refer to comparisons within groups between time points; lowercase letters indicate comparisons between groups at the same time point; different letters indicate values that differ significantly (P < 0.001). Data are shown as mean values; bar, 1 SD.
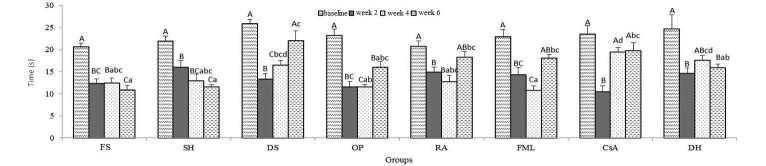
TBUT.
For all groups, TBUT was lower at 2 wk than at baseline (P < 0.001, Figure 8); this decrease persisted throughout the study in the SH and FS groups, whereas TBUT consistently increased in the DS and CsA mice (Figure 8). Comparing between groups at week 4 revealed a significantly decreased TBUT for CsA compared with FS, SH, OP, RA, and FML but an increased TBUT in the FML group compared with DS and DH. At week 6, the TBUT was lower in DS compared with DH but increased in FS compared with DS, RA, FML, or CsA and in SH compared with DS, RA, FML, or CsA (P < 0.001, Figure 8).
Figure 8.
TBUT. Uppercase letters refer to comparisons within groups between time points; lowercase letters indicate comparisons between groups at the same time point; different letters indicate values that differ significantly (P < 0.001). Data are shown as mean values; bar, 1 SD.
Overall, DS and CsA were the most effective agents for the treatment of induced dry eye in mice, followed by FML, OP, RA, SH and DH, and FS, respectively (Table 1).
Discussion
Animal models that accurately mimic human DES facilitate the investigation of the many factors that play a role in the disease process and treatment options.6,9,29,43 Currently available models induce DES through the inhibition of lacrimal secretion by using mechanical,29 hormonal,43 or neural9 mechanisms or by initiating evaporative stress on the ocular surface.6 The precorneal tear film (PTF) is continuously exposed to potential stressors including humidity, temperature, and air flow, with resultant dryness of the ocular surface in the case of low rates of tear production.6 People now typically live and work in air-conditioned environments and are exposed to extreme temperature fluctuations as they move between these indoor and outdoor environments—these conditions of temperature and humidity are considered the most important risk factors in the increased prevalence of DES leading to PTF impairment.17 Therefore a model that incorporates all of these factors—that is, low relative humidity, decreased temperature, and high air flow—is preferable to those used in previous studies, which tended to focus on a single environmental factor or a limited combination of factors to induce DES6. For the present study, we designed a dry eye cabinet that optimally coordinated low relative humidity, high air flow, and decreased environmental temperature to generate ocular surface dryness in our test mice. In studies using DES models, disease process and therapeutic outcome typically are evaluated by assessing, for example, the tear production rate, impression cytology, blink rate, and TBUT, as we have done in the current and a previous study.24 Comparing the baseline values with those at week 2 revealed decreases in the tear production rate, goblet cell density, and TBUT coupled with an increase in the blink rate in the absence of treatment, thus demonstrating the effectiveness of evaporative stress factors in the dry eye cabinet. The therapeutic effects of the 8 agents we used were compared between time points. Throughout the 6-wk course of the experiment, the average goblet cell density increased in all groups (except OP), mean tear production quantities increased in all groups (except SH, FML, and DH), the average TBUT increased in the DS and CsA groups, and the average blink rate decreased in mice treated with DS and FML.
The mucin layer, the innermost layer of the tear film, prevents desiccation of the ocular surface by protecting the integrity of the tear film.32 The conjunctival goblet cells are primarily responsible for the production of the mucin layer.12,32 A reduction in goblet cells may cause corneal epithelial damage, because of tear film instability due to disintegration of the mucin layer.1,32 Many previous experimental and clinical studies in humans38 and animals,1 as well as our present study, have used impression cytology to assess goblet cell density. The decrease in conjunctival goblet cell density in DES models reportedly is induced through evaporative stress factors, that is, high air velocity, low relative humidity, and low temperature.7 In the current study, which incorporated these same factors, impression cytology analysis revealed similar decreases in goblet cell density in all groups at week 2 (in the absence of treatment) compared with baseline and only in the FS group at weeks 4 and 6. These findings are supported by the results of another model study14 that induced DES by using a high air flow and an anticholinergic agent. Furthermore, goblet cell density increased for all agents except OP at week 4 and for all agents including OP at week 6, thus indicating that OP has a beneficial effect on goblet cell density over a prolonged period.11 In parallel with the results of the current study, SH,3 FML,4,46 RA,23 and CsA25 have been reported to increase goblet cell number during a short period. DS, a NSAID, is mainly used for the treatment of ocular inflammation26 and, according to goblet cell density, was one of the most effective medicines we tested. DS may decrease the synthesis of endogenous prostaglandins, which initiate inflammatory processes, by inhibiting cyclooxygenase.40 Our results suggest that the beneficial effect of DS on goblet cell number in mice exposed to evaporative stress in the dry eye cabinet is related to its prevention of ocular surface inflammation.14,26 This association supports the idea13 that inflammatory processes play an important role in DES pathogenesis.
An increased blink rate is an important clinical sign of DES18 and reportedly occurs due to stinging,2 burning and foreign-body sensations, pain, and associated blepharospasm15 that develop during the disease process. In the present study, the significant increase in the blink rate in all groups (except FS) at week 2, the first time point after their exposure to evaporative stress, supports the claim2 that the evaporative stress causing DES may increase blink rate. The blink rate in the FS group increased markedly over time, contrary to the decline in the other groups. We attributed this result for the FS-treated mice to continued evaporative stress, which appeared to have been alleviated with the administration of the other agents. The positive action of these therapeutic agents reduced the blink rate that is characteristic of DES15 and other ocular disturbances.18
When the agents were evaluated between time points and groups by using the blink rate data, FML, a corticosteroid antiinflammatory agent,26,27 yielded the most encouraging result. FML produces its antiinflammatory action by diminishing the production of proinflammatory cytokines and chemokines, lipid mediators including matrix metalloproteinase 9, and prostaglandins;26 by inhibiting the release of intercellular adhesion molecule 1;34 through the transcriptional regulation of various proinflammatory molecules including nuclear factors;26 and by stimulating the apoptosis of lymphocytes.34 Reportedly27 inflammation plays an important role in DES pathogenesis and thus its treatment depends on ceasing or preventing this process. For this reason, many antiinflammatory agents have been advocated in the treatment of DES.46 FML, one such compound, has been reported to ameliorate the ocular symptoms of foreign-body sensation, dryness, burning, pain, and photophobia46 and to have beneficial effects on the tear production rate, corneal fluorescein staining score,27 TBUT,46 and impression cytology.4 As previously mentioned, some ocular signs, such as burning,15 foreign-body sensation, and pain,15 increase the blink rate. In the current study, we surmise that FML diminished the blink rate by reducing the ocular signs due to inflammatory processes.46 This current finding further supports the idea27 that inflammation has a role in the pathogenesis of the DES model that is induced through evaporative stress.
The PTF is very important in maintaining the health of the ocular surface, and a minor change in its mucin, aqueous, and lipid layers may remarkably affect tear function and physiology.35 Aqueous tear deficiency, an important clinical sign of DES,18 is usually assessed by using Schirmer tear test in humans and animals with large globes, such as cats, dogs, rabbits, and monkeys and by using the phenol red cotton thread test in smaller laboratory species.39 Some DES model studies7,14 have demonstrated marked reductions in tear production in the subjects exposed to evaporative stress. We found a similar result in the current study when the baseline and week 2 values were compared with those of week 4, and this reduction reversed in all groups (except FS). Among the agents we evaluated, FML,26 CsA,27 and RA21 have been reported to have prompt, favorable effects on increasing the tear production rate in DES model cases. The lowest tear production rate occurred at week 6 in the FS group, thus demonstrating the effect of prolonged exposure to evaporative stress, similar to the result of a previous study.10 In addition to that for the FS group, tear production at week 6 was decreased in the SH, FML, and DH groups. Previous studies have indicated that whereas DH fails to increase in tear production with prolonged usage,26 FML has a beneficial effect at all stages of DES.27 Except for DH and FML, all other groups showed no significant increase in the mean tear production rate over time. RA21 and CsA27 reportedly have beneficial effects on the tear production rate within 4 weeks of treatment initiation in other studies. In regard to tear production, DS26 was reported to have no effect, whereas OP had a negative effect in some studies45 but a favorable action in others.28 Some researchers26,45 have suggested that the favorable effect of OP on DES could be recognized as reduced clinical signs. In our study, OP significantly increased the tear production rate, contrary to previous findings.45 Another study28 found that OP prevented the sensations of stinging, itching, and burning in mild and moderate cases of DES. As previously noted, these sensations occur frequently in DES cases and are associated with aqueous tear production rates.18 Tear replacement and refreshment agents often are used to ease this signs.17 In the present study, we attributed the favorable action of OP on these clinical signs of DES to its inductive effect on the tear production rate.28
The TBUT is a routine diagnostic test used to determine the stability of the PTF in DES cases;39 the TBUT is decreased in cases with PTF instability20 and is adversely affected by evaporative stress.22 In the current study, the TBUT at week 2 was decreased markedly compared with baseline values, in agreement with earlier findings.20,22 In our study, the TBUT was prolonged for DS, CsA, and DH at week 4 and for all agents (except FS, SH, and DH) at week 6. These results prompted us to conclude that DS, CsA, and DH help to preserve the integrity of the PTF. Similar actions of CsA33 and DS3 on the TBUT have been reported previously. In contrast, the shortest TBUT we noted was for FS at week 6 and, as mentioned previous,22 may reveal impairment of PTF stability owing to prolonged exposure to evaporative stress. With regard to the TBUT values of in our current study, CsA appeared to be the most effective agent, which conclusion is supported by several previous studies.21,32 A prolonged TBUT is associated with increased tear production27 and goblet cell density.21
In conclusion, this study determined that DS and CsA were the most effective therapeutic agents in a mouse model of DES. Both DS and CsA have antiinflammatory properties.27,30 That this characteristic is shared between both effective agents suggests that inflammation plays a key role in the pathogenesis of this particular DES model. In addition, the present findings imply that these 2 antiinflammatory agents should considered for the treatment of DES. Further studies are needed to determine whether one of these agents is superior to the other.
References
- 1.Altinors DD, Bozbeyoglu S, Karabay G, Akova YA. 2007. Evaluation of ocular surface changes in a rabbit dry eye model using a modified impression cytology technique. Curr Eye Res 32:301–307. [DOI] [PubMed] [Google Scholar]
- 2.American Optometric Association. [Internet] 2002. Optometric clinical practice guideline: care of the patient with ocular surface disorders [Cited: 10 May 2011]. Available at: http://www.aoa.org/documents/CPG-10.pdf.
- 3.Aragona P, Stilo A, Ferreri F, Mobrici M. 2005. Effects of the topical treatment with NSAIDs on corneal sensitivity and ocular surface of Sjögren's syndrome patients. Eye (Lond) 19:535–539. [DOI] [PubMed] [Google Scholar]
- 4.Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. 2003. The comparison of efficacies of topical corticosteroids and nonsteroidal antiinflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol 136:593–602. [DOI] [PubMed] [Google Scholar]
- 5.Azcarate PM, Venincasa VD, Feuer W, Stanczyk F, Schally AV, Galor A. 2014. Androgen deficiency and dry eye syndrome in the aging male. Invest Ophthalmol Vis Sci 55:5046–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barabino S, Dana MR. 2004. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci 45:1641–1646. [DOI] [PubMed] [Google Scholar]
- 7.Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. 2005. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci 46:2766–2771. [DOI] [PubMed] [Google Scholar]
- 8.Bilkhu PS, Wolffsohn JS, Tang GW, Naroo SA. 2014. Management of dry eye in UK pharmacies. Cont Lens Anterior Eye 37:382 –387. [DOI] [PubMed] [Google Scholar]
- 9.Burgalassi S, Panichi L, Chetoni P, Saettone MF, Boldrini E. 1999. Development of a simple dry eye model in the albino rabbit and evaluation of some tear substitutes. Ophthalmic Res 31:229–235. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Zhang X, Zhang J, Chen J, Wang S, Wang Q, Qu J. 2008. A murine model of dry eye induced by an intelligently controlled environmental system. Invest Ophthalmol Vis Sci 49:1386–1391. [DOI] [PubMed] [Google Scholar]
- 11.Corum I, Yeniad B, Bilgin LK, Ilhan R. 2005. Efficiency of olopatadine hydrochloride 0.1% in the treatment of vernal keratoconjunctivitis and goblet cell density. J Ocul Pharmacol Ther 21:400–405. [DOI] [PubMed] [Google Scholar]
- 12.Davidson HJ, Kuonen VJ. 2004. The tear film and ocular mucins. Vet Ophthalmol 7:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Paiva CS, Pflugfelder SC. 2008. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol 71:89–95. [DOI] [PubMed] [Google Scholar]
- 14.Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, Stern ME, Pflugfelder SC. 2002. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci 43:632–638. [PubMed] [Google Scholar]
- 15.Foster CS, Yuksel E, Anzaar F, [Internet] 2010. Dry eye syndrome. [Cited 10 January 2015]. Available at: www.emedicine.medscape.com/article/1210417-overview.
- 16.Foulks GN. 2008. Pharmacological management of dry eye in the elderly patient. Drugs Aging 25:105–118. [DOI] [PubMed] [Google Scholar]
- 17.Gayton JL. 2009. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol 3:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelatt KN. 1991. Veterinary ophthalmology, 2nd ed. London (United Kingdom): Lea and Febijer. [Google Scholar]
- 19.Hartley C, Williams DL, Adams VJ. 2006. Effect of age, gender, weight, and time of day on tear production in normal dogs. Vet Ophthalmol 9:53–57. [DOI] [PubMed] [Google Scholar]
- 20.Johnson ME, Murphy PJ. 2005. The effect of instilled fluorescein solution volume on the values and repeatability of TBUT measurements. Cornea 24:811–817. [DOI] [PubMed] [Google Scholar]
- 21.Kim EC, Choi JS, Joo CK. 2009. A comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am J Ophthalmol 147:206–213. [DOI] [PubMed] [Google Scholar]
- 22.Kjaergaard SK, Hempel-Jørgensen A, Mølhave L, Andersson K, Juto JE, Stridh G. 2004. Eye trigeminal sensitivity, tear film stability, and conjunctival epithelium damage in 182 nonallergic, nonsmoking Danes. Indoor Air 14:200–207. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi TK, Tsubota K, Takamura E, Sawa M, Ohashi Y, Usui M. 1997. Effect of retinol palmitate as a treatment for dry eye: a cytological evaluation. Ophthalmologica 211:358–361. [DOI] [PubMed] [Google Scholar]
- 24.Kulualp K, Kilic S. 2012. Evaluation of the effects of different therapeutic agents on experimental dry eye for the purposes of ocular surface impairment in mice. J Anim Vet Adv 11:1555–1563. [Google Scholar]
- 25.Kunert KS, Tisdale AS, Gipson IK. 2002. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol 120:330–337. [DOI] [PubMed] [Google Scholar]
- 26.Lekhanont K, Leyngold IM, Suwan-Apichon O, Rangsin R, Chuck RS. 2007. Comparison of topical dry eye medications for the treatment of keratoconjunctivitis sicca in a botulinum toxin B-induced mouse model. Cornea 26:84–89. [DOI] [PubMed] [Google Scholar]
- 27.Lekhanont K, Park CY, Smith JA, Combs JC, Preechawat P, Suwan-Apichon O, Rangsin R, Chuck RS. 2007. Effects of topical antiinflammatory agents in a botulinum toxin b-induced mouse model of keratoconjunctivitis sicca. J Ocul Pharmacol Ther 23:27–34. [DOI] [PubMed] [Google Scholar]
- 28.Mah FS, O'Brien T, Kim T, Torkildsen G. 2008. Evaluation of the effects of olopatadine ophthalmic solution, 0.2% on the ocular surface of patients with allergic conjunctivitis and dry eye. Curr Med Res Opin 24:441–447. [DOI] [PubMed] [Google Scholar]
- 29.Maitchouk DY, Beuerman RW, Ohta T, Stern M, Varnell RJ. 2000. Tear production after unilateral removal of the main lacrimal gland in squirrel monkeys. Arch Ophthalmol 118:246–252. [DOI] [PubMed] [Google Scholar]
- 30.McCabe E, Narayanan S. 2009. Advancements in antiinflammatory therapy for dry eye syndrome. Optometry 80:555–566. [DOI] [PubMed] [Google Scholar]
- 31.Nelson JD, Havener VR, Cameron JD. 1983. Cellulose acetate impressions of the ocular surface. Dry eye states. Arch Ophthalmol 101:1869–1872. [DOI] [PubMed] [Google Scholar]
- 32.Perry HD, Solomon R, Donnenfeld ED, Perry AR, Wittpenn JR, Greenman HE, Savage HE. 2008. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol 126:1046–1050. [DOI] [PubMed] [Google Scholar]
- 33.Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. 2008. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor β2 production. Cornea 27:64–69. [DOI] [PubMed] [Google Scholar]
- 34.Pflugfelder SC. 2004. Antiinflammatory therapy for dry eye. Am J Ophthalmol 137:337–342. [DOI] [PubMed] [Google Scholar]
- 35.Rolando M, Zierhut M. 2001. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol 45:S203–S210. [DOI] [PubMed] [Google Scholar]
- 36.Rothschild CM, Sellon DC, Bryan GM, Gay JM, Hines MT. 2004. Effects of trimethoprim–sulfadiazine on tear production and the fluctations of Schirmer tear test values in horses. Vet Ophthalmol 7:385–390. [DOI] [PubMed] [Google Scholar]
- 37.Sahai A, Malik P. 2005. Dry eye: prevalence and attributable risk factors in a hospital-based population. Indian J Ophthalmol 53:87–91. [DOI] [PubMed] [Google Scholar]
- 38.Sahli E, Hosal BM, Zilelioglu G, Gulbahce R, Ustun H. 2010. The effect of topical cyclosporine A on clinical findings and cytological grade of the disease in patients with dry eye. Cornea 29:1412–1416. [DOI] [PubMed] [Google Scholar]
- 39.Savini G, Prabhawasat P, Kojima T, Grueterich M, Espana E, Goto E. 2008. The challenge of dry eye diagnosis. Clin Ophthalmol 2:31–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schalnus R. 2003. Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica 217:89–98. [DOI] [PubMed] [Google Scholar]
- 41.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. 2003. Prevalence of dry eye syndrome among US women. Am J Ophthalmol 136:318–326. [DOI] [PubMed] [Google Scholar]
- 42.Schein OD, Muñoz B, Tielsch JM, Bandeen-Roche K, West S. 1997. Prevalence of dry eye among the elderly. Am J Ophthalmol 124:723–728. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan DA, Allansmith MR. 1986. Hormonal modulation of tear volume in the rat. Exp Eye Res 42:131–139. [DOI] [PubMed] [Google Scholar]
- 44.Tear Film and Ocular Surface Society. [Internet]. 2007. Report of the Definition, Classification, Management, and Therapy Subcommittee of the International Dry-Eye Workshop. [Cited: 4 March 2011]. Available at: http://www.tearfilm.org/dewsreport/
- 45.Villareal AL, Farley W, Pflugfelder SC. 2006. Effect of topical epinastine and olopatadine on tear volume in mice. Eye Contact Lens 32:272–276. [DOI] [PubMed] [Google Scholar]
- 46.Yang CQ, Sun W, Gu YS. 2006. A clinical study of the efficacy of topical corticosteroids on dry eye. J Zhejiang Univ Sci B 7:675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]